Introduction
Pulmonary fibrosis is a progressive and fatal
disease characterized by pulmonary interstitial fibrosis, which may
result in diffuse alveolar inflammation and structural disorder
with decreasing lung volumes and hypoxemic respiratory failure
(1,2). Despite intense research efforts, there
are currently no effective therapies to reduce the high fatality
rate of pulmonary fibrosis (3).
Bone marrow-derived mesenchymal stem cells (BMSCs)
are easy to separate, purify and augment in vitro, and are
targeted to and differentiate in damaged tissue. Furthermore, BMSCs
are able to regulate immune function and promote the expression of
various growth factors locally in the damaged tissue, thus reducing
inflammation and collagen deposition and potentially exerting a
therapeutic effect against lung fibrosis (4,5).
Survivin is a member of the apoptosis inhibitory
protein family, and exerts an antiapoptotic effect primarily by
inhibiting the activity of cysteine-aspartic acid protease-3
(caspase-3) (6). Lentiviruses are
among the most commonly used vectors of gene therapy, possessing
high transfection efficiency and a high level of exogenous gene
expression. Green fluorescent protein (GFP) is characterized by
stability, high efficiency and non-toxicity and is easy to detect.
GFP can used to observe living cells directly and to trace stem
cells in vivo. In the present study, BMSCs containing GFP
and the survivin gene were introduced into a bleomycin-induced
mouse model of pulmonary fibrosis. The effect of BMSCs and survivin
on the pulmonary fibrosis was then assessed.
Materials and methods
Reagents and kits
BMSCs were obtained from Cyagen Biosciences, Inc.
(Santa Clara, CA, USA). Cell culture medium and fetal bovine serum
(FBS) were purchased from Gibco Life Technologies (Carlsbad, CA,
USA). Bleomycin hydrochloride for injection was purchased from
Nippon Kayaku Co., Ltd. (Tokyo, Japan). An hydroxyproline ELISA
detection kit was purchased from Wuhan ColorfulGene Biological
Technology Co., Ltd. (Wuhan, China). Quantitative polymerase chain
reaction (qPCR) reagents were obtained from Takara Biotechnology
Co., Ltd. (Dalian, China), rabbit anti-mouse surfactant protein A
(SP-A) immunohistochemistry kits from Wuhan Boster Biological
Technology, Ltd. (Wuhan, China) and Beyotime Institute of
Biological Technology (Haimen, China). Secondary antibodies for
western blot analysis were purchased from Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA).
Animals
All procedures in this study were approved by the
Animal Studies Committee of Chongqing Medical University
(Chongqing, China) and were conducted in accordance with the Guide
for the Care and Use of Laboratory Animals published by the
National Institutes of Health. Male C57BL/6 mice, 6–8 weeks old and
weighing 22 g, were supplied by the animal center of Chongqing
Medical University and were housed in a specific pathogen-free
facility.
BMSC culture
This study was conducted in the Respiratory
Department of the First Affiliated Hospital of Chongqing Medical
University. BMSCs derived from C57BL/6 mice were cultured in
F12-Dulbecco's modified Eagle's medium (DMEM) medium containing 10%
FBS at 37°C in 5% CO2. The medium was exchanged every
two days and the cells were digested with 0.1% pancreatin for
subculture. The cellular morphology and apoptosis rate of
unmodified BMSCs and BMSCs transfected with the survivin gene were
detected for comparison. Subsequently, the BMSC populations were
collected for injection into the animals.
Establishment of viral vector and
transfection into BMScs
The viral vector (pLV-EX3d-P/hygro-EF1A-IRES-EmGFP,
a GFP-marked vector) was purchased from Saiye (Guangzhou) Wusheng
Technology Co., Ltd. (Guangzhou, China). The mouse survivin gene
was cloned into the pLV-EX3d-P/hygro-EF1A-IRES-EmGFP viral vector.
This successfully established the
pLV-EX3d-P/hygro-EF1A-mSurvivin-IRES-EmGFP viral vector. This viral
vector was transfected into BMSCs cultured in DMEM medium, followed
by screening and amplification in the cells. The blank
pLV-EX3d-P/hygro-EF1A-IRES-EmGFP vector was used to transfect the
cells in the control group.
Pulmonary fibrosis model
establishment
Fifty-four C57BL/6 mice with normal breeding were
allocated at random into three groups: BMSC-survivin group (group
A), BMSC group (group B) and the model group (group C). Anesthesia
was induced by an intraperitoneal injection of 10% chloral hydrate
(300 mg/kg). Then, the neck skin was disinfected and incised and
0.2 ml bleomycin solution (5 mg/kg) was gradually administered
dropwise into the trachea to induce the pulmonary fibrosis model.
