Introduction
Heart rate variability (HRV) analysis is a reliable
tool indicating cardiac autonomic modulation, which is useful in
the clinical diagnosis of cardiovascular and autonomic diseases
(1). Typically, HRV indices
calculated based on the RR intervals (RRI) of electrocardiography
(ECG), where R is the peak point of the QRS complex, are considered
as a clinical standard. In recent years, photoplethysmography
(PPG), a simple and ubiquitous technology used to measure the blood
volume changes in the microvascular bed, has been widely used in
clinical monitoring (2). Since the
pulsatile component is synchronous with the heartbeat, PPG has been
strongly recommended as an alternative approach to obtain HRV
indices.
Due to the variation of pulse transit time (PTT),
the pulse intervals of PPG are not exactly the same as the RRIs of
ECG. To date, numerous studies have evaluated the agreement between
pulse rate variability (PRV) and HRV; however, the results are
still disputed (3,4). This uncertainty may be due to deficient
methods of analysis or diverse experimental settings. For instance,
several studies (5) involved ≤4 HRV
indices, which is insufficient since ≥7 HRV indices (2 in the time
domain and 5 in the frequency domain) are frequently used in
clinical practice (6). In addition,
the majority of the existing studies have used Pearson's
correlation coefficients (CC) to measure the agreement between PRV
and HRV (5); however, CC reflects
the linear correlation instead of the agreement between them, and
thus Bland-Altman (BA) analysis may be a more cogent method
(7). Furthermore, since HRV is a
sensitive indicator reflecting autonomic nervous function, the key
point for the accuracy of PRV should be that it accurately
indicates the changes in the sympathovagal balance. Experimental
conditions must lead to the bidirectional shifts of the
sympathovagal balance, which is the shift toward sympathetic
predominance and parasympathetic predominance. However, certain
studies were only concerned with the accuracy of PRV at resting
state (8) or during particular tasks
(9). A previous study used activity
to alter the autonomic function (10); however, since PPG is susceptible to
motion artifacts, the results were not fully convincing. It can
thus be seen that accurate analysis methods and the appropriate
experimental settings are important for evaluating the accuracy of
PRV.
In the present study, the use of different
respiratory modes to stimulate sympathetic and parasympathetic
function was investigated. Initially, the shifts of sympathovagal
balance were analyzed. Subsequently, the BA and the CC methods were
used to evaluate the agreement between PRV and HRV under different
autonomic nervous conditions. The study aimed to achieve a more
universal conclusion on the accuracy of HRV from PPG.
Subjects and methods
Subjects
Experiments were performed on 33 healthy subjects
[male, 26; female, 7; median age, 22 years (lower quartile, 22
years; upper quartile, 23 years); age range, 22–25 years]. This
study was conducted in accordance with the Declaration of Helsinki
and with approval from the Ethics Committee of Xi'an Jiaotong
University (Xi'an, China). Written informed consent was obtained
from all participants.
Data acquisition
A PPG transducer (TSD 200; Biopac Systems Inc.,
Goleta, CA, USA) was attached to the left index finger of each
individual. The PPG and lead II ECG signals were recorded
simultaneously using a multi-channel system (MP 150; Biopac Systems
Inc.) at a sampling rate of 1 kHz. In addition, a thoracic belt
(TSD 201; Biopac Systems Inc.) was used to help the operator judge
whether the individuals performed the correct respiratory protocol.
The AcqKnowledge software (version 4.2; Biopac Systems Inc.) was
used to extract the RRI time series from the ECG signals and to
extract the peak-to-peak intervals (PPI) pulse cycle interval time
series from the PPG signals. The RRI and PPIs were manually
verified beat-by-beat to guarantee detection accuracy. In total,
7,159 beats were obtained during spontaneous respiration, 6,803
beats during paced respiration and 6,755 beats during breath
holding.
Experimental protocol
Trials were performed between 2:00 and 5:00 pm. Each
subject was asked to refrain from consuming coffee or alcohol from
8 h prior to the trials. The room was maintained at a temperature
of 22±2°C. The subjects were instructed to lie in a supine position
with their hands comfortably placed at their sides. Subsequent to
testing the tolerance to paced respiration and breath holding,
3-min data measurements under each respiratory condition were
performed for each subject. Initially, ECG and PPG signals were
recorded for 3 min while the subjects maintained a relaxed state.
Next, the subjects followed an audio guide instructing them to
breathe with a fixed frequency of 15 breaths/min, and 3 min signals
were recorded. Subsequently, the subjects took a deep breath, held
that breath for as long as possible, breathed normally for 30 sec,
and then began holding their breath again. The same procedure was
repeated various times within 3 min, and the signals during normal
breathing and breath holding were recorded.
