Introduction
Cardiomyocyte apoptosis is important in left
ventricular (LV) remodeling and dysfunction following myocardial
infarction (MI) (1–3), which induces symptomatic heart failure.
The large amount of reactive oxygen species (ROS) produced in the
ischemic myocardium can induce cardiomyocyte apoptosis (3,4).
Accumulating evidence suggests that there is an upregulated
expression and activity of xanthine oxidase (XO), which mediates
myocardial dilatation by increased production of catalytic ROS
(5–7).
The Qili qiangxin capsule was developed according to
the theory on main and collateral channels in traditional Chinese
medicine, which consisted of extraction or powder from 11
components (8). As a medicine for
chronic heart failure, Qili qiangxin was approved by the State Food
and Drug Administration of China in 2009 (9,10).
Previous studies indicated that the major active ingredients of
Qili qiangxin, such as Ginseng, Radix Astragali and Salvia
miltiorrhiza, may significantly inhibit the enzymatic activity
of XO (11–13) and decrease the production of ROS
(13–15).
However, the effect of Qili qiangxin on
cardiomyocyte apoptosis remains to be determined. Therefore, the
aim of the current study was to investigate the effect of Qili
qiangxin on cardiomyocyte apoptosis in rats after MI, as well as
its potential mechanism of action.
Materials and methods
Components and preparation of Qili
qiangxin
Qili qiangxin powder was provided by Shijiazhuang
Yiling Pharmaceutical Co., Ltd (Shijiazhuang, Hebei, China), and
comprises Ginseng, Radix Astragali, Salvia miltiorrhiza,
Aconite root, Semen Lepidii Apetali, Cortex Periplocae Sepii
Radicis, Rhizoma Alismatis, Carthamus tinctorius,
Polygonatum odorati, Seasoned Orange Peel and Ramulus
Ginnamomi. The herbal drugs were authenticated and standardized on
marker compounds according to the Chinese Pharmacopoeia 2010. The
drug powder was dissolved in normal saline at the concentration of
2.67 g/ml (10).
Rat models and experimental
protocol
MI was induced in 50 adult male Sprague-Dawley rats
weighing 180–230 g (supplied by the Experimental Animal Centre of
the Third Affiliated Hospital of the Third Military Medical
University, Chongqing, China), by permanent ligation of the left
anterior descending coronary artery, as previously described
(10). Briefly, the rats were
anesthetized (pentobarbital sodium; 30 mg/kg; i.p.), intubated, and
mechanically ventilated with a rodent ventilator (TKR-200; Jiangxi
Teli Anesthesia Ventilator Co., Ltd, Jiangxi, China). Following
thoracotomy on the left chest, the heart was exposed. A superficial
suture line (7–0) was passed around the proximal left coronary
artery and the suture was tied, and successful occlusion was
confirmed by visual cyanosis. The left chest was closed in three
layers (ribs, muscles, and skin), 15 min after occlusion, and the
rats were allowed to recover from anesthesia on their own.
Thirty-one rats survived the operation and were randomly divided
into the MI group (n=16) and the Qili qiangxin group (4 g/kg per
day by gavage, n=15). Five rats were subjected to the same surgical
procedure, except for the ligation of the coronary artery, and
served as the sham group. Administration of Qili qiangxin commenced
24 h after the operation, and continued for 28 successive days. The
rat experiments were approved by the local institutional animal
research committee.
Echocardiographic measurements
Transthoracic Doppler echocardiographic experiments
were performed with a commercially available echocardiographic
system (GE Vivid 7; GE Healthcare, Fairfield, Connecticut, USA)
equipped with a 13 MHz transducer. Briefly, a two-dimensional
short-axis view of the left ventricle was obtained at the level of
the papillary muscle, in order to record M-mode tracing. The
average of left ventricular end-diastolic dimension (LVEDD),
factional shortening (FS), as well as ejection faction (EF) were
calculated, on the basis of three successive cardiac cycles. The
observer was blind to the experimental group assignment.
Measurement of infarct size
After 28 days of surgery, all the rats were weighed
and sacrificed by cervical dislocation and the hearts were
immediately removed. Hearts of rats were removed and the atria,
vasculature and right ventricles were dissected and removed.
Infarct size (IS) was determined as previously described (7). Briefly, the sections of the left
ventricle were immersed in fixative solution, dehydrated and then
embedded in paraffin. Histological sections 5 µm were subsequently
obtained and stained with hematoxylin and eosin. Endocardial and
epicardial circumferences of the infarcted tissue and the left
ventricle were determined using image analysis software (Image Pro
Plus 4.5; Media Cybernetics, Inc., Silver Spring, MD, USA). IS was
calculated as: (endocardial + epicardial circumference of the
infarcted tissue)/(endocardial + epicardial circumference of the
left ventricle) and expressed as a percentage. The remaining
myocardial tissue was frozen in liquid nitrogen and stored at
−80°C.