After 24 h, mice in group A received 1×106 survivin
gene-expressing BMSCs-in 200 µl normal saline via the caudal vein,
mice in group B received 1×106 BMSCs, while group C mice
were treated with 200 µl normal saline. A selection of 6 mice from
each group were sacrificed on days 7, 14 and 28, respectively.
Following anesthesia, blood was collected from the vena jugularis
externa, allowed to settle for 10 min, centrifuged at 10,000 × g
for 10 min at 4°C, then stored at −80°C. The left lung was used for
pathological section, while the right lung was stored in liquid
nitrogen for reverse transcription (RT)-qPCR and western blot
analysis.
Analysis of pathology in lung
tissue
Lung tissue was fixed in 4% paraformaldehyde for 24
h, then subjected to dehydration, paraffin, embedding, sectioning
and hematoxylin and eosin (H&E) staining. Finally, the lung
tissue was observed under a microscope in order to assess the
degree of pulmonary fibrosis. The cells were also observed using a
BX53 fluorescence microscope (Olympus Corporation, Tokyo,
Japan).
SP-A determination in the lung tissue
using immunohistochemistry
Lung tissue slices were dewaxed in xylene, treated
with citric acid for antigen retrieval and then treated for 30 min
with H2O2 to inactivate endogenous
peroxidase. Slices were treated for 30 min with normal goat serum
and incubated with a rabbit anti-mouse SP-A primary antibody
(1:3,000; sc-7700; Santa Cruz Biotechnology, Inc.) overnight at
4°C. Samples were rewarmed for 1 h at 37°C, then incubated with a
biotinylated goat anti-rabbit secondary antibody (1:2,000;
sc-45101; Santa Cruz Biotechnology, Inc.) at 37°C for 30 min. Next,
streptavidin-biotin complex was added at 37°C for 30 min, and the
samples were stained using diaminobenzidine. Next, the samples were
subjected to nuclear staining with hematoxylin, xylene dehydration,
transparency (using Triton-X 100) and fixation with neutral balata.
Images were obtained under a CX22 light microscope (Olympus
Corporation) and analyzed using Image-Pro Plus software, version
2.0 (Media Cybernetics, Inc., Rockville, MD, USA). The average
optical density (OD) represents the SP-A content.
Hydroxyproline content
determination
Following anesthesia, blood was collected via the
vena jugularis externa, allowed to stand for 10 min, centrifuged
for 10 min at a temperature of 4°C, then kept at −80°C until
analysis. The hydroxyproline content was determined according to
the instructions provided by the manufacturer of the hydroxyproline
ELISA kit. OD values were obtained for each sample at 450 nm. A
standard curve was produced, and the concentration of
hydroxyproline in each sample was determined from the standard
curve, where the volume of the sample (10 µl) was increased to 50
µl with phosphate buffer solution.
Flow cytometry
Cell viability was evaluated by flow cytometry using
Annexin V-fluorescein isothiocyanate (FITC) and propidium iodide
(PI) double staining (Trevigen, Minneapolis, MN, USA). Briefly, the
lung cells were suspended in Annexin V-FITC binding buffer and
treated with Annexin V-FITC and PI at room temperature overnight.
The stained cells were observed and analyzed using FACScan flow
cytometry (Becton-Dickinson, Oxford UK).
RT-qPCR analysis
The total RNA of lung tissues was isolated using an
RNAsimple Total RNA kit according to the manufacturers instructions
(Tiangen Biotech Co., Ltd., Beijing, China). RT was performed using
the SuperScript™ III First-Strand Synthesis System (Invitrogen Life
Technologies, Carlsbad, CA, USA) according to the manufacturer's
instructions. The procedure and conditions of the RT-qPCR analysis
were as previously reported (4).
RT-qPCR was performed using SYBR Premix Ex Taq II (Takara
Biotechnology Co., Ltd., Dalian, China) using an ABI7500 Real-time
PCR instrument (Applied Biosystems, Foster City, CA, USA). The
products of the RT reaction were used for qPCR. Matrix
metalloprotease (MMP)-9 and transforming growth factor (TGF)-β1
levels were detected by qPCR. Primer-BLAST (http://www.ncbi.nlm.nih.gov/tools/primer-blast/)
was used to design the primer sequences. The qPCR primer sequences
were as follows: MMP-9, 5′-GCG CCA CCA CCG CCA ACT AT-3′ (upstream)
and 5′-CTC GTG CGC TGC CAC CAG AA-3′ (downstream); TGF-β1, 5′-TAA
CCG GCT GCT GAC CCC-3′ (upstream) and 5′-ATC CAG GGC TCT CCG GTG
CC-3′ (downstream); β1-actin, 5′-AGG CTG TGC TGT CCC TGT ATG-3′
(upstream) and 5′-GAG GTC TTT ACG GAT GTC AAC G-3′ (downstream).
Following qPCR, the products were subjected to 2% agarose gel
electrophoresis and the resulting images were analyzed using
Quantity One software (Bio-Rad Laboratories, Inc., Hercules, CA,
USA), which calculated the gray area value of electrophoresis. The
gray area ratios MMP-9/β-actin and TGFβ-1/β-actin represent the
relative quantities of MMP-9 and TGF-β1, respectively.
Western blot assay
The lung tissues were isolated, snap-frozen in
liquid nitrogen and immediately homogenized in ice-cold RIPA lysis
buffer (Sigma-Aldrich, St. Louis, MO, USA) containing a cocktail of
protease inhibitors (Roche, Basel, Switzerland). Caspase-3 and
caspase-9 proteins were then detected using a western blot assay.
Extracted total proteins (25 g) from lung tissue were resolved by
SDS-PAGE, then transferred to a polyvinylidene difluoride membrane,
which was blocked with 5% skimmed milk powder solution for 1.5 h.
The membranes were treated with rabbit anti-mouse caspase-3
monoclonal antibody (1:3,000; Santa Cruz Biotechnology, Inc.) and
rabbit anti-mouse caspase-9 antibody (1:4,000; Santa Cruz
Biotechnology, Inc.) overnight at 4°C. The membranes were washed,
treated with goat anti-rabbit polyclonal secondary antibody
(1:2,000; Santa Cruz Biotechnology, Inc.) for 2 h at room
temperature, and then developed using an enhanced chemiluminescent
(ECL) reagent (Thermo Scientific Pierce, Rockford, IL, USA).
Analysis of the average OD value of images was performed using
Quantity One software. The OD ratios of caspase-3/β-actin and
caspase-9/β-actin represent the protein expression levels of
caspase-3 and caspase-9, respectively.
Statistical analysis
Experimental data were analyzed using SPSS
statistical software, version 17.0 (SPSS, Inc., Chicago, IL, USA).
Measurement values are expressed as the mean ± standard error of
the mean. Inter-group comparisons were performed using one-way
analysis of variance. P<0.05 was considered to indicate a
statistically significant difference.
Results
Morphology of BMSCs
BMSC distribution showed significant aggregation.
Although the cells were predominantly single, 2–3-cell clusters of
small cells were also present (Fig.
1). The BMSCs appeared ovular or cuboid in shape, some with a
spindle shape on one side with a large nucleus which was circular
or elliptic (Fig. 1). Cells with
normal and uniform distribution presented with visible green
fluorescence when observed under a fluorescence inverted
microscope.
Survivin decreases the rate of
apoptosis of BMSCs
The apoptosis rate of the BMSCs was detected using a
flow cytometry assay with Annexin V-FITC and PI double staining.
The results were that the apoptosis rate of BMSCs carrying the
survivin gene was 7.718±0.493%, which was significantly reduced
compared with that of the BMSC group (14.466±1.953%, P<0.05;
Fig. 2), indicating that the
survivin gene reduces the apoptosis rate of BMSCs and prolongs the
survival time of these cells in vitro.
Survivin enhances the anti-fibrotic
effect of BMSCs
Fibrosis in group A was significantly inhibited
compared with that in groups B and C at days 14 and 28 following
treatment (Fig. 3). However, no
significant differences were detected among the three groups at day
7.
Survivin mitigates the inflammatory
response in the alveoli
In group C, numerous inflammatory cells had
infiltrated around the alveoli by day 7; on day 14, the alveolar
interval had thickened, inflammatory cell infiltration was
extensive, and the alveolar structure was observed to have
collapsed; and on day 28 the alveolar structure was collapsed and
degraded more seriously, and the alveolar spaces and intervals were
occupied by collagen and fibrin. The alveolar inflammation in group
B was less severe on day 7 compared with that in group C. The
amount of fibrin was also relatively less in group B than in group
C. In group A, the inflammatory reaction was lighter than that in
group B, and a small amount of fibrosis was distributed in the
alveolar interval on the day 7. On day 28, some of the alveolar
structure had collapsed, there was a little collagen in the
alveolar interval, and alveolar damage was rare (Fig. 4 and Table
I).
 | Table I.Comparison of mean optical density of
surfactant protein A expression among the groups (mean ± standard
deviation). |
Table I.
Comparison of mean optical density of
surfactant protein A expression among the groups (mean ± standard
deviation).
| Group | Day 7 | Day 14 | Day 28 |
|---|
| A |
0.718±0.008a,b |
0.643±0.013a,b |
0.433±0.012a,b |
| B |
0.669±0.013c |
0.550±0.024c |
0.372±0.009c |
| C |
0.581±0.007 |
0.362±0.011 |
0.277±0.013 |
Survivin decreases hydroxyproline
levels in BMSCs
The ELISA results indicated statistically
significant differences among the groups in the levels of
hydroxyproline. At each time point the hydroxyproline levels in
group A were reduced compared with those in group B, while group B
hydroxyproline levels were reduced compared with those in group C
(Table II).
 | Table II.Comparison of hydroxyproline content
(µg/ml) among the groups (mean ± standard deviation) as determined
by ELISA. |
Table II.
Comparison of hydroxyproline content
(µg/ml) among the groups (mean ± standard deviation) as determined
by ELISA.
| Group | Day 7 | Day 14 | Day 28 |
|---|
| A |
0.106±0.002a,b |
0.328±0.004a,b |
0.457±0.006a,b |
| B |
0.245±0.009c |
0.472±0.012c |
0.752±0.012c |
| C |
0.324±0.002 |
0.794±0.007 |
1.372±0.035 |
Survivin suppresses the expression
levels of TGF-β1 and increases those of MMP-9
The expression levels of TGF-β1 and MMP-9 mRNA were
detected using an RT-qPCR assay. The expression levels of TGF-β1
were significantly decreased in group A compared with groups B and
C (Fig. 5; P<0.05). Furthermore,
the TGF-β1 expression levels in group B were significantly
decreased compared with those in group C (Fig. 5, P<0.05). The mRNA expression
levels of MMP-9 in group A were significantly increased compared
with those in groups B and C (Fig.
5; P<0.05). Furthermore, the MMP-9 expression levels in
group B were significantly increased compared with those in group C
(P<0.05).
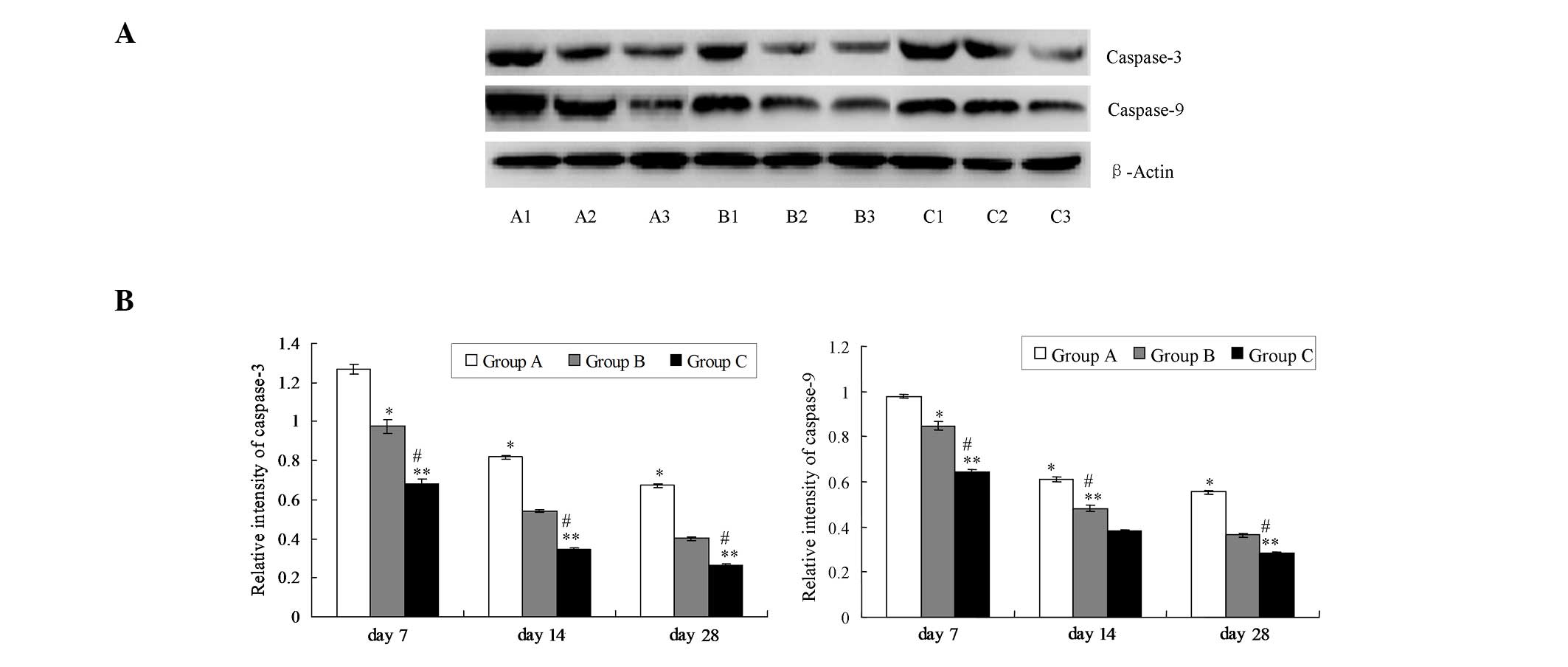
Survivin increases the expression of
caspase-3 and caspase-9
The expression levels of caspase-3 and caspase-9
were detected using a western blot assay. The results indicate that
survivin significantly elevated caspase-3 and caspase-9 expression
in group A compared with that in groups B and C (Fig. 6; P<0.05). Furthermore, the levels
of caspase-3 and caspase-9 in group B were significantly elevated
compared with those in group C (Fig.
6; P<0.05).
Discussion
As the incidence of pulmonary fibrosis is
increasing, a number of recent studies have indicated a range of
factors that may cause damage to alveolar epithelial and
endothelial cells. The abnormal repair response to this damage may
lead to fibroblast activation and excessive accumulation of
extracellular matrix (ECM) in the lung parenchyma (7,8), which
may ultimately lead to the occurrence of pulmonary fibrosis. At
present, glucocorticoids are the preferred drug treatment for
pulmonary fibrosis; however, the curative effect of glucocorticoids
is not sufficient to reverse or retard the course of pulmonary
fibrosis. Furthermore, the long-term use of glucocorticoids may
result in a series of complications, such as bacterial or fungal
lung infection and metabolic disorders. A number of previous
studies have investigated the use of agents targeting cytokines
(9), such as pirfenidone, imatinib
and interferon (IFN) (10),
particularly IFN-α, as a treatment for pulmonary fibrosis. Liu
et al (11) reported that
IFN-α may inhibit the expression of TGF-β1 and connective tissue
growth factor, and thus reduce the generation of ECM in a
bleomycin-induced model of pulmonary fibrosis. However, IFN-α is an
expensive treatment, which limits its potential for widespread use.
In addition, IFN-α exhibits a number of potential side-reactions.
N-acetyl cysteine is able to notably increase glutathione levels in
the lung tissue and exhibits strong resistance to oxidation and
cell detoxification. Hence, N-acetyl cysteine may improve the lung
function of patients with idiopathic pulmonary fibrosis and is well
tolerated, but is not able to reduce mortality (12). Lung transplantation is the primary
method for treating end-stage pulmonary fibrosis and can markedly
improve the lung function of patients (13); however, lung transplantation is not
applicable to a wide range of clinics. Certain traditional Chinese
medicines may exert curative effects against lung fibrosis
(14), but the underlying mechanisms
of these treatments require further study. Thus, the treatment of
pulmonary fibrosis remains challenging, and a safer and more
effective therapy for pulmonary fibrosis is urgently required.
A number of studies have demonstrated that BMSCs are
able to grow and participate in the development of lung tissue. The
administration of BMSCs has been shown to ameliorate fibrotic
injuries, suggesting the possibility that stem cell-based therapies
may be developed as an effective intervention against pulmonary
fibrosis (15–18). BMSCs exist in numerous types of human
tissue, possess multidirectional differentiation potential and may
be obtained from a variety of tissues. Currently, the studies
investigating BMSC separation, differentiation, expansion, immune
phenotype, features and mechanism, including immune regulation and
anti-inflammatory function, have increased significantly (19). Previous studies have confirmed that
exogenous BMSCs may be transplanted into impaired lung tissue, and
are able to differentiate into alveolar type II epithelial cells,
which possess a higher migration rate in the early stages of lung
injury (1,18,20).
Furthermore, a previous animal study confirmed that BMSCs are able
to develop in lung tissue afflicted with bleomycin-induced
pulmonary fibrosis, and to reduce the extent of the pulmonary
fibrosis (21).
The primary function of the survivin gene is to
inhibit cell division and apoptosis. Survivin is the regulatory
gene of the cell cycle in the G2/M phase, causing cells to exit the
G2/M phase checkpoint and accelerate conversion to the S phase, in
addition to inhibiting stationary G phase. Furthermore, survivin
participates in the regulation of cellular mitosis by binding with
the microtubule proteins of the mitotic spindle (22), promoting the abnormal proliferation
of transformed cells and mitigating the occurrence of apoptosis.
Furthermore, previous studies have reported that survivin is
associated with tumor angiogenesis and multiple drug resistance
(23,24). Therefore, it may be speculated that
survivin enhances the antifibrotic effect of BMSCs by inhibiting
the occurrence of apoptosis.
In the present study, BMSCs transfected with
survivin were transferred into the lung tissue of mice modeling
pulmonary fibrosis as a result of treatment with bleomycin 24 h
previously. This was conducted to evaluated the hypothesis that the
apoptosis of such BMSCs may be inhibited and the repair of damaged
lung tissue promoted, thus mitigating the progression of pulmonary
fibrosis.
The results of the present experiments indicate that
the BMSCs containing GFP and survivin were successfully
transplanted into the mice modeling bleomycin-induced pulmonary
fibrosis. Green fluorescence was detected in the lung tissue,
demonstrating that the BMSCs targeted damaged lung tissue. The
degree of pulmonary fibrosis in group B was evidently reduced
compared with that in group C, while the damage in group A was
lessened compared with that in group B. Immunohistochemical
detection showed that SP-A expression in group B was increased
compared with that in group C, while SP-A expression in group A was
higher compared with that in group B. Collectively, these results
indicate that BMSCs may differentiate into epithelial cells and
alveolar type II epithelial cells in damaged lung tissue,
participate in the repair of lung injury and reduce pulmonary
fibrosis, which is consistent with the previous results of Chen
et al (25). In vitro,
the results of flow cytometry indicate that the apoptosis rate of
BMSCs transfected with survivin was significantly lower compared
with that of BMSCs that were not transfected with survivin.
Fluorescence intensity in the lung tissue of group A was markedly
increased compared with that in group B. Western blot analysis
results indicate that the protein expression levels of caspase-3
and caspase-9, which are effectors in the functional pathway of the
survivin gene, were increased in group A compared with the levels
in the other two groups, indicating that the expression of survivin
gene was increased in group A. Hence, the survivin gene may delay
BMSC apoptosis in vivo and in vitro, and sequentially
block the process of pulmonary fibrosis in the initial stages.
The RT-qPCR results suggest that the mRNA expression
levels of MMP-9 were increased and those of TGF-β1 were reduced in
group A compared with those in group B. Since MMP-9 and TGF-β1 are
crucial factors associated with pulmonary fibrosis (3,26), these
results demonstrate that BMSCs, particularly when transfected with
survivin, may be able to reduce bleomycin-induced pulmonary
fibrosis in mice, which is consistent a previous report (21). Furthermore, previous studies have
indicated that BMSCs secrete a variety of cell factors that
participate in vascular regeneration and endothelial cell
remodeling in lung tissue, and these factors may induce BMSCs to
differentiate (6,27). In addition, it has been reported that
BMSCs are able to promote the expression of growth factors in local
damaged tissue, inhibiting cell apoptosis, reinforcing cell repair
and promoting cell proliferation and differentiation (28). However, further studies are required
to confirm the present and previous findings.
BMSCs possess multidirectional differentiation
potential; a previous study suggested that stem cells contain gene
sequences that resemble those of cancer cells (29). Human mesenchymal stem cells may
transform spontaneously and exhibit long-term proliferation and
differentiation in vivo (30). These observations suggest that
ensuring the safety assessment of BMSC-based treatment is crucial
(31). Survivin is expressed in the
majority of tumor tissues, in which it is able to inhibit tumor
cell apoptosis and induce cancer cells to proliferate and
differentiate constantly, thus enhancing tumor growth. In addition,
survivin may cause loss of the normal cell cycle checkpoint,
leading to disorders of the normal cell cycle (32).
In summary, the results of the present study
indicate that BMSCs are effective in preventing bleomycin-induced
lung fibrosis, and that survivin is able to enhance the protective
effects of BMSCs. However, further safety assessments are required
to evaluate the potential clinical value of this intervention,
including further investigation into cancerous characteristics of
the survivin gene.
References
|
1
|
Liu X, Qian L, Nan HY, Cui M, Hao XY and
Du YF: Function of the transforming growth factor-β 1/c-Jun
N-terminal kinase signaling pathway in the action of thalidomide on
a rat model of pulmonary fibrosis. Exp Ther Med. 7:669–674.
2014.PubMed/NCBI
|
|
2
|
Moodley Y, Atienza D, Manuelpillai U,
Samuel CS, Tchongue J, Ilancheran S, Boyd R and Trounson A: Human
umbilical cord mesenchymal stem cells reduce fibrosis of
bleomycin-induced lung injury. Am J Pathol. 175:303–313. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Selman M, King TE and Pardo A: Idiopathic
pulmonary fibrosis: prevailing and evolving hypotheses about its
pathogenesis and implications for therapy. Ann Intern Med.
134:136–151. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ortiz LA, Gambelli F, McBride C, Gaupp D,
Baddo M, Kaminski N and Phinney DG: Mesenchymal stem cell
engraftment in lung is enhanced in response to bleomycin exposure
and ameliorates its fibrotic effects. Proc Natl Acad Sci USA.
100:8407–8411. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang ZH, Lu Y, Luan Y and Zhao JJ: Effect
of bone marrow mesenchymal stem cells on experimental pulmonary
arterial hypertension. Exp Ther Med. 4:839–843. 2012.PubMed/NCBI
|
|
6
|
Kondo K, Shintani S, Shibata R, Murakami
H, Murakami R, Imaizumi M, Kitagawa Y and Murohara T: Implantation
of adipose-derived regenerative cells enhances ischemia-induced
angiogenesis. Arterioscler Thromb Vasc Biol. 29:61–66. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tzouvelekis A, Harokopos V, Paparountas T,
Oikonomou N, Chatziioannou A, Vilaras G, Tsiambas E, Karameris A,
Bouros D and Aidinis V: Comparative expression profiling in
pulmonary fibrosis suggests a role of hypoxia-inducible factor-1α
in disease pathogenesis. Am J Respir Crit Care Med. 176:1108–1119.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu Z, Wang X, Yang R, Liu Y, Zhao W, Si J,
Ma X, Su C, Liu Y, Tan Y, Liu W, et al: Effects of carbon ion beam
irradiation on lung injury and pulmonary fibrosis in mice. Exp Ther
Med. 5:771–776. 2013.PubMed/NCBI
|
|
9
|
Sun YW, Li BP, Wang Xuan, Zhang L, Lu PY
and Zhao XY: Oriented migration of intravenously administrated
mesenchymal stem cells transfected with adenovirus vector mediated
green fluorescence protein in the lung tissue of pulmonary
emphysema rats. Zhongguo Zu Zhi Gong Cheng Yan Jiu Yu Lin Chuang
Kang Fu. 14:2528–2532. 2010.(In Chinese).
|
|
10
|
Cai Y: Pulmonary fibrosis drug treatment
progress. Shi Jie Lin Chuang Yao Wu. 29:17–19. 2008.(In
Chinese).
|
|
11
|
Liu T, Xie M, Wang HL and Ling W: Effect
of matrine and IFN-γ on bleomycin-induced pulmonary fibrosis.
Nanjing Yi Ke Da Xue Xue Bao. 28:592–596. 2008.(In Chinese).
|
|
12
|
Bhatt N, Baran C, Allen J, Magro C and
Marsh CB: Promising pharmacologic innovations in treating pulmonary
fibrosis. Curr Opin Pharmacol. 6:284–292. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lettieri CJ, Veerappan GR, Helman DL,
Mulligan CR and Shorr AF: Outcomes and safety of surgical lung
biopsy for interstitial lung disease. Chest. 127:1600–1605. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hu F: Research progress of Chinese
medicine treatment of pulmonary fibrosis. Yi Yao Dao Bao.
11:1471–1474. 2011.(In Chinese).
|
|
15
|
Xu L, Xiao W, Ma DD, Zhou SY and Zhang QH:
Ulcerative colitis combined with acute interstitial lung disease
and airway disease: A case report and literature review. Exp Ther
Med. 8:1229–1236. 2014.PubMed/NCBI
|
|
16
|
Kotton DN, Ma BY, Cardoso WV, Sanderson
EA, Summer RS, Williams MC and Fine A: Bone marrow-derived cells as
progenitors of lung alveolar epithelium. Development.
128:5181–5188. 2001.PubMed/NCBI
|
|
17
|
Krause DS, Theise ND, Collector MI,
Henegariu O, Hwang S, Gardner R, Neutzel S and Sharkis SJ:
Multi-organ, multi-lineage engraftment by a single bone
marrow-derived stem cell. Cell. 105:369–377. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Teke T, Maden E, Kiyici A, Korkmaz C, Gok
Z, Ozer F, Imecik O and Uzun K: Cigarette smoke and
bleomycin-induced pulmonary oxidative stress in rats. Exp Ther Med.
4:121–124. 2012.PubMed/NCBI
|
|
19
|
Tzouvelekis A, Antoniadis A and Bouros D:
Stem cell therapy in pulmonary fibrosis. Curr Opin Pulm Med.
17:368–373. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cui A, Dai HP and Dai JW: Effects of bone
marrow mesenchymal stem cells on bleomycin induced pulmonary
fibrosis in rats. Zhonghua Jie He He Hu Xi Za Zhi. 30:677–682.
2007.(In Chinese). PubMed/NCBI
|
|
21
|
Rojas M, Xu J, Woods CR, Spears W, Roman J
and Brigham KL: Bone marrow-derived mesenchymal stem cells in
repair of the injured lung. Am J Respir Cell Mol Biol. 33:145–152.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Beardmore VA, Ahonen LJ, Gorbsky GJ and
Kallio MJ: Survivin dynamics increases at centromeres during G2/M
phase transition and is regulated by microtubule-attachment and
Aurora B kinase activity. J Cell Sci. 117:4033–4042. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang M, Liu Y, Lu S, Wang Z, Wang R, Zi Y
and Li J: Analysis of the expression levels of surviving and VEGF
in patients with acute lymphoblastic leukemia. Exp Ther Med.
5:305–307. 2013.PubMed/NCBI
|
|
24
|
Liu F, Liu S, He S, Xie Z, Zu X and Jiang
Y: Survivin transcription is associated with P-glycoprotein/MDR1
over expression in the multidrug resistance of MCF-7 breast cancer
cells. Oncol Rep. 23:1469–1475. 2010.PubMed/NCBI
|
|
25
|
Chen Y, Ma N, Mei J, Xiao HB, Lu SW, Xu HY
and Zhong H: In vitro induction of human bone marrow
mesenchymal stem cells to differentiate into type II alveolar
epithelial cells. Zhong Guo Zu Zhi Gong Cheng Yan Jiu.
16:1737–1741. 2012.(In Chinese).
|
|
26
|
Guo T, Fang M, Zhang D and Li X:
Combination treatment with asiaticoside and repamycin: A new hope
for in-stent restenosis. Exp Ther Med. 6:557–561. 2013.PubMed/NCBI
|
|
27
|
Jiang PC, Xiong WP, Wang G, Ma C, Yao WQ,
Kendell SF, Mehling BM, Yuan XH and Wu DC: A clinical trial report
of autologous bone marrow-derived mesenchymal stem cell
transplantation in patients with spinal cord injury. Exp Ther Med.
6:140–146. 2013.PubMed/NCBI
|
|
28
|
Rubio D, Garcia S, Paz MF, De la Cueva T,
Lopez-Fernandez LA, Lloyd AC, Garcia-Castro J and Bernad A:
Molecular characterization of spontaneous mesenchymal stem cell
transformation. PLoS One. 3:e13982008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
de la Fuente R, Bernad A, Garcia-Castro J,
Martin MC and Cigudosa JC: Retraction: Spontaneous human adult stem
cell transformation. Cancer Res. 70:66822010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lin ZX and Xu H: Current understanding
into the biological characteristics of mesenchymal stem cells.
Zhongguo Zu Zhi Gong Cheng Yan Jiu Yu Lin Chuang Kang Fu.
15:3577–3580. 2011.(In Chinese).
|
|
31
|
Wang LZ, Xu L, Tan DM and Tan Y: The
progress of survivin. Yi Xue Zong Shu. 12:1089–1092. 2006.(In
Chinese).
|
|
32
|
Kobayashi M, Huang CL, Sonobe M, Kikuchi
R, Ishikawa M, Kitamura J, Miyahara R, Menju T, Iwakiri S, Itoi K,
Yasumizu R and Date H: Intratumoral Wnt2B expression affects tumor
proliferation and survival in malignant pleural mesothelioma
patients. Exp Ther Med. 3:952–958. 2012.PubMed/NCBI
|