Data processing
For each subject, the RRI and PPI time series were
obtained under the three respiratory conditions, and HRV analysis
was performed. A total of 11 HRV indices were calculated from the
RRI and PPIs, including the following values: Mean value; heart
rate (HR), which is the reciprocal of average intervals (mean);
SDNN, which is the standard deviation of normal beat intervals;
rMSSD, which is the square root of the mean squared differences of
successive normal beat intervals; SD1 and SD2, which are the
standard deviations of points perpendicular to and along
line-of-identity in a Poincaré plot, respectively; LF
(msec2) and HF (msec2), which are the power
values in the low (0.04–0.15 Hz) and high (0.15–0.4 Hz) frequency
band ranges, respectively; LF (n.u.) and HF (n.u.), which are the
normalized values of LF (msec2) and HF
(msec2), respectively; and LF/HF, which is the ratio of
LF (n.u.) and HF (n.u.) (6).
It has been generally accepted that LF
(msec2), SDNN and SD2 represent the long-term
variability and are associated with the combination of sympathetic
and parasympathetic function. By contrast, HF (msec2),
rMSSD and SD1 represent the short-term variability and reflect
vagus tone, while LF (n.u), HF (n.u) and LF/HF are proportional
indices and reflect sympathovagal balance.
Statistical analysis
Data are expressed as the mean ± standard error.
Three statistical analysis methods were used to perform a
comprehensive evaluation. A paired-sample t-test was used to assess
the difference between the corresponding indices of HRV and PRV.
This method was sensitive to the systematic bias, but not to random
errors. Pearson's CC was also used to directly measure the
correlation between any two indices. In contrast to the t-test, the
CC is sensitive to random errors rather than systematic errors.
The agreements between the HRV and PRV variables
were mainly assessed using the BA method (7). BA plots show the differences between
PRV and HRV versus the average as the best estimator of the true
value. Confidence intervals (CI) for the differences were
determined for the mean bias (d) and for the upper (UL) and lower
limits (LL) of agreement, using the equations UL=d+1.96·SD and
LL=d-1.96·SD, where SD is the standard deviation of differences.
The 95% CIs of bias were calculated as d±t0.05 ·SD/√n.
Similarly, the 95% CIs of the UL and LL were calculated as
(d±1.96·SD)±t0.05√3SD2/n, where
t0.05 is the critical value for a 5% two-sided test
drawn from tables of t distribution with n-1 degrees of freedom,
where n is the sample size. The BA ratio (BAr), which has
already been used in a previous study (5), was used to assess the quality of
agreement. BA r was defined as BAr=(UL-LL)/2Apm (equation 5), where
Apm is the average of the pairwise means. BAr<0.01 is considered
as an excellent agreement, values between 0.01 and 0.1 are
considered as a good agreement, values between 0.1 and 0.2 as a
moderate agreement, and values of >0.2 are defined as
insufficient agreement. The BA plots were produced using MedCalc
software, version 12.7.5 (MedCalc Software bvba, Ostend,
Belgium).
Results
Respiration rates
Respiration rates were determined during: i)
Spontaneous respiration, which was the normal breathing; ii) paced
respiration, with a fixed frequency of 15 breaths/min; and iii)
apnea, which was intermittent breath holding. For all subjects, the
lowest average respiratory rate was 10 breaths/min, and the highest
was 21 breaths/min. The mean respiratory rate of the subjects was
15.2±3.6 breaths/min. Among the 33 subjects, the respiratory rates
of 15 subjects was <15 breaths/min, and the respiratory rate of
the remaining 18 subjects was >15 breaths/min. In addition, the
shortest duration of breath holding was 35 sec and the longest
duration was 80 sec. The mean duration of breath holding for all
the subjects was 63.2±13.2 sec.