Measurement of
·O2−, ·OH-scavenging
and XO activity by colorimetry
High ·O2− and
·OH-scavenging activity, and low
·O2− and ·OH levels are
usually observed in the myocardium. As per the manufacturer's
instructions for the Superoxide Anion Free Radical Detection kit,
Hydroxyl Free Radical Detection kit, and XO Detection kit (Nanjing
Jiancheng Bioengineering Institute, Nanjing, China), 100 mg of the
frozen myocardial tissue was produced into a 10% homogenate, and
the supernatant was removed and mixed with appropriate reagents.
When the reaction was finished, absorbance was detected with a 752
Ultraviolet Spectrometer (Qinghua Tech-Apparatus Company, Beijing,
China), to calculate the ·O2− and
·OH-scavenging activity, as well as XO activity.
In situ nick end-labeling
Apoptotic nuclei were detected by in situ
terminal deoxynucleotidyl transferase (TdT)-mediated
digoxigenin-conjugated dUTP nick end-labeling (TUNEL), using an In
Situ Cell Apoptosis Detection kit (MK1020; Boster Biotechnology
Co., Ltd., Wuhan, China). The sections were processed according to
the manufacturer's instructions, with modifications. Briefly, the
sections were incubated for 10 min and quenched in 3%
H2O2 buffer blocked with endogenous
peroxidase, followed by a 10-min proteolytic digestion in
pre-diluted proteinase K (Sigma, St Louis, MO, USA)-exposed antigen
binding sites. The slides were then incubated in equilibration
buffer for 2 min, followed by incubation in working strength TdT
enzyme at 37°C for 2 h. The TdT working strength enzyme comprised
TdT enzyme and digoxigenin-11-dUTP for extension of the 3′-OH ends
of double-or single-stranded DNA of fragmented DNA. This process
was followed by stop/wash and anti-digoxigenin-peroxidase steps,
which were performed according to the manufacturer's instructions.
Color was developed using 3,3′-diaminobenzidine (DAB), which
generated a brown reaction product. Positive controls were prepared
by treating selected slides with 0.5 mg/ml DNase I for 10 min at
room temperature. dUTP labelling was not observed when TdT was
omitted from the reaction. The number of apoptotic cardiomyocytes
and their percentage of total cardiomyocytes were counted under a
light microscope. For each slide, five fields were randomly
selected, and by using a defined rectangular field area at a
magnification of ×200, a total of 200 cells per field were counted.
The apoptotic index (AI) was determined as the number of apoptotic
cardiomyocytes divided by the total number of cardiomyocytes
counted ×100%, from a total of 15 fields per heart, with assays
performed in a blinded manner.
Agarose gel electrophoresis of
DNA
To detect the internucleosomal cleavage of genomic
DNA, a hallmark of apoptotic cell death, DNA was isolated from LV
tissue and subjected to ethidium bromide (0.4 mg/l) agarose gel
(1.5%) electrophoresis.
Immunohistochemical analysis of
Fas
The streptavidin- biotin complex (SABC)
immunohistochemical technique was used to detect Fas using an
SABC-POD kit (SA2002; Boster Biotechnology Co., Ltd). Samples were
fixed within 5 min of excision in 10% neutral buffered formalin and
embedded in paraffin blocks. After deparaffinization and
rehydration, the samples underwent 10-min incubation in 3%
H2O2 in buffer blocked with endogenous
peroxidase. The slides were treated with 0.01 M target retrieval
solution (natrium citricum buffer; Beijing Leagene Biotechnology
Co., Ltd., Beijing, China) at 95°C for 10 min as per the
manufacturer's instructions. Non-specific binding was blocked using
goat serum in PBS buffer (Boster Biotechnology Co., Ltd.) for 20
min. Primary antibody for rat Fas (sc-7886; 1:400; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) was applied overnight at
4°C. Following incubation with the initial primary antibody, the
slides were placed in biotinylated secondary anti-rabbit (goat
absorbed) polyclonal antibody (BA1003; 1:1200; Boster Biotechnology
Co., Ltd.) for 20 min. This antibody complex was then detected
after 20 min incubation in a streptavidin-biotin-horseradish
peroxidase complex. Color was developed using DAB, which generated
a brown reaction product. The slides were counterstained with
hsematoxylin. Control slides included isotype-matched host-specific
antibodies at a dilution of 1:100, 10% primary antibody host-serum,
and single (no primary antibody) and double (no primary or
secondary antibody) negative controls. For analysis of the positive
expression of Fas, the slides were evaluated and Fas cytoplasm
staining was quantified from five non-consecutive tissue sections
at a magnification of ×200 in 10 random fields in a blinded manner.
The integrated optical density (IOD) of the positively expressed
area was measured as a semiquantitative parameter with image
analysis software (Image Pro Plus 4.5).
Western blot analysis of XO and
caspase-3
For all the groups, equal amounts (40 µg) of protein
extracts were loaded and separated, according to their size, by
sodium dodecyl sulfate polyacrylamide gel electrophoresis
(SDS-PAGE; Boster Biotechnology Co., Ltd.) using 10% acrylamide
gradient. Separated proteins were transferred electrophoretically
to a polyvinylidene difluoride membrane (Boster Biotechnology Co.,
Ltd.). Non-specific sites were blocked by incubation of the
membrane in blocking buffer (5% non-fat dry milk in T-TBS) for 2 h.
The membranes were incubated with the indicated primary antibodies
[XO: (1:400), sc-20991; Santa Cruz Biotechnology, Inc.; caspase-3:
(1:400), CPP32; NeoMarkers, Fremont, CA, USA; β-actin: (1:200);
Boster Biotechnology Co., Ltd)] overnight at 4°C. Horseradish
peroxidase-conjugated anti-rabbit immunoglobulin IgG (1:1500;
Boster Biotechnology Co., Ltd) was used as a secondary antibody and
incubated for 2 h at 37°C. Immunoreactive bands were visualized by
DAB and photographed. Absorbance analysis of the images was
performed using image analysis software (Quantity One 4.4.0). The
densities of XO and caspase-3 in relation to β-actin were,
respectively, expressed as XO/β-actin and caspase-3/β-actin.
Statistical analysis
Data are presented as mean ± SEM. The parameters
were compared using one-way ANOVA, followed in case of
significance, by a two-sided Tukey's test for multiple comparisons.
P<0.05 was considered statistically significant.
Results
Echocardiography and infarct size
After 28 days, 6 rats in the MI group and 1 rat in
the Qili qiangxin group died. The rats in the sham group survived.
Compared to the sham group, LVEDD was increased and FS and EF were
significantly decreased in the MI group (P<0.01). However,
compared to the MI group LVEDD was decreased, and FS and EF were
significantly increased in the Qili qiangxin group (P<0.01).
Although IS was smaller in the Qili qiangxin group than that in the
MI group, the difference was not statistically significant
(P>0.05), (Table I, Figs. 1 and 2). No infarct was detected in the sham
group since none of the rats underwent ligation of the coronary
artery.
 | Table I.Effect of Qili qiangxin on
ventricular remodeling, cardiomyocyte apoptosis and oxidative
stress (mean ± SD). |
Table I.
Effect of Qili qiangxin on
ventricular remodeling, cardiomyocyte apoptosis and oxidative
stress (mean ± SD).
| Group | Sham | MI | Qili qiangxinz |
|---|
| No. | 5 | 10 | 14 |
| LVEDD (mm) |
5.8±0.9 |
7.2±0.8a |
6.2±0.5b |
| FS (%) | 53.94±8.41 |
31.97±5.88a |
46.50±7.82b |
| EF (%) | 74.86±5.66 |
35.47±2.25a |
56.38±5.45a,b |
| IS (%) |
| 30.6±3.2 | 28.4±2.9 |
| AI (%) |
0.5±0.2 |
21.1±1.4a |
6.2±0.8a,b |
| Fas (IOD) | 10681±2079 |
77,328±5,151a |
31,925±3,263a,b |
|
Caspase-3/β-actin |
0.04±0.01 |
0.45±0.08a |
0.12±0.04c,b |
| XO/β-actin |
0.15±0.06 |
0.53±0.09a |
0.51±0.07a |
| Activity of XO
(u/mgpro) |
0.21±0.05 |
1.62±0.19a |
0.43±0.13b |
| Activity of
·O2−-scavenging (u/gpro) | 151.38±9.57 |
64.77±8.50a |
97.72±8.99a,b |
| Activity of
·OH-scavenging (u/mgpro) | 253.03±17.39 |
91.67±16.21a |
177.02±9.56a,b |
Cardiomyocyte AI
The apoptotic cardiomyocytes were mainly distributed
in the border zones of the infarction area (Fig. 3). The AI was markedly higher
(P<0.01) in the MI and Qili qiangxin groups, when compared to
the sham group (Table I). However,
the AI in the Qili qiangxin group was significantly lower than that
in the MI group (P<0.01).
DNA ladder
Following agarose gel electrophoresis, the
myocardial tissue DNA of the sham group exhibited a late band close
to the sample well, which was recognized as the normal band pattern
for cardiomyocyte DNA. The distribution of DNA fragments in the MI
group was dispersed and a typical DNA ladder was observed while the
relatively minor dispersion of the DNA fragments was detected in
the Qili qiangxin group which made the DNA ladder disappear
(Fig. 4).
Fas expression
Fas expression was located on the membrane or in the
cytoplasm of the cardiomyocytes (Fig.
5). Compared to the sham group, the IOD of Fas was
significantly higher in the MI and the Qili qiangxin groups
(P<0.01). However, IOD in the Qili qiangxin group was markedly
lower than that in the MI group (P<0.01) (Table I).
Caspase-3 expression
Caspase-3 expression was similar to that of Fas and
was markedly increased in the myocardial tissue of the MI and Qili
qiangxin groups (P<0.01). However, the increase was much lower
in the Qili qiangxin group compared to the MI group, although a
significant difference was evident between the Qili qiangxin and
sham groups (P<0.05) (Table I and
Fig. 6).
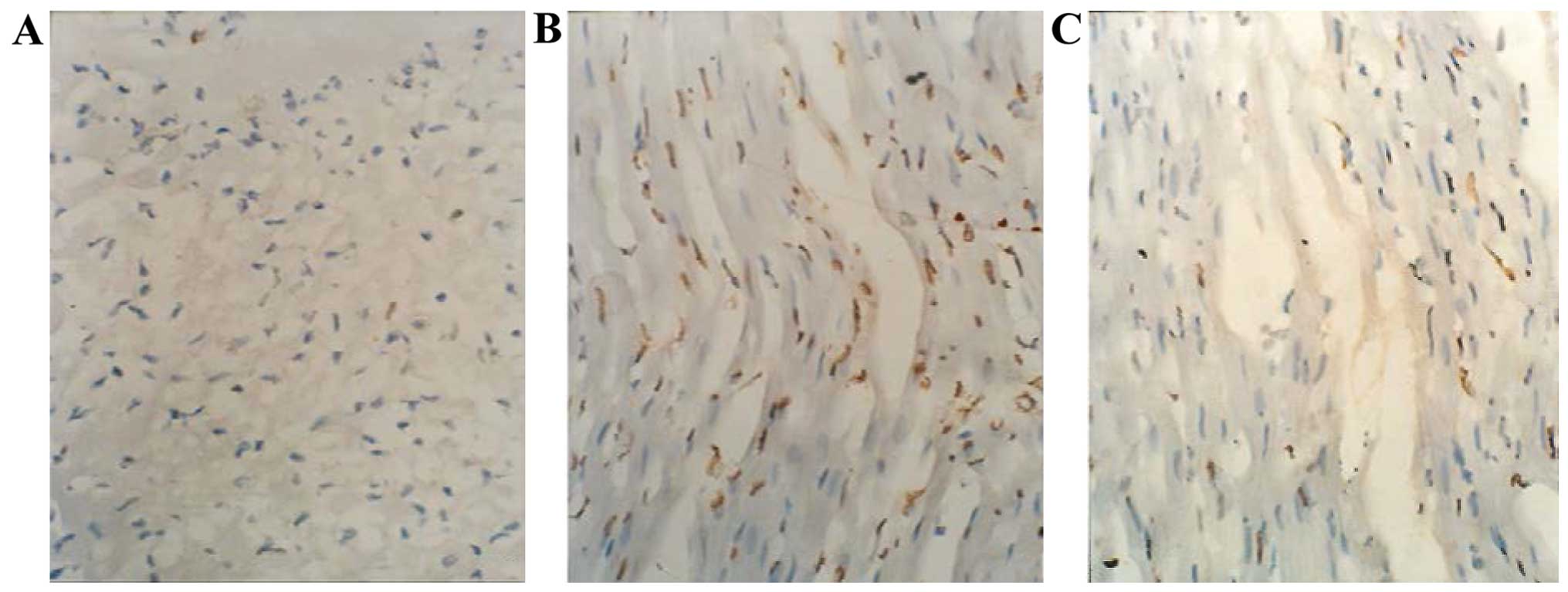
Activity and expression of XO
Compared to the sham group, XO activity was
significantly increased and the expression was also enhanced in the
MI and Qili qiangxin groups (P<0.01). However, XO activity in
the Qili qiangxin group was significantly lower than that in the MI
group (P<0.01), while no significant difference was identified
in the expression of XO between the two groups (P>0.05)
(Table I and Fig. 6).
·O2−
and ·OH-scavenging activity of myocardial tissue
The .O2- and
.OH-scavenging activity of the myocardial tissue
decreased significantly in the MI and Qili qiangxin groups
(P<0.01 or P<0.05). However, the two indicators were
significantly higher in the Qili qiangxin group compared to the MI
group (P<0.05) (Table I).
Discussion
Pharmaceutics of herbal medicine is undergoing rapid
development in China. With the progression of modern technology,
herbal compound extracts are increasingly being authenticated,
standardized, and administered successfully in clinical practice.
Qili qiangxin, derived from a group of herbal medicine including
Radix Astragali, Ginseng, Salvia miltiorrhiza, Aconite root,
and Semen Lepidii Apetali have been used to treat patients with
chronic heart failure for two years (8–10). It
has been demonstrated that the major active constituents of Qili
qiangxin, such as Ginseng, Radix Astragali and Salvia
miltiorrhiza, significantly inhibit the enzymatic activity of
XO (11–13) and decrease the production of ROS
(13–15). However, whether the improvement of
ventricular remodeling by Qili qiangxin is associated with the
inhibition of cardiomyocyte apoptosis remains to be determined.
Ventricular remodeling after MI involves expansion
of the infarcted area, ventricular dilatation, as well as thinning
of the ventricular wall (16,17). Our
study was designed to assess whether the administration of Qili
qiangxin affected ventricular remodeling. Echocardiography
performed after 28 days of surgery showed that LEVDD was decreased
while FS and EF were significantly increased in rats that had
undergone the MI operation. However, no significant difference was
observed for IS between the MI and Qili qiangxin groups.
Considering the trend towards a smaller IS in the Qili qiangxin
group, this finding may be due to the relatively short duration or
small sample size of our study.
Accumulating evidence suggests that a higher level
of ROS plays a pathological role through cell signaling pathways,
inducing apoptosis (18). ROS is
generated intracellularly by activation of nicotinamideadenine
dinucleotide phosphate oxidase or XO, uncoupling of nitric oxide
synthase, and electron transport and ‘leakage’ during oxidative
phosphorylation in the mitochondria. Biochemical and
pharmacological studies suggest that XO acts as a major source of
ROS in the cardiovascular system, and XO-derived ROS has been
demonstrated in experimental and clinical heart failure (5–7).
Apoptosis is a distinct type of cell death
characterized by a series of typical morphological events, such as
shrinkage of the cell, fragmentation into membrane-bound apoptotic
bodies and rapid phagocytosis into neighboring cells without the
induction of inflammatory response, while the biochemical hallmark
of apoptosis is internucleosomal DNA fragmentation. Cardiomyocyte
apoptosis has been identified in viable myocardial areas after MI
in experimental and human ischemic heart failure, and was regarded
as an important factor leading to ventricular remodeling and
dysfunction (1–4). As an independent factor, apoptosis in
the expansion of the infarcted area is thought to lead to the
partial ventricular wall becoming thinner and cavity dilation,
i.e., an earlier remodeling (19).
By contrast, apoptosis in the border and remote zones of the
infarction is the main factor leading to the LV remodeling and
dysfunction in the advanced stage (20).
The Fas death pathway is critical for cardiomyocyte
apoptosis, which is easily activated by oxidative stress (21). Fas ligand, an integral membrane
protein binding to a Fas trimer can induce a conformational change
in Fas that enables its cytoplasmic tail to recruit Fas-associated
death domain protein (FADD) through interactions involving death
domains in the two molecules. FADD, in turn recruits procaspase-8
through homotypic interactions involving death effector motifs. The
approximation of procaspase-8 stimulates its autoactivation,
followed by the activation of downstream caspase-3 by caspase-8
which induces apoptosis (22). Zhu
et al reported that the conformity between the degree of Fas
expression and the phase of an increase in apoptosis indicated that
the change in the expression of Fas was closely associated with
cardiomyocyte apoptosis (2).
Caspase-3 has been confirmed as a dominant executor in the Fas
death pathway, a key protease in apoptosis, which can result in DNA
degradation and apoptosis by activating CAD (23). Thus inhibition of the activity or
function of caspase-3 may depress apoptosis (24,25). Sam
et al reported an increase in cell death in the
non-infarcted zones (NIZ) after MI along with an increase in
caspase-3 activity (20).
Results of the present study suggest that after 28
days of treatment with Qili qiangxin, the AI and expression of Fas
and caspase-3 were markedly reduced in the rats that had undergone
the MI operation. The DNA ladder, a hallmark indicator of
apoptosis, disappeared in these rats, suggesting the anti-apoptotic
activity of Qili qiangxin in myocardial tissue in the NIZ.
The potentially cytological and molecular mechanisms
by which Qili qiangxin inhibits cardiomyocyte apoptosis remain to
be elucidated. These mechanisms may be due to the cumulative or
synergistic effects of multiple compounds present in the herbal
extract. One possible mechanism is that it relieves apoptosis by
reducing LV end-diastolic pressure and mitigates the tension of the
ventricular wall (26). For example,
Aconite root, a Chinese medicinal herb, has been shown to have
positive inotropic, vasodilation and diuretic effects in the
management of congestive heart failure (27). Another possible explanation is
associated with inhibition of the high production of ROS in the
ischemic myocardium. It has been reported that Ginseng, Radix
Astragali and Salvia miltiorrhiza, may significantly inhibit
the production of ROS (13–15).
ROS (.O2−,
.OH and H2O2) which, through
multiple intracellular redox signaling pathways (18,28–30),
directly damages DNA and mitochondria, promotes the expression of
redox sensitive proapoptotic genes (such as Fas, and p53) (31), and activates the inflammatory
cytokine-like mitogen-activated protein kinases (32) to manipulate cardiomyocyte apoptosis.
It has been previously confirmed that the direct scavenging of
.O2− or .OH attenuates
LV remodeling and dysfunction (33,34).
Glutathione peroxidase, which removes H2O2
and detoxifies lipid hydroperoxides, is also overexpressed in mouse
heart and has been shown to ameliorate post-MI remodeling (35).
XO, a potent enzymatic source of ROS, has an
upregulated expression and activity in the ischemic myocardium,
while the major active constituents of Qili qiangxin, such as
Ginseng, Radix Astragali and Salvia miltiorrhiza may
significantly inhibit XO activity (11–13).
Results of the present study showed that following administration
of Qili qiangxin for 28 days, XO activity was reduced and
.O2−, .OH-scavenging
activity of myocardial tissue was enhanced in the rats that had
undergone the MI resection, whereas the expression level of XO
remained the same in the untreated MI group.
In summary, our results in the rats indicate that
following administration of Qili qiangxin for 28 successive days,
dilation of the left ventricle caused by MI was markedly
attenuated. By contrast, in the non-infarct zones XO activity was
inhibited and .O2−,
.OH-scavenging activity of myocardial tissue was
enhanced by Qili qiangxin. In addition, the AI, and expression
levels of Fas and caspase-3 were also reduced in the same area. The
expression level of XO remained the same in the MI group,
suggesting that Qili qiangxin acts following the translation
process, rather than at the transcription and translation levels.
These results confirm our hypothesis that through the combination
of XO after MI, Qili qiangxin was able to reduce the generation of
ROS and inhibit cardiomyocyte apoptosis in the non-infarct zone,
and prevent remodeling of the left ventricle.
Qili qiangxin may also inhibit cardiomyocyte
apoptosis through other means, for example, by inhibiting the
expression of p53 (31), or by
reducing the activity of the stress-activated protein kinase
(36), or by inhibiting the
apoptosis signal-regulating kinase 1 (37). However, these possible mechanisms
require further investigation.
Acknowledgements
This study was partially supported by Chongqing
Municipal Health and Family Planning Commission Medical Research
Fund, Chongqing, China (20142083).
Abbreviations:
|
MI
|
myocardial infarction
|
|
ROS
|
reactive oxygen species
|
|
XO
|
xanthine oxidase
|
|
LVEDD
|
left ventricular end-diastolic
dimension
|
|
FS
|
factional shortening
|
|
EF
|
ejection faction
|
|
IS
|
infarct size
|
|
TUNEL
|
terminal deoxynucleotidyl transferase
(TdT)-mediated digoxigenin-conjugated dUTP nick-end labeling
|
|
DAB
|
3,3′-diaminobenzidine
|
|
AI
|
apoptotic index
|
|
IOD
|
integrated optical density
|
|
SDS-PAGE
|
sodium dodecyl sulfate polyacrylamide
gel electrophoresis
|
|
FADD
|
Fas-associated death domain
protein
|
References
|
1
|
Shah AM and Mann DL: In search of new
therapeutic targets and strategies for heart failure: Recent
advances in basic science. Lancet. 378:704–712. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhu YZ, Zhu YC, Wang ZJ, Lu Q, Lee HS and
Unger T: Time-dependent apoptotic development and pro-apoptotic
genes expression in rat heart after myocardial infarction. Jpn J
Pharmacol. 86:355–358. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Savateev AV and Savateeva-Liubimova TN:
Apoptosis-universal mechanisms of cell death and survival in
ischemia and reperfusion: Ways to pharmacological control. Eksp
Klin Farmakol. 73:44–49. 2010.(In Russian). PubMed/NCBI
|
|
4
|
Abbate A, Bussani R, Amin MS, Vetrovec GW
and Baldi A: Acute myocardial infarction and heart failure: Role of
apoptosis. Int J Biochem Cell Biol. 38:1834–1840. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gladden JD, Ahmed MI, Litovsky SH, Schiros
CG, Lloyd SG, Gupta H, Denney TS Jr, Darley-Usmar V, McGiffin DC
and Dell'Italia LJ: Oxidative stress and myocardial remodeling in
chronic mitral regurgitation. Am J Med Sci. 342:114–119. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Doehner W and Landmesser U: Xanthine
oxidase and uric acid in cardiovascular disease: Clinical impact
and therapeutic options. Semin Nephrol. 31:433–440. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mellin V, Isabelle M, Oudot A,
Vergely-Vandriesse C, Monteil C, Di Meglio B, Henry JP, Dautreaux
B, Rochette L, Thuillez C, et al: Transient reduction in myocardial
free oxygen radical levels is involved in the improved cardiac
function and structure after long-term allopurinol treatment
initiated in established chronic heart failure. Eur Heart J.
26:1544–1550. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kang LP, Zhao Y, Yu HS, Liu YX, Xiong CQ,
Tan DW, Jia JM, Wang HT, Tian SY and Ma BP: Identification of
chemical constituents in qiliqiangxin capsule by UPLC-Q-TOF/MS(E).
Yao Xue Xue Bao. 46:1231–1236. 2011.[In Chinese]. PubMed/NCBI
|
|
9
|
Liu W, Chen J, Xu T, Tian W, Li Y, Zhang Z
and Li W: Qiliqiangxin improves cardiac function in spontaneously
hypertensive rats through the inhibition of cardiac chymase. Am J
Hypertens. 25:250–260. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xiao H, Song Y, Li Y, Liao YH and Chen J:
Qiliqiangxin regulates the balance between tumor necrosis
factor-alpha and interleukin-10 and improves cardiac function in
rats with myocardial infarction. Cell Immunol. 260:51–55. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shen L, Han JZ, Li C, Yue SJ, Liu Y, Qin
XQ, Liu HJ and Luo ZQ: Protective effect of ginsenoside Rg1 on
glutamate-induced lung injury. Acta Pharmacol Sin. 28:392–397.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yu DH, Bao YM, Wei CL and An LJ: Studies
of chemical constituents and their antioxidant activities from
Astragalus mongholicus Bunge. Biomed Environ Sci. 18:297–301.
2005.PubMed/NCBI
|
|
13
|
Liu X, Chen R, Shang Y, Jiao B and Huang
C: Superoxide radicals scavenging and xanthine oxidase inhibitory
activity of magnesium lithospermate B from Salvia miltiorrhiza. J
Enzyme Inhib Med Chem. 24:663–668. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Karmazyn M, Moey M and Gan XT: Therapeutic
potential of ginseng in the management of cardiovascular disorders.
Drugs. 71:1989–2008. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen R, Shao H, Lin S, Zhang JJ and Xu KQ:
Treatment with Astragalus membranaceus produces antioxidative
effects and attenuates intestinal mucosa injury induced by
intestinal ischemia-reperfusion in rats. Am J Chin Med. 39:879–887.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Konstam MA, Kramer DG, Patel AR, Maron MS
and Udelson JE: Left ventricular remodeling in heart failure:
Current concepts in clinical significance and assessment. JACC
Cardiovasc Imaging. 4:98–108. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gajarsa JJ and Kloner RA: Left ventricular
remodeling in the post-infarction heart: A review of cellular,
molecular mechanisms, and therapeutic modalities. Heart Fail Rev.
16:13–21. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Finkel T: Signal transduction by reactive
oxygen species. J Cell Biol. 194:7–15. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Abbate A, Biondi-Zoccai GG, Bussani R,
Dobrina A, Camilot D, Feroce F, Rossiello R, Baldi F, Silvestri F,
Biasucci LM, et al: Increased myocardial apoptosis in patients with
unfavorable left ventricular remodeling and early symptomatic
post-infarction heart failure. J Am Coll Cardiol. 41:753–760. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sam F, Sawyer DB, Chang DL, Eberli FR,
Ngoy S, Jain M, Amin J, Apstein CS and Colucci WS: Progressive left
ventricular remodeling and apoptosis late after myocardial
infarction in mouse heart. Am J Physiol Heart Circ Physiol.
279:H422–H428. 2000.PubMed/NCBI
|
|
21
|
Lee P, Sata M, Lefer DJ, Factor SM, Walsh
K and Kitsis RN: Fas pathway is a critical mediator of cardiac
myocyte death and MI during ischemia-reperfusion in vivo. Am J
Physiol Heart Circ Physiol. 284:H456–H463. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Foo RS, Mani K and Kitsis RN: Death begets
failure in the heart. J Clin Invest. 115:565–571. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schwarz K, Simonis G, Yu X, Wiedemann S
and Strasser RH: Apoptosis at a distance: Remote activation of
caspase-3 occurs early after myocardial infarction. Mol Cell
Biochem. 281:45–54. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Holly TA, Drincic A, Byun Y, Nakamura S,
Harris K, Klocke FJ and Cryns VL: Caspase inhibition reduces
myocyte cell death induced by myocardial ischemia and reperfusion
in vivo. J Mol Cell Cardiol. 31:1709–1715. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Saitoh T, Nakajima T, Takahashi T and
Kawahara K: Changes in cardiovascular function on treatment of
inhibitors of apoptotic signal transduction pathways in left
ventricular remodeling after myocardial infarction. Cardiovasc
Pathol. 15:130–138. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hsieh MH and Nguyen HT: Molecular
mechanism of apoptosis induced by mechanical forces. Int Rev Cytol.
245:45–90. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lin JS, Chan CY, Yang C, Wang YH, Chiou HY
and Su YC: Zhi-fuzi, a cardiotonic Chinese herb, a new medical
treatment choice for portal hypertension? Exp Biol Med (Maywood).
232:557–564. 2007.PubMed/NCBI
|
|
28
|
Shah AM and Channon KM: Free radicals and
redox signalling in cardiovascular disease. Heart. 90:486–487.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Murdoch CE, Zhang M, Cave AC and Shah AM:
NADPH oxidase-dependent redox signalling in cardiac hypertrophy,
remodelling and failure. Cardiovasc Res. 71:208–215. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Oktyabrsky ON and Smirnova GV: Redox
regulation of cellular functions. Biochemistry (Mosc). 72:132–145.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li PF, Dietz R and von Harsdorf R: p53
regulates mitochondrial membrane potential through reactive oxygen
species and induces cytochrome c-independent apoptosis blocked by
Bcl-2. EMBO J. 18:6027–6036. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
McCubrey JA, Lahair MM and Franklin RA:
Reactive oxygen species-induced activation of the MAP kinase
signaling pathways. Antioxid Redox Signal. 8:1775–1789. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kinugawa S, Tsutsui H, Hayashidani S, Ide
T, Suematsu N, Satoh S, Utsumi H and Takeshita A: Treatment with
dimethylthiourea prevents left ventricular remodeling and failure
after experimental myocardial infarction in mice: Role of oxidative
stress. Circ Res. 87:392–398. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Matsushima S, Ide T, Yamato M, Matsusaka
H, Hattori F, Ikeuchi M, Kubota T, Sunagawa K, Hasegawa Y, Kurihara
T, et al: Overexpression of mitochondrial peroxiredoxin-3 prevents
left ventricular remodeling and failure after myocardial infarction
in mice. Circulation. 113:1779–1786. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shiomi T, Tsutsui H, Matsusaka H, Murakami
K, Hayashidani S, Ikeuchi M, Wen J, Kubota T, Utsumi H and
Takeshita A: Overexpression of glutathione peroxidase prevents left
ventricular remodeling and failure after myocardial infarction in
mice. Circulation. 109:544–549. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Verheij M, Bose R, Lin XH, Yao B, Jarvis
WD, Grant S, Birrer MJ, Szabo E, Zon LI, Kyriakis JM, et al:
Requirement for ceramide-initiated SAPK/JNK signalling in
stress-induced apoptosis. Nature. 380:75–79. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nagai H, Noguchi T, Takeda K and Ichijo H:
Pathophysiological roles of ASK1-MAP kinase signaling pathways. J
Biochem Mol Biol. 40:1–6. 2007. View Article : Google Scholar : PubMed/NCBI
|