Introduction
Adenomyosis, one of the most common debilitating
diseases, affects women in the reproductive age group (1). Uterine adenomyosis, by definition, is
the presence of endometrial tissue, including stroma and glands,
≥2.5 mm below the endometrial-myometrial junction and widely
distributed within the myometrium layer of the uterus. An
adenomyoma is a circumscribed, nodular aggregate of smooth muscle,
and endometrial glands and stroma within the myometrium (2). Common clinical symptoms of adenomyosis
include metrorrhagia, dysmenorrhea, pelvic pain, early
pregnancy-stage miscarriage, menorrhagia and subfertility (3). The management of adenomyosis has been a
major challenge, with hysterectomy comprising the treatment of
choice (4). The ontogeny of
adenomyosis is clearly important for the development of new
alternatives to hysterectomy. Despite the frequency of the disease,
its precise etiology and physiopathology remain unknown. The
current theory is that the disease is developed through the
downgrowth and invagination of the basalis endometrium into the
myometrium (5). According to the
aforementioned theory, the increased invasiveness of the
endometrial cells may result in the development of adenomyosis.
Several studies have reported that estrogen-induced
epithelial-to-mesenchymal transition (EMT) is critical to the
pathogenesis of adenomyosis (6,7). EMT is
an important developmental program exploited by cancer cells in
their acquisition of invasive and metastatic capacity (8). Its distinctive characteristics are the
loss of E-cadherin and apical-basal cell polarity, accompanied by
the acquisition of cell migration and invasion abilities and an
increased expression of mesenchymal markers, including fibronectin,
N-cadherin and vimentin (9). A
number of recent studies have implicated focal adhesion kinase
(FAK) in the regulation of EMT (10–12). FAK
was shown to mediate cell invasion and metastasis through the
promotion of EMT; however, whether FAK is involved in the pathology
of adenomyosis has yet not been explored.
The aims of the present study were therefore to
investigate whether there was a difference in FAK expression in the
eutopic endometria of women with and without adenomyosis, and to
examine whether FAK expression in adenomyosis of the eutopic
endometrium was associated with pelvic pain and dysmenorrhea.
Materials and methods
Patients and sample collection
The ethical approval for the present study was
obtained by the Ethics Committee of School of Medicine, Zhejiang
University (Hangzhou, China). Written informed consent was obtained
from all the patients prior to tissue collection. A total of 47
females in reproductive age volunteered to participate in the
present study. All the participants had normal menstrual cycles
(28–32 days) and had not received any anti-inflammatory or hormonal
treatment for ≥6 months prior to enrollment and surgery.
Of the 47 patients, 22 females (aged 39–45 years)
had been diagnosed with adenomyosis by laparoscopy and subsequent
histological analysis. Information on the history of pelvic pain
and dysmenorrhea was obtained from the patients' clinical records.
The standard visual analogue scale was used to measure pain
intensity (13). The patients were
taught to rate their pain intensity in a scale of 1–10. The control
group comprised 25 females (aged 38–43 years), who were undergoing
hysterectomy for benign indications and had no visible evidence of
adenomyosis or endometriosis. The mean ages of the patients with
adenomyosis and the control group were 41.7 ± 3.8 years and 40.9 ±
2.9 years, respectively, and no statistically significant age
difference was observed between them (P>0.05).
Endometrial tissues were obtained by endometrial
curettage (Pipelle, Laboratoire CDD, Paris, France) simultaneously
with the surgery. Shortly after the tissue collection, the
endometrial tissues were either snap-frozen in liquid nitrogen and
stored at −80°C for mRNA and protein extraction, or fixed for 24 h
in 4% paraformaldehyde for pathological examination and embedded in
paraffin for immunohistochemical analysis.
Immunohistochemistry
The endometrial samples were sectioned at 4-µM
intervals, and the slides were heated at 60°C for 1 h,
deparaffinized in xylene and washed with graded ethanol solutions
followed by distilled water. Next, the slides were heated to
92–98°C in 0.01 mol/l sodium citrate buffer for 15 min and then
placed at room temperature for 30 min. Subsequently, the slides
were incubated with 3% (v/v) hydrogen peroxide for 10 min.