Representative example of waveform
measurements under paced respiration and apnea
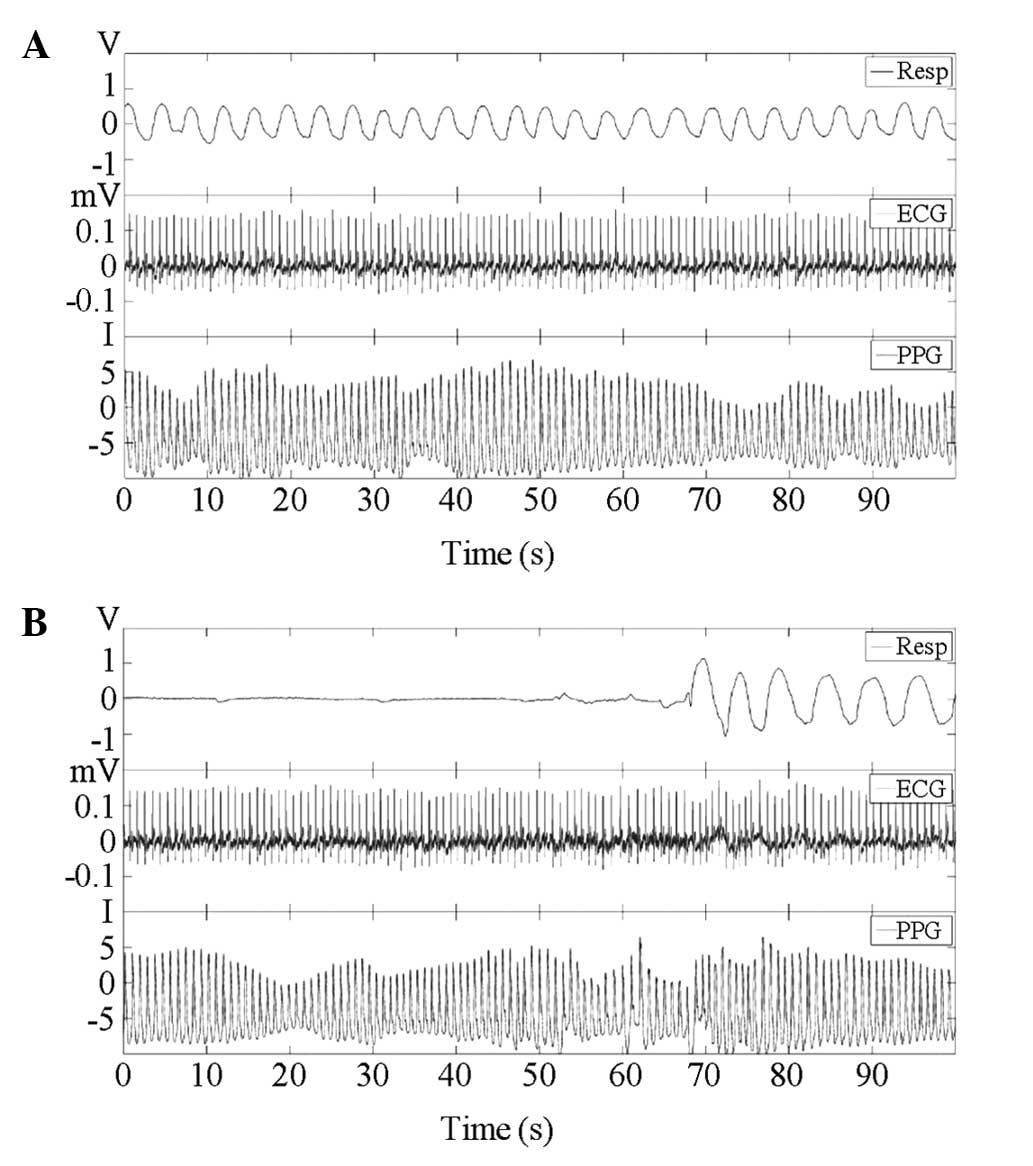
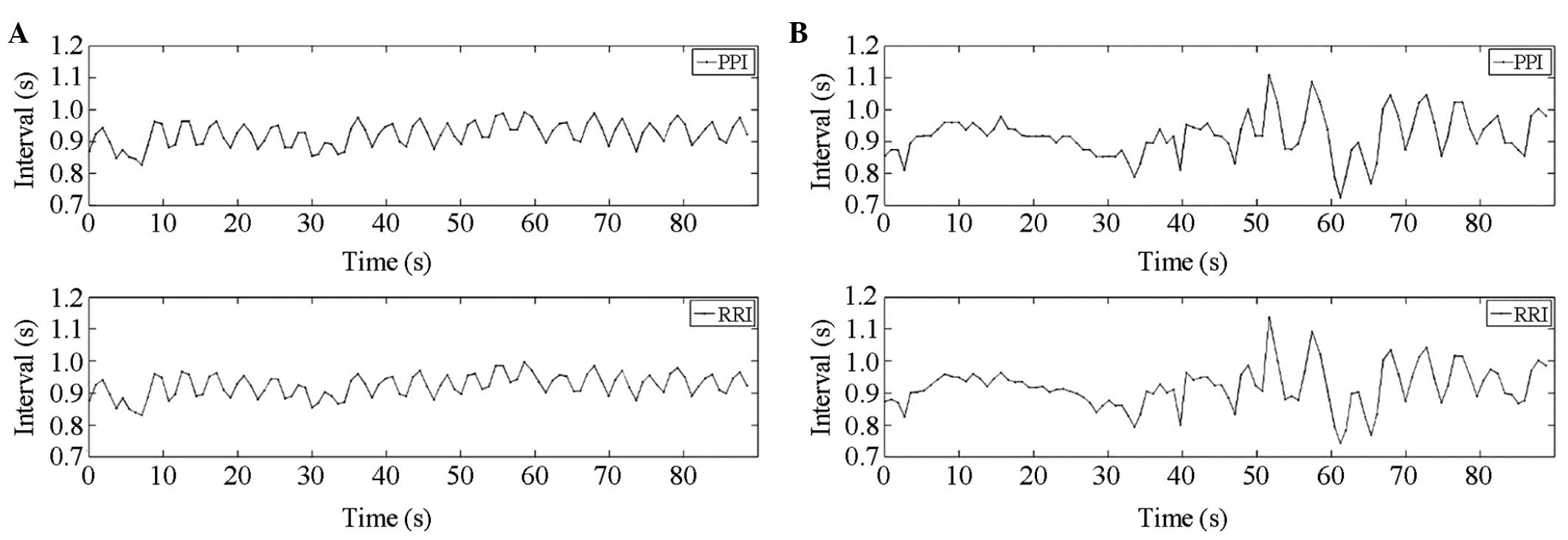
A representative example of the waveform
measurements from a subject included in the present study is shown
in Fig. 1, which includes 90-sec
segments of the respiratory, ECG and PPG waveforms. The RRI time
series obtained from the ECG signal and PPI time series from PPG
are shown in Fig. 2. The amplitude
of PPG is the quantified level of intensity.
Descriptive statistics of HRV indices
during spontaneous respiration, paced respiration and apnea
The descriptive statistics of HRV indices during
spontaneous respiration, paced respiration and apnea are shown in
Table I. There were significant
differences (P<0.05) for the indices of the mean, HR, SD2, LF
(msec2), LF (n.u.), HF (n.u.) and LF/HF values between
paced and spontaneous respiration. In particular, a highly
significant decrease (P<0.001) was observed for the LF/HF ratio
from 0.9 (0.4–1.2) during spontaneous respiration to 0.3 (0.1–0.7)
during paced respiration, which conventionally implied the shift of
sympathovagal balance toward vagal predominance. All other indices,
with the exception of HF (msec2), presented
statistically significant differences (P<0.05) between apnea and
spontaneous respiration (Table I).
In particular, the significant increase in the LF/HF ratio implied
a sympathovagal balance shift toward sympathetic predominance
during apnea. The results indicated the reverse changes in
sympathovagal balance during paced respiration and apnea.
 | Table I.Descriptive statistics of HRV indices
during spontaneous respiration, paced respiration and apnea. |
Table I.
Descriptive statistics of HRV indices
during spontaneous respiration, paced respiration and apnea.
|
| Respiratory
conditions | Spontaneous-paced
respiration | Spontaneous
respiration-apnea |
|---|
|
|
|
|
|
|---|
| HRV indices | Spontaneous | Paced | Apnea | Difference | P-value | Difference | P-value |
|---|
| Mean (msec) | 835 (749,
910) | 900 (808,
941) | 901 (815,
959) | 29 (−5, 107) |
0.002 | 42 (7, 84) | <0.001 |
| HR (bps) | 72 (66, 80) | 66 (63, 74) | 67 (63, 74) |
−2(−9.1, 0.5) |
0.004 | −3 (−6, 0) | <0.001 |
| SDNN (msec) | 53 (39, 68) | 48 (37, 58) | 76 (61, 101) | −7 (−14, 5) |
0.068 | 28 (9, 48) | <0.001 |
| SD2 (msec) | 69 (52, 87) | 57 (45, 69) | 99 (78,
131) | −7 (−23, 8) |
0.043 | 40 (12,
68) | <0.001 |
| LF
(msec2) |
536 (356,
1,152) | 353 (154,
594) |
995 (718,
1,505) | −228 (−641, 76) |
0.009 |
510 (165,
737) |
0.002 |
| rMSSD (msec) | 41 (33, 60) | 44 (34, 59) | 50 (39, 74) | 2 (−12,
9) |
0.549 | 8 (0,
24) |
0.001 |
| SD1 (msec) | 29 (23, 42) | 31 (24, 42) | 35 (27, 52) | 29 (23, 42) |
0.544 | 6 (0,
17) |
0.001 |
| HF
(msec2) |
799
(463, 1,422) |
834 (531,
1,557) |
981 (429,
2,353) |
18
(−290, 565) |
0.561 |
96
(−165, 791) |
0.081 |
| LF (n.u.) | 0.4 (0.3,
0.5) | 0.2 (0.1, 0.4) | 0.5 (0.3, 0.7) | −0.1
(−0.2, 0.0) | <0.001 | 0.1 (0.0,
0.2) |
0.016 |
| HF (n.u.) | 0.5 (0.4,
0.6) | 0.7 (0.5, 0.8) | 0.4 (0.2, 0.6) | 0.1 (0.0,
0.2) | <0.001 | −0.1
(−0.2, .0.0) |
0.016 |
| LF/HF | 0.9 (0.4,
1.2) | 0.3 (0.1, 0.7) | 1.4 (0.5, 2.3) | −0.3
(−0.8, 0.0) | <0.001 | 0.4
(−0.2, 1.4) |
0.004 |
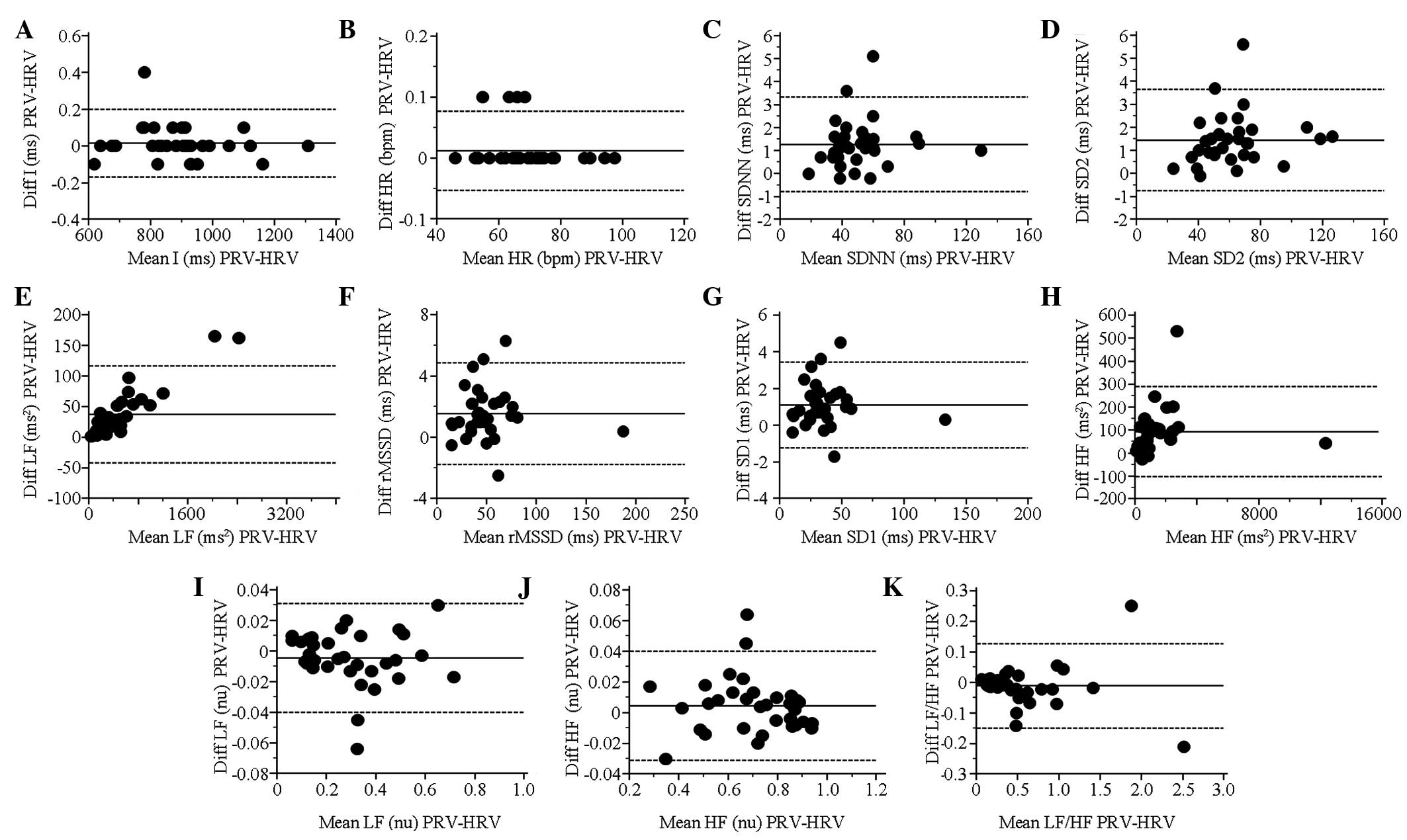
BA plots and statistical results of
PRV and HRV indices during paced respiration
All long-term and short-term variability indices,
including SDNN, SD2, LF (msec2), rMSSD, SD1, HF
(msec2) and PRV, presented a highly significant
(P<0.001) increase compared with HRV. In addition, HR showed a
statistically significant difference. The indices had strong
correlations (CC>0.99) between PRV and HRV. All other indices
had an acceptable agreement (BAr<0.2) between PRV and HRV, with
the exception of the ratio of low to high frequency power (LF/HF,
BA r=0.25; Table II). The BA plots
for each index during paced respiration are shown in Fig. 3.
 | Table II.Comparison of PRV and HRV indices
during paced respiration. |
Table II.
Comparison of PRV and HRV indices
during paced respiration.
| A, CC
correlations |
|
|
|
|
|---|
|
|---|
| Type of index | Index | PRV | HRV | CC |
|---|
| Heart rate | Mean (msec) | 890±26 | 890±26 | 1.00a |
|
| HR (bpm) | 69.4±1.9 | 69.3±1.9c | 1.00a |
| Long-term
variability | SDNN (msec) | 51.8±3.6 | 50.6±3.5a | 0.99a |
|
| SD2 (msec) | 62.9±4.0 | 61.4±3.9a | 0.99a |
|
| LF
(msec2) | 519±94 | 481±88a | 1.00a |
| Short-term
variability | rMSSD (msec) | 50.8±5.2 |
49.2±5.2a | 0.99a |
|
| SD1 (msec) | 36.0±3.7 |
34.9±3.7a | 0.99a |
|
| HF
(msec2) | 1,489±366 |
1,396±363a | 0.99a |
| Sympathovagal
balance | LF (n.u.) | 0.29±0.03 | 0.30±0.03 | 0.99a |
|
| HF (n.u.) | 0.70±0.03 | 0.69±0.03 | 0.99a |
|
| LF/HF | 0.54±0.09 | 0.55±0.09 | 0.99a |
|
| B, BA ratios |
|
|
|
|
|
| Index | Bias (CI) | Lower limits of
agreement (CI) | Upper limits of
agreement (CI) | BA ratio |
|
| Mean (msec) |
0.01 (−0.01 to
0.04) | −0.17
(−0.23 to −0.11) | 0.20
(0.14 to 0.26) | 0.00d |
| HR (bpm) | 0.01
(0.00 to 0.02) | −0.05
(−0.07 to −0.03) | 0.07
(0.05 to 0.09) | 0.00d |
| SDNN (msec) | 1.27
(0.89 to 1.64) | −0.78
(−1.43 to −0.14) | 3.32
(2.68 to 3.97) | 0.04e |
| SD2 (msec) | 1.45
(1.05 to 1.84) | −0.74
(−1.42 to −0.05) | 3.64
(2.96 to 4.33) | 0.03e |
| LF
(msec2) | 37.2
(22.9 to 51.6) | −41.9
(−66.7 to −17.2) | 116 (91 to
141) | 0.15f |
| rMSSD (msec) | 1.5 (0.9 to
2.1) | −1.7 (−2.7 to
−0.7) | 4.8 (3.8 to
5.8) | 0.06e |
| SD1 (msec) | 1.1 (0.6 to
1.5) | −1.2 (−1.9 to
−0.5) | 3.4 (2.7 to
4.1) | 0.06e |
| HF
(msec2) | 92 (57 to 128) | −103 (−164 to
−41) | 288 (227 to
350) | 0.13f |
| LF (n.u.) | −0.00 (0.01 to
0.00) | −0.04
(−0.05 to −0.02) | 0.03 (0.02 to
0.04) | 0.11f |
| HF (n.u.) | 0.00
(−0.00 to 0.01) | −0.03
(−0.04 to −0.02) | 0.04 (0.02 to
0.05) | 0.05e |
| LF/HF | −0.01
(−0.03 to −0.01) | −0.14
(−0.19 to −0.10) | 0.12 (0.08 to
0.17) | −w0.25g |
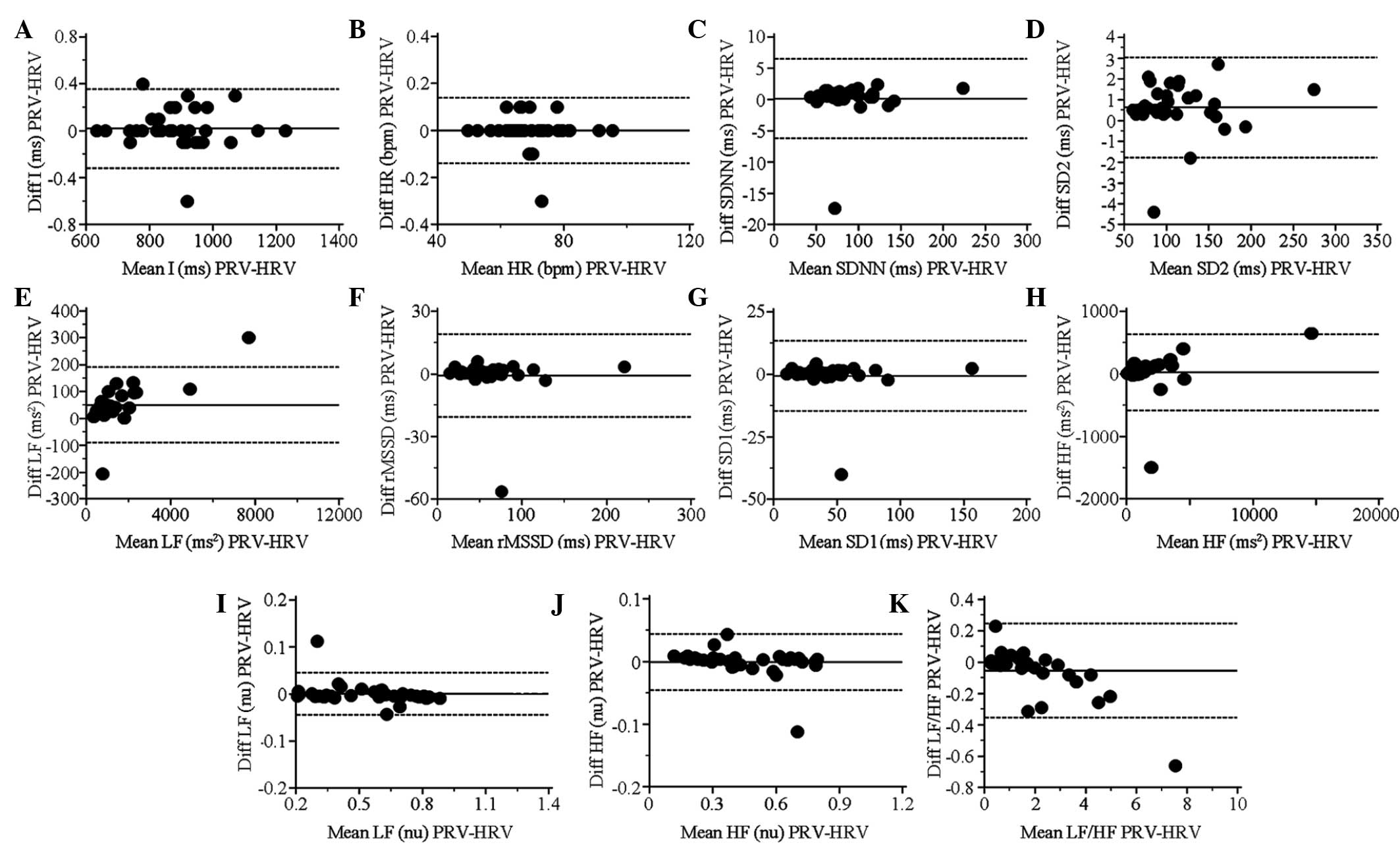
BA plots and statistical results of
PRV and HRV indices during apnea
Statistically significant differences between PRV
and HRV were identified only for LF (msec2) (P<0.001)
and SD2 (P<0.05). The linear correlation was weakened for the
short-term variability indices, rMSSD (CC=0.96) and SD1 (CC=0.96).
Furthermore, the agreement between PRV and HRV for all short-term
variability indices [rMSSD, SD1 and HF (msec2)] exceeded
the acceptable limit with BAr>0.3, but the other indices
remained in acceptable agreement (BAr<0.2; Table III). The BA plots for each index
under apnea are shown in Fig. 4.
 | Table III.Comparison of PRV and HRV indices
during Apnea. |
Table III.
Comparison of PRV and HRV indices
during Apnea.
| A, CC
correlations |
|
|
|
|
|---|
|
|---|
| Type of index | Index | PRV | HRV | CC |
|---|
| Heart rate | Mean (msec) | 891±21 | 891±21 | 1.00a |
|
| HR (bpm) | 69.3±1.6 | 69.3±1.7 | 1.00a |
| Long-term
variability | SDNN (msec) | 85.9±6.2 | 85.7±6.2 | 0.99a |
|
| SD2 (msec) | 112±7 | 112±7b | 1.00a |
|
| LF
(msec2) | 1,464±249 |
1,414±240a | 0.99a |
| Short-term
variability | rMSSD (msec) | 60.9±6.7 | 61.7±6.8 | 0.96a |
|
| SD1 (msec) | 43.2±4.7 | 43.7±4.8 | 0.96a |
|
| HF
(msec2) | 1,790±464 | 1,762±446 | 0.99a |
| Sympathovagal
balance | LF (n.u.) | 0.53±0.03 | 0.53±0.03 | 0.99a |
|
| HF (n.u.) | 0.46±0.03 | 0.46±0.03 | 0.99a |
|
| LF/HF | 1.75±0.28 | 1.80±0.30 | 0.99a |
|
| B, BA ratios |
|
|
|
|
|
| Index | Bias (CI) | Lower limits of
agreement (CI) | Upper limits of
agreement (CI) | BA ratio |
|
| Mean (msec) |
0.01
(−0.04 to 0.07) | −0.32 (−0.42 to
−0.21) | 0.35 (0.25 to
0.46) | 0.00d |
| HR (bpm) |
0.00
(−0.02 to 0.02) | −0.13 (−0.18 to
−0.09) | 0.13 (0.09 to
0.18) | 0.00d |
| SDNN (msec) |
0.11
(−1.02 to 1.26) | −6.22 (−8.20 to
−4.24) | 6.45 (4.47 to
8.43) | 0.07e |
| SD2 (msec) |
0.63
(0.19 to 1.07) | −1.78 (−2.53 to
−1.02) | 3.04 (2.29 to
3.80) | 0.02e |
| LF
(msec2) | 50 (25
to 76) | −90 (−133 to
−46) | 191 (147 to
235) | 0.09e |
| rMSSD (msec) | −0.8
(−4.3 to 2.7) | −20.6 (−26.8 to
−14.4) | 19.0 (12.8 to
25.2) | 0.32g |
| SD1 (msec) | −0.5
(−3.1 to 1.9) | −14.6 (−19.0 to
−10.2) | 13.5 (9.1 to
17.9) | 0.32g |
| HF
(msec2) |
27 (−81 to
137) | −578 (−768 to
−389) | 634 (445 to
824) | 0.34g |
| LF (n.u.) |
0.00 (−0.00 to
0.00) | −0.04 (−0.05 to
−0.03) | 0.04 (0.03 to
0.06) | 0.08e |
| HF (n.u.) |
0.00 (−0.00 to
0.00) | −0.04 (−0.06 to
−0.03) | 0.04 (0.03 to
0.05) | 0.09e |
| LF/HF |
−0.05 (−0.10 to
0.00) | −0.35 (−0.44 to
−0.25) | 0.24 (0.15 to
0.34) | 0.16f |
Discussion
Due to deficient methods of analysis and the diverse
experimental settings, the accuracy of HRV obtained from PPG is
mostly incommensurable (5). The
present study aimed to achieve a more universal conclusion on the
accuracy of PRV. Different respiratory modes were used to stimulate
the sympathetic and parasympathetic functions. The effect of
respiratory-induced changes in sympathovagal balance on the
agreement between PRV and HRV was then evaluated. Changes in the
LF/HF of HRV indicated that there was a sympathovagal balance shift
toward vagal predominance during paced respiration, but toward
sympathetic predominance during apnea. In addition, the results
demonstrated that during paced respiration, the other indices had
an acceptable agreement (BAr<0.2) between PRV and HRV, with the
exception of LF/HF that showed an insufficient agreement (BA
r=0.25). The indices had very strong correlations (CC>0.99) and
PRV had a highly significant (P<0.001) increase for the majority
of the variability indices, when compared with HRV. During apnea,
the agreement of the indices reflecting short-term variability
(rMSSD, SD1 and HF) was reduced and was below the acceptable limit,
although statistically significant differences only existed in the
SD2 and LF values between PRV and HRV.
The present study revealed that paced respiration
resulted in the shift of the sympathovagal balance toward vagal
predominance, and this result differed from the findings of a
previous study indicating that no significant changes were observed
in the spectral indices of HRV during paced breathing at 0.25 Hz
(11). The different findings may be
due to the different physiological conditions. In the current
study, the data during paced respiration were recorded in the first
3 min of the paced respiration. The subjects were in the adaptation
of the new respiratory state, during which the cardiopulmonary and
autonomic nervous systems were in the process of establishing a new
equilibrium state. By contrast, in the previous study (11), the subjects were trained to adapt the
paced respiration prior to the data measurement and, in order to
make the subjects comfortable, an adjustment within ±10% of 0.25 Hz
respiratory frequency was used, while the data were recorded for 8
min. Thus, the autonomic nervous system was possibly much closer to
a relatively stable state. However, other contradictory results
have also been observed in previous studies, where the paced
breathing increased parasympathetic activity during a ≤5 min time
period (12,13).
The beat-to-beat variability of PTT is primarily
responsible for the possible difference between PRV and HRV. It has
been verified that PTT variation had an inversely proportional
association with changes in blood pressure, which may result from
the autonomic regulation on HR, cardiac contractility, vascular
resistance and compliance (14). For
spontaneous respiration at resting, during which sympathetic and
vagal branches are in a relatively balanced state, PTT variability
keeps a relative consistency with HRV; thus, PRV and HRV show good
agreement for all HRV indices, as previous studies stated (15,16).
However, the paced respiration produced decreases in sympathetic
activation that would lead to a reduction in variability of PTT
(17). Based on this notion, the
random error due to PTT variation decreases, and the difference
between PRV and HRV indices tends to be constant. In the present
study, the systematic discrepancy caused by different measurements
became predominant, but remained within an acceptable limit. In the
present study, a significant difference for long-term and
short-term indices was observed between PRV and HRV and at the same
time, BA r for most indices was <0.2 during paced respiration.
The only exception was the LF/HF ratio that showed an insufficient
agreement (BAr=0.25), which may be due to the notable decrease in
the value of LF/HF. Specifically, the average of the pairwise means
Apm during paced respiration became lower, so even if there was no
increase in UL-LLr, (UL-LL)/2Apm was still high [see equation
5].
By contrast, apnea resulted in sympathetic
predominance. Based on a previous study (17), the enhancement of sympathetic
activity during breath holding should have led to an increase in
the beat-to-beat variability of PTT. Accordingly, the beat-to-beat
discrepancy between RRI and PPI increased. Thus, the indices
associated with short-term variability, including rMSSD and SD1 and
HF, exhibited an insