Introduction
Quantitative polymerase chain reaction (qPCR), the
method by which a small sample of genetic material can be
exponentially amplified and quantitatively measured in real time,
is now a mainstay of research and medical laboratories. As the
process has evolved, the applications for qPCR have increased
rapidly, and include the detection of infectious diseases,
paternity identification, forensic analysis and food processing.
The PCR process necessitates the cycling of test samples through a
temperature profile, typically 95, 55 and 72°C, multiple times. The
time taken to change the temperature of the samples between these
levels is a key determinant of the speed of the process and thus of
the duration of a test (1). A
typical 40-cycle PCR can take ~2 h to complete and improvements in
that time have not been achieved as rapidly as the advances in
other areas. Therefore, some of the potential benefits of the qPCR
process remain limited by speed.
Thermal uniformity, the absence of which can cause
discrepancies in the cycling conditions between different samples
on the same plate, is directly linked to speed. In many PCR
instruments, conductive blocks are used to connect the heating or
cooling source(s) to the test samples. When heating and cooling the
system, it is necessary to drive heat into and out of the block.
Temperature gradients are eliminated by the natural flow of heat
within the blocks; therefore, over time the same conditions should
be delivered to all the test samples. However, block-based systems
are vulnerable to greater heat losses on the edges and surfaces
that tend to distort the thermal distribution. The conductivity of
the blocks affects the rate of heat flow and thus the uniformity of
heating of the samples. In addition, the larger the thermal mass of
the block, the greater the amount of heat to be transferred and the
longer this will take. The faster heat is driven into or out of the
system, the less time is available for the temperature distribution
of the conductive block to even out and for thermal uniformity to
be maintained. Ultimately, such a system can only maintain thermal
uniformity if the rate of change of temperature is slower than the
time it takes for the temperature of the conductive block to even
out. To achieve quick cycle times, large temperature gradients are
applied to the block, which can lead to the target temperatures of
samples being over- or undershot. Thus, in these types of systems,
the requirement for uniformity of temperature directly conflicts
with the desire for speed; they are able to deliver one feature or
the other but not both (2).
The ABI Prism 7900HT (Applied Biosystems, Thermo
Fisher Scientific, Waltham, MA, USA) is perhaps the industry
standard peltier/block-based thermal cycler. The CFX96 (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) provided an upgrade to the
conventional system by reducing the thermal mass of the block.
Alternatives to the block-based system have also been developed.
The Rotor-Gene Q (Qiagen, Hilden, Germany) combines a centrifugal
set-up with an air-based thermal system. Ensuring that samples are
continuously rotated through heated air removes the edge effect to
provide superior thermal uniformity. xxpress® (BJS Biotechnologies,
Perivale, London, UK) employs a different system in which an
‘active heating’ method is combined with a block of low thermal
mass, precisely controlling the amount and location of additional
heating to avoid temperature discrepancies (Table I). The present study investigated and
compared the efficiency and thermal uniformity of four of the qPCR
thermal cyclers currently available that use the conventional
block/peltier system or novel methods.
 | Table I.Ramp rate and thermal uniformity of
qPCR instruments. |
Table I.
Ramp rate and thermal uniformity of
qPCR instruments.
| qPCR platform | Thermal system | Advertised fastest
ramp rate (°C/sec) | Advertised thermal
uniformity (°C) |
|---|
| ABI Prism 7900HT | Block/peltier | 1.5 | ±0.5 (measured 30 sec
after timing starts) |
| Bio-Rad CFX96 | Block/peltier |
3.3
(average) | ±0.4 (well-to-well
within 10 sec of reaching 90°C) |
| Qiagen Rotor-Gene
Q | Air |
15
(peak) | ±0.02 |
| BJS biotechnologies
xxpress® | Resistive
heating | 10 | ±0.3 |
Materials and methods
qPCR
The expression of 18S rRNA in human genomic DNA was
assessed and compared by qPCR using an ABI Prism 7900HT, a Bio-Rad
CFX96 System, a Qiagen Rotor-Gene Q and a BJS Biotechnologies
xxpress®. Human genomic DNA was purchased from Bioline (Meridian
Bioscience, London, UK) and input in concentrations of 100, 10, 1,
0.1 and 0.01 ng/µl to give final concentrations of 5, 0.5, 0.05,
0.005 and 0.0005 ng/µl, generating a standard curve. Eukaryotic 18S
rRNA gene primers were used as follows: forward, 3′-AAA CGG CTA CCA
CAT CCA AG-5′ and reverse, 3′-CCT CCA ATG GAT CCT CGT TA-5′. SYBR
FAST qPCR master mix (Kapa Biosystems, Wilmington, MA, USA) was
used across all platforms using the following thermal profile: 20
sec hot start at 95°C followed by 40 cycles of 95°C for 1 sec and
60°C for 10 sec. Heating and cooling rates and all other parameters
were at the manufacturers' pre-set levels.
Thermal variability was assessed in qPCR by
measuring the amplification of 18S rRNA in a selection of wells
covering all areas of the sample plate on ABI Prism 7900HT, Bio-Rad
CFX96 System, Qiagen Rotor-Gene Q and BJS Biotechnologies xxpress
instruments. Human genomic DNA at 100 ng/µl (final concentration, 5
ng/µl) was used with the protocol detailed above.
A standard curve was generated by amplifying 18S
rRNA in human genomic DNA at concentrations of 5, 0.5, 0.05, 0.005
and 0.0005 ng/µl and plotting Ct against log
concentration. Efficiency was calculated by the following equation:
Efficiency = 10(−1/slope)-1. Efficiency of reaction
values between 90 and 110% are considered acceptable for qPCR
reactions.
Statistical analysis
Statistical tests commonly used to determine the
reliability and accuracy of a quantitative PCR assay include
performing a standard curve experiment with each dilution series
run in triplicate. The Ct value was plotted against the
log of the nucleic acid input level to generate a linear graph. The
slope or gradient of this graph was used to determine the PCR
reaction efficiency and a linear regression analysis with a
correlation coefficient or R2 value was included to
determine the accuracy and repeatability of the standard curve. The
ideal result is a PCR reaction efficiency of 100% and an
R2 value of 1. An efficiency of <90 or ≥110% is
unacceptable and indicates that further optimisation is required.
If the R2 value is ≤0.985, this raises questions about
assay reliability with respect to pipetting accuracy and the range
of the assay (3).
Results
Amplification efficiency and thermal
variability
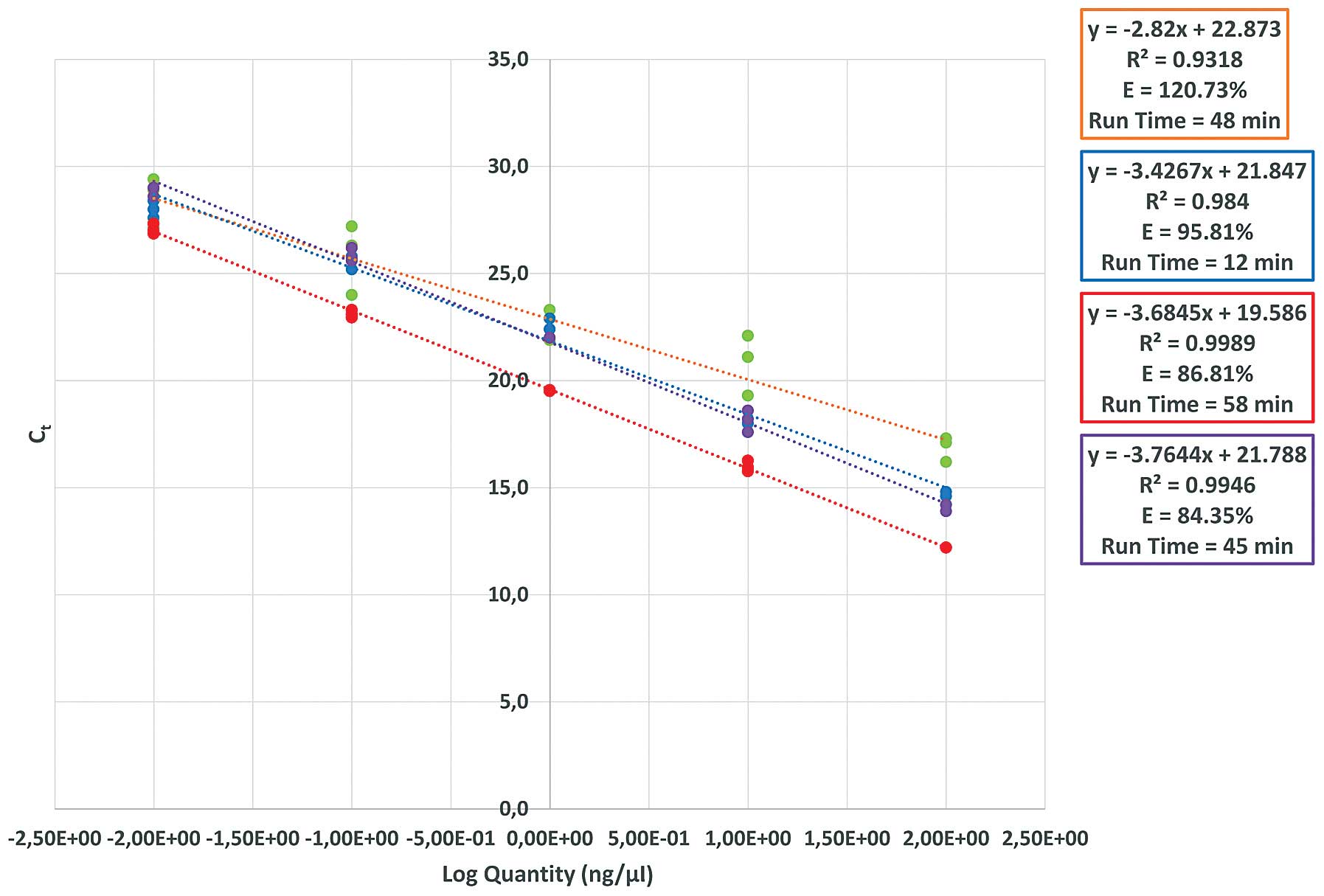
The fastest instrument was the xxpress®, which
completed 40 cycles in 12 min (Fig.
1). In terms of amplification efficiency there was a variation
of ≤36 min among the different cyclers (Fig. 1). The time required to complete a PCR
run may be of particular interest to clinicians, as it may be used
as a point of care testing platform. This is of increasing
significance, particularly for diseases that currently require
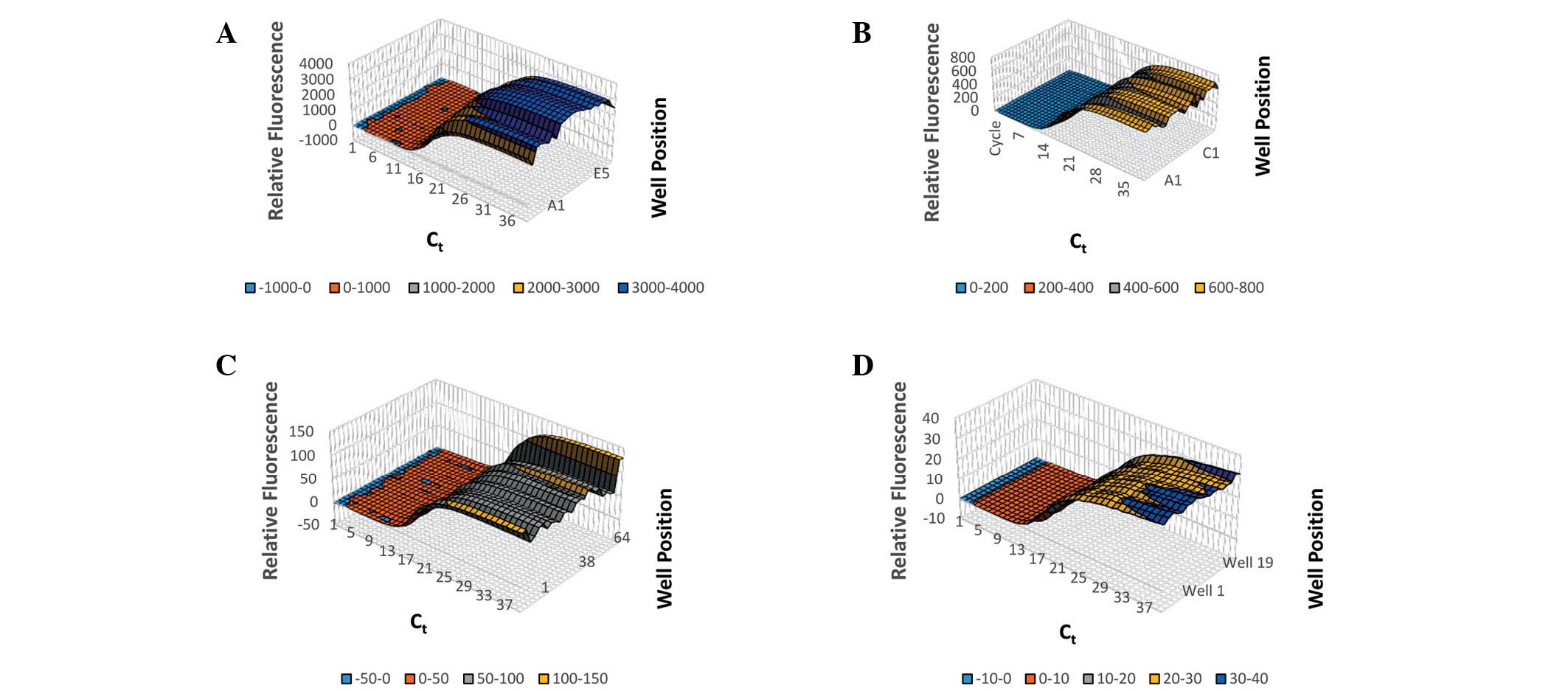
hours or days to diagnose. Thermal variability was assessed by
measuring the amplification of 18S rRNA in 5 ng/µl human genomic
DNA in a selection of wells covering all areas of the sample plate
on ABI Prism 7900HT, Bio-Rad CFX96 System, Qiagen Rotor-Gene Q and
BJS Biotechnologies xxpress instruments (Fig. 2). The average Ct,
Ct spread and Ct standard deviation were for
CFX: 16.0, 1.315 and 0.34; for xxpress: 13.6, 1.2 and 0.29; for
Prism 7900HT: 14.4, 4.526, and 1.91; and for Rotor-Gene: 16.8,
1.319, and 0.43 (Fig. 3).
Discussion
qPCR instrumentation is rapidly evolving not only to
meet the needs of basic science but also in an attempt to address
some of the needs of the current healthcare system, in terms of
diagnosis as well as prognosis. For example, qPCR technology has
been widely used in the field of molecular diagnostics for a number
of infectious diseases (4). Food and
Drug Administration-approved qPCR-based screening tests include
group A Streptococcus and methicillin-resistant
Staphylococcus aureus (MRSA), HIV-1, human metapneumovirus
and H1N1 influenza virus (5,6). More recently, Qiagen received FDA
approval of a therascreen® KRAS RGQ PCR kit, paired with a
colorectal cancer drug. KRAS mutations occur in ~40% of colorectal
cancer patients (7,8). Therefore, screening patients by PCR
will detect the most frequent mutations in the KRAS gene and should
aid with the selection of therapeutic interventions.
Over the past decade, there has been a shift from
testing in reference hospitals/centres to clinical/diagnostic
laboratories worldwide (4). Point of
care testing (POCT) allows a test to be carried out and results
obtained in a single visit to a primary or secondary care health
provider (9). In developing
countries, POCT is perhaps even more effective. The requirement for
expensive, central laboratories, highly trained technicians and a
reliable method of specimen and data transport can all be removed
with the implementation of a well-designed, multifunctional POCT
system. Bringing the test into the clinic allows treatment to
commence without delay and, in areas of high displacement, reduces
the likelihood of losing patient contact before the condition has
been effectively treated. This is particularly important for
communicable diseases such as HIV/AIDS, measles and typhoid fever
(10). An effective POC test in a
low resource setting is inexpensive to use and maintain. The test
must be easy to operate, requiring little to no training or
specialist knowledge to both generate and interpret results. In a
recent study of sub-Saharan Africa, only 34% of hospitals had
reliable electricity access (11).
Since energy access for healthcare facilities in this region varies
markedly, and as electrical sources may be unreliable, low
electrical consumption or even the ability to run on battery or
solar power is desirable.
The results of the present study demonstrate that
the performance of new technologies in qPCR instrumentation such as
Rotor-Gene Q and xxpress, is equally as good as that of
conventional qPCR instruments, in terms of amplification efficiency
and thermal uniformity. Notably, an advantage of the new
technologies is the fast delivery, as rapid testing and diagnosis
may be lifesaving. For example, rapid diagnostic tests can help in
the diagnosis and management of patients who present with signs and
symptoms compatible with influenza. These technologies can reduce
the time from 3–10 days for conventional viral cell cultures, and
requires minutes rather than hours to perform, which may be of
clinical benefit (12). Infections
with MRSA are known to be associated with considerable morbidity
and mortality (13). Current sample
preparation/testing times based on blood samples can take up to 5
h. However, in an emergency situation this process might be too
long if the patient admitted is positive for MRSA and therefore has
the potential to infect others. Equally, an early diagnosis of
tuberculosis will assist not only in the initiation of appropriate
treatment but also limit the spread of this highly contagious
disease (14). A test that could be
administered either at admission to the clinic, or even in an
ambulance on the way to the hospital, and takes only 10 min could
be of real benefit. Moreover, given the unreliability of
electricity in the developing world, diagnostic instrumentation
that is rapid is vital.
To date, qPCR-based diagnosis is often associated
with high cost, time-consuming procedures, scientists and
clinicians trained in qPCR analysis, lack of specificity and
sensitivity or even standardisation for certain tests. In the
future (Fig. 4), a standardised,
rapid, scalable, affordable and easy-to-use qPCR platform for use
in POCT should provide an invaluable platform in the field of
diagnostic/prognostic testing that will complement the current
conventional methods, including microscopy, cell culture and
immunological-based methods.
Acknowledgements
BJS partially funded a PhD studentship and
collaborates with Brunel University for developing rapid diagnostic
testing for sepsis.
References
|
1
|
Dooms M, Chango A and Abdel-Nour A:
Quantitative PCR (qPCR) and the guide to good practices MIQE:
adapting and relevance in the clinical biology context. Ann Biol
Clin (Paris). 72:265–269. 2014.(In French). PubMed/NCBI
|
|
2
|
Burroughs N and Karteris E:
Ultra-high-speed PCR instrument development. PCR, Technology
Current Innovations. Nolan T and Bustin SA: CRC Press; Florida: pp.
143–157. 2013, View Article : Google Scholar
|
|
3
|
Kavanagh I, Jones G and Nayab SN:
Significance of controls and standard curves in PCR. PCR
Troubleshooting and Optimization: The Essential Guide. Kennedy S
and Oswald N: Caister Academic Press. (Norfolk, UK). 67–78.
2011.
|
|
4
|
Muldrew KL: Molecular diagnostics of
infectious diseases. Curr Opin Pediatr. 21:102–111. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Emmadi R, Boonyaratanakornkit JB,
Selvarangan R, et al: Molecular methods and platforms for
infectious diseases testing. A review of FDA-approved and cleared
assays. J Mol Diagn. 13:583–604. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Peterson LR, Hacek DM and Robicsek A: 5
million lives campaign. Case study: An MRSA intervention at
Evanston Northwestern Healthcare. Jt Comm J Qual Patient Saf.
33:732–738. 2007.PubMed/NCBI
|
|
7
|
Rodriguez R: Biomarker testing for
treatment of metastatic colorectal cancer: Role of the pathologist
in community practice. J Community Support Oncol. 12:27–32.
2014.PubMed/NCBI
|
|
8
|
Therkildsen C, Bergmann TK,
Henrichsen-Schnack T, Ladelund S and Nilbert M: The predictive
value of KRAS, NRAS, BRAF, PIK3CA and PTEN for anti-EGFR treatment
in metastatic colorectal cancer: A systematic review and
meta-analysis. Acta Oncol. 53:852–864. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Niemz A, Ferguson TM and Boyle DS:
Point-of-care nucleic acid testing for infectious diseases. Trends
Biotechnol. 29:240–250. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wynberg E, Toner S, Wendt JK, Visser LG,
Breederveld D and Berg J: Business travelers' risk perception of
infectious diseases: Where are the knowledge gaps and how serious
are they? J Travel Med. 20:11–16. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Adair Rohani, Zukor K, Bonjour S, Wilburn
S, Kuesel AC, Hebert R and Fletcher ER: Limited electricity access
in health facilities of sub-Saharan Africa: A systematic review of
data on electricity access, sources and reliability. Glob Health
Sci Pract. 1:249–261. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Centers for Disease Control and
Prevention. Rapid Diagnostic Testing for Influenza: Information for
Health Care Professionals. http://www.cdc.gov/flu/professionals/diagnosis/rapidclin.htmAccessed.
September 5th–2014
|
|
13
|
Cosgrove SE, Sakoulas G, Perencevich EN,
Schwaber MJ, Karchmer AW and Carmeli Y: Comparison of mortality
associated with methicillin-resistant and methicillin-susceptible
Staphylococcus aureus bacteremia: A meta-analysis. Clin
Infect Dis. 36:53–59. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Niemz A and Boyle DS: Nucleic acid testing
for tuberculosis at the point-of-care in high-burden countries.
Expert Rev Mol Diagn. 12:687–701. 2012. View Article : Google Scholar : PubMed/NCBI
|