Non-specific binding was blocked by 10% (v/v) normal goat serum in
PBS for 10 min at room temperature, and the slides were incubated
with the anti-FAK primary antibody (dilution, 1:100) in PBS for 2 h
at room temperature.
The anti-FAK antibody used in the present study was
a rabbit polyclonal antibody of mouse origin, raised against amino
acids 903–1052 of FAK (cat. no. sc-932; Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA). Slides incubated with a rabbit
immunoglobulin (IgG) antibody at the same dilution as the primary
antibody were used as negative controls. Following three washes
with PBS for 5 min each, slides were incubated with anti-IgG
horseradish peroxidase (HRP)-conjugated secondary antibody (cat.
no. P0488; Dako Cytomation, Inc., Carpinteria, CA, USA) for 30 min.
After a further wash, the sections were treated with
diaminobenzidine (Dako Cytomation, Inc.), counterstained with
hematoxylin (Sigma-Aldrich, St. Louis, MO, USA), dehydrated and
mounted in DPX mounting medium (Merck Millipore, Darmstadt,
Germany).
Western blotting
The homogenization of the endometrial samples was
performed in 1X radioimmunoprecipitation assay buffer
(Sigma-Aldrich) that contained 1% Nonidet P-40, 0.1% SDS, 0.05%
deoxycholate and protease inhibitors (1 µg/ml phenylmethylsulfonyl
fluoride and 1 µg/ml leupeptin). The homogenate was incubated for
40 min on ice and subsequently centrifuged at 15,000 × g for 5 min
at 4°C. The insoluble fraction was discarded. A Bradford assay
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) was used to
determine the protein concentration in the supernate and then 50 µg
proteins were added into 2X sodium dodecyl sulfate (SDS) buffer and
heated for 5 min at 95°C. Samples at 50 µg/lane were separated on a
8% SDS-polyacrylamide gel and then transferred to an immune-blot
nitrocellulose transfer membrane (Protran®; Schleicher &
Schuell BioScience GmbH, Dassel, Germany).
The membranes were blocked with 5% non-fat milk
powder in Tris-buffered saline/Tween-20 (TBST), and then incubated
with the anti-FAK (dilution, 1:200) and β-actin (dilution, 1:400;
sc-47778; Santa Cruz Biotechnology, Inc.) primary antibodies at 4°C
overnight. Following several washes with TBST, incubation of the
membranes was performed for 2 h at room temperature with the
secondary HRP-labeled antibody (dilution, 1:5,000). The bound
antibody was detected using an enhanced chemiluminescence detection
reagent (Santa Cruz Biotechnology, Inc.). The bands were scanned
using Quantity One software, version 4.62 (Bio-Rad Laboratories,
Inc.). Normalized densities were determined using the ratio of the
band density of FAK to the band density of β-actin.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
In order to validate the results of the array,
semi-quantitative RT-qPCR was used to examine the FAK mRNA
expression. Total RNA was extracted from each endometrial sample
using TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA,
USA). Reverse transcription was performed with 3 µg RNA using
random primers and M-MLV reverse transcriptase (Promega
Corporation, Madison, WI, USA) according to the manufacturer's
instructions. The PCR reaction for FAK was performed in a final
volume of 25 µl, using 0.25 units Taq DNA polymerase (Sangon
Biotech Co., Ltd., Shanghai, China), 0.2 µM of each primer and 200
µM deoxynucleotide triphosphate in 10 nM Tris-HCl buffer (pH 8.3),
containing 50 nM KCl and 1.5 nM MgCl2. GADPH was used as
the endo-reference control. The FAK primer sequences used were as
follows: Sense, 5′-AAT ACG GCG ATC ATA CTG GG-3′, and anti-sense,
5′-CATGCCTTGCTTTTCGCTGT-3′, amplifying a 620 bp product; GAPDH
sense, 5′-ACC ACA GTC CAT GCC ATC AC-3′, and anti-sense, 5′-TCC ACC
ACC CTG TTG CTG TA-3′, amplifying a 452 bp product. PCR was
performed in a DNA thermal cycler using the following conditions: 1
cycle of 95°C for 5 min, then 40 cycles of 95°C for 30 sec, 54°C
for 30 sec and 72°C for 30 sec, and finally 1 cycle of 72°C for 10
min. Subsequently, 10 µl PCR product mixed with 2 µl loading buffer
were electrophoretically separated on a 2% agarose gel and
visualized with ethidium bromide (Life Technologies, Carlsbad, CA,
USA).
Statistical analysis
All data were normally distributed and the results
are expressed as the mean ± standard deviation. Statistical
analysis of the FAK to endo-reference ratios was performed by
one-way analysis of variance using SPSS software, version 11.5
(SPSS, Inc., Chicago, IL, USA). Linear regression was used to
analyze the correlation between FAK protein expression and the
score of pelvic pain and dysmenorrhea. P<0.05 was considered to
indicate a statistically significant difference.
Results
Immunohistochemistry
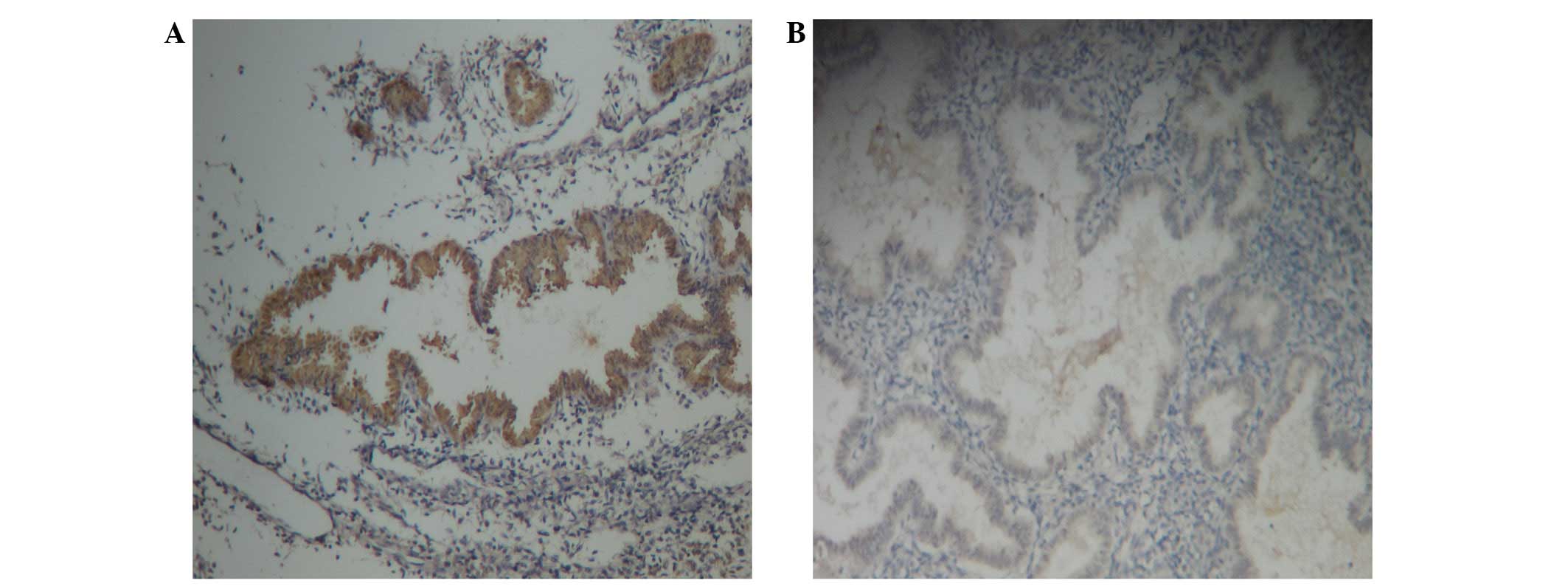
FAK immunoreactive staining was present in the
cytoplasm of glandular epithelial and stromal cells in females with
and without adenomyosis (Fig. 1A and
B, respectively). The immunostaining in the glandular
epithelium was more evident.
Western blot analysis
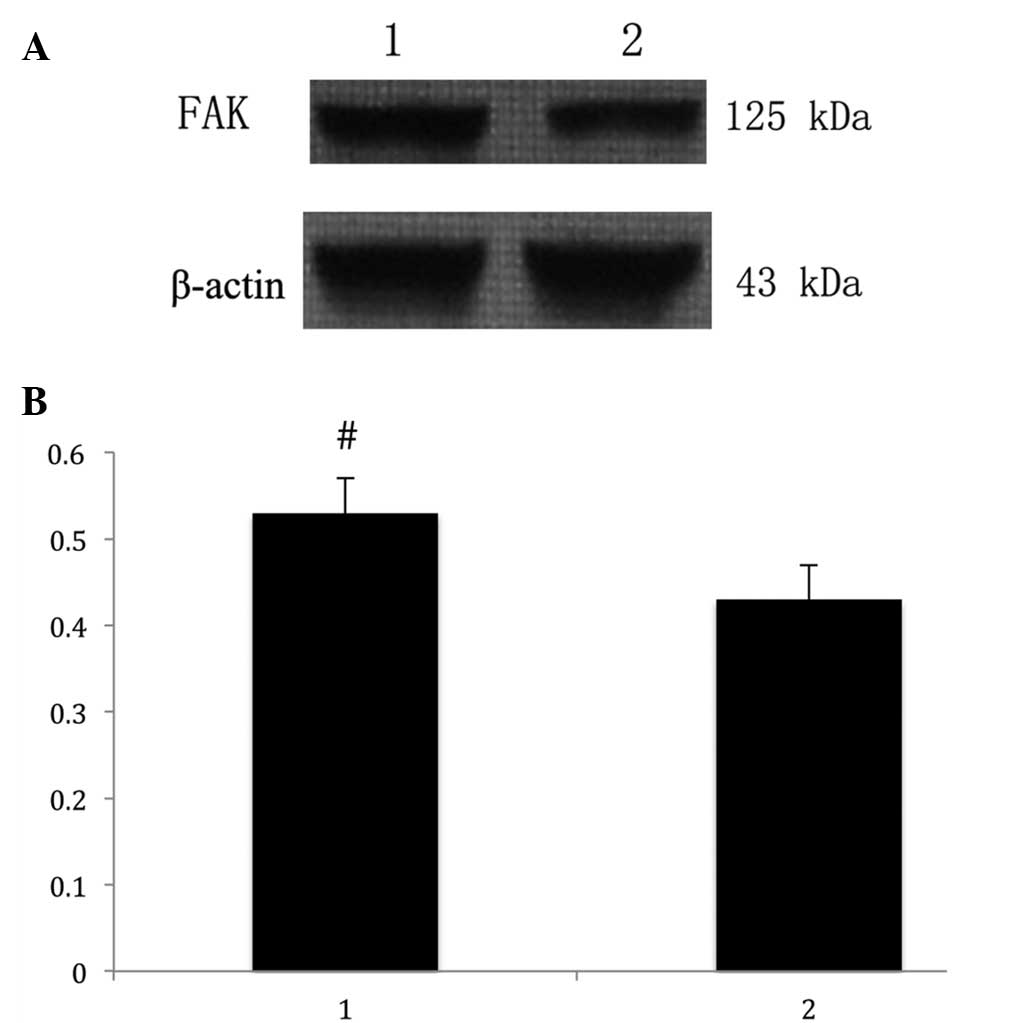
The anti-FAK antibody detected a band at 125 kDa in
protein extracts from all the endometrial tissues (Fig. 2A). Following the normalization of
each band of FAK with β-actin from different samples, the average
FAK expression in patients with adenomyosis (0.53 ± 0.04) was found
to be significantly higher compared with that of the control group
(0.43 ± 0.05; P<0.05; Fig.
2B).
RT-qPCR analysis
RT-qPCR was used to assess the FAK mRNA expression,
and a 620-bp product of FAK mRNA was visualized with ethidium
bromide following agarose gel electrophoresis. GADPH was used as a
control to assess the volume of RNA in each sample (Fig. 3A).
Semi-quantitative PCR analysis identified that FAK
mRNA expression in the endometrial samples of patients with
adenomyosis was significantly higher (0.67 ± 0.12) compared with
that of the control individuals (0.57 ± 0.11; P<0.05; Fig. 3B).
Correlation of FAK protein expression
with dysmenorrhea and pelvic pain in adenomyosis
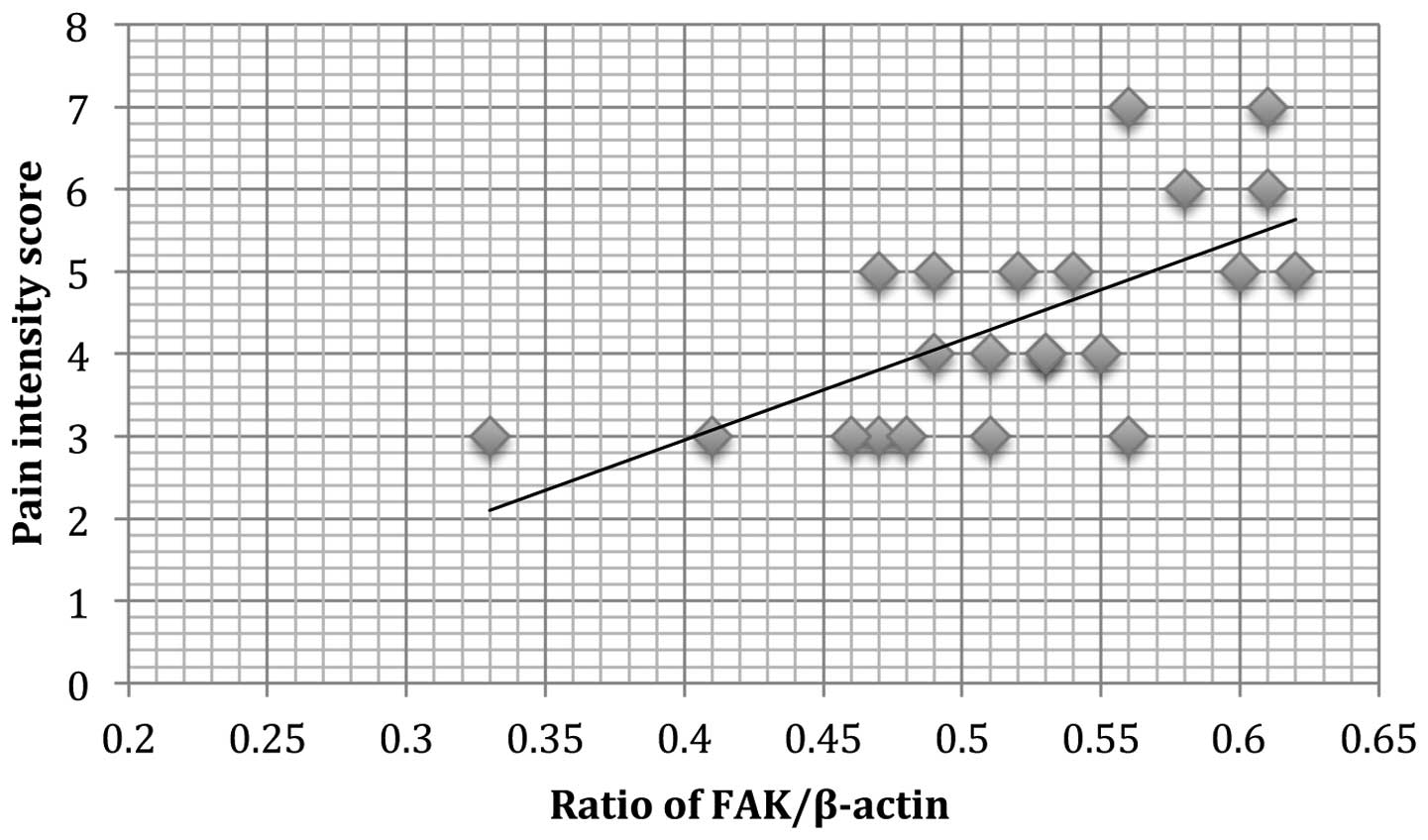
A positive correlation was observed between FAK
protein expression and dysmenorrhea (r2=0.121, P=0.011;
Fig. 4) in patients with
adenomyosis. In addition, a positive correlation of pelvic pain and
FAK protein expression (r2=0.110, P=0.009; Fig. 5) was observed.
Discussion
In the present study, an elevation in FAK expression
was observed in the eutopic endometrium of patients with
adenomyosis. In addition, an association was identified between FAK
protein expression in endometrial tissues from patients with
adenomyosis and the scores of dysmenorrhea and pelvic pain. These
results provide further evidence that the eutopic endometrium of
patients with adenomyosis is aberrant, and the downgrowth and
invagination of the basalis endometrium into the myometrium is
critical to the pathogenesis of adenomyosis.
FAK is a part of the integrin-stimulated signaling
network and is localized to sites of integrin clustering at focal
adhesions through indirect protein-protein interactions (14). The N-terminus is a FERM domain
reported to be in direct association with the cytoplasmic tail of
integrins (15). The focal adhesion
targeting sequence that mediates discrete localization to focal
adhesions is known to reside in the C-terminal non-catalytic domain
of FAK. The remaining region in this domain is more adjacent to the
catalytic domain and consists of two proline-rich sequences
functioning as binding sites for a variety of Src (16), which may result in the activation of
multiple intracellular signaling cascades. Through the
aforementioned binding sites, FAK controls several biological
processes, such as cell survival, proliferation, invasion and
migration (17). FAK is
overexpressed in several types of solid and non-solid tumors,
mediating survival and other important functions (18). Due to the fact that high percentages
of FAK overexpression have been reported in different types of
tumors, FAK has been proposed as a therapeutic target (19).
In addition to determining the role of FAK in cell
proliferation, survival and migration, recent studies have also
revealed potentially novel functions of FAK in the regulation of
EMT (20), an important
developmental program exploited by cancer cells in their process of
acquiring invasive and metastatic capacity. Chen et al have
suggested that oestrogen-induced EMT of endometrial epithelial
cells contributes to the development of adenomyosis (7). According to the results of the present
study, we postulate that FAK may be involved in the EMT of
adenomyosis. The connection between the loss of E-cadherin
expression by cancer cells and passage through an EMT has been
established by numerous studies (21,22). FAK
was shown to affect E-cadherin expression through different
mechanisms. The phosphorylation of FAK was required for the
Src-induced downregulation of E-cadherin in colon cancer cells
(23). Thus, we speculate that the
higher expression of FAK in adenomyosis caused the deregulation of
E-cadherin, promoting EMT and the invasion and metastasis of
endometrial cells. Based on this speculation, it is very likely
that FAK plays a critical role in transforming the eutopic
endometrium of adenomyosis in order to be more susceptible to
surviving, adhering and growing in the ectopic sites.
In conclusion, to the best of our knowledge, the
present study revealed for the first time a significant increase in
FAK expression in the endometrial tissues of female patients with
adenomyosis, as well as an association of FAK expression with
dysmenorrhea and pelvic pain. These findings suggest that FAK may
contribute to the pathogenesis of adenomyosis. Further research is
warranted on the application of FAK as a clinical marker and
therapeutic target.
Acknowledgements
This study was supported by a grant from the
National Natural Science Foundation of China (grant no.
81100407).
References
|
1
|
Vercellini P, Vigano P, Somigliana E,
Daguati R, Abbiati A and Fedele L: Adenomyosis: Epidemiological
factors. Best Pract Res Clin Obstet Gynaecol. 20:465–477. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bergeron C, Amant F and Ferenczy A:
Pathology and physiopathology of adenomyosis. Best Pract Res Clin
Obstet Gynaecol. 20:511–521. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Peric H and Fraser IS: The symptomatology
of adenomyosis. Best Pract Res Clin Obstet Gynaecol. 20:547–555.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Farquhar C and Brosens I: Medical and
surgical management of adenomyosis. Best Pract Res Clin Obstet
Gynaecol. 20:603–616. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Parrott E, Butterworth M and Green A:
Adenomyosis-a result of disordered stromal differentiation. Am J
Pathol. 159:623–630. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Oh SJ, Shin JH and Kim TH: β-Catenin
activation contributes to the pathogenesis of adenomyosis through
epithelial-mesenchymal transition. J Pathol. 231:210–222. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen YJ, Li HY and Huang CH:
Oestrogen-induced epithelial-mesenchymal transition of endometrial
epithelial cells contributes to the development of adenomyosis. J
Pathol. 222:261–270. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Acloque H, Adams MS and Fishwick K:
Epithelial - mesenchymal transitions: The importance of changing
cell state in development and disease. J Clin Invest.
119:1438–1449. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Polyak K and Weinberg RA: Transitions
between epithelial and mesenchymal states: Acquisition of malignant
and stem cell traits. Nat Rev Cancer. 9:265–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Scheel C and Weinberg RA: Phenotypic
plasticity and epithelial-mesenchymal transitions in cancer and
normal stem cells? Int J Cancer. 129:2310–2314. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fan H, Zhao X, Sun S, Luo M and Guan JL:
Focal adhesion kinase scaffolding function to mediate endophilin A2
phosphorylation promotes epithelial-mesenchymal transition and
mammary cancer stem cell activities in vivo. J Biol Chem.
288:3322–3333. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Serrels A, Canel M, Brunton VG and Frame
MC: Src/FAK-mediated regulation of E-cadherin as a mechanism for
controlling collective cell movement: Insights from in vivo
imaging. Cell Adh Migr. 5:360–365. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Knop C, Oeser M, Bastian L, Lange U,
Zdichavsky M and Blauth M: Development and validation of the Visual
Analogue Scale (VAS) Spine Score. Unfallchirurg. 104:488–497.
2001.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cox BD, Natarajan M, Stettner MR and
Gladson CL: New concepts regarding focal adhesion kinase: Promotion
of cell migration and proliferation. J Cell Biochem. 99:35–52.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schaller MD: Biochemical signals and
biological responses elicited by the focal adhesion kinase. Biochim
Biophys Acta. 1540:1–21. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schaller MD, Hildebrand JD and Shannon JD:
Autophosphorylation of the focal adhesion kinase, pp125FAK, directs
SH2-dependent binding of pp60src. Mol Cell Biol. 14:1680–1688.
1994.PubMed/NCBI
|
|
17
|
Zhang X, Chattopadhyay A and Ji QS: Focal
adhesion kinase promotes phospholipase C-gamma1 activity. Proc Natl
Acad Sci USA. 96:9021–9026. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gabarra-Niecko V, Schaller MD and Dunty
JM: FAK regulates biological processes important for the
pathogenesis of cancer. Cancer Metastasis Rev. 22:359–374. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
McLean GW, Avizienyte E and Frame MC:
Focal adhesion kinase as a potential target in oncology. Expert
Opin Pharmacother. 4:227–234. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Canel M, Serrels A, Frame MC and Brunton
VG: E-cadherin-integrin crosstalk in cancer invasion and
metastasis. J Cell Sci. 126:393–401. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tepass U, Truong K, Godt D, Ikura M and
Peifer M: Cadherins in embryonic and neural morphogenesis. Nat Rev
Mol Cell Biol. 1:91–100. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Edelman GM, Gallin WJ and Delouvee A:
Early epochal maps of two different cell adhesion molecules. Proc
Natl Acad Sci. 80:4384–8. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Avizienyte E, Wyke AW and Jones RJ:
Src-inducd de-regulation of E-cadherin in colon cancer cells
requires integrin signalling. Nat Cell Biol. 4:632–638.
2002.PubMed/NCBI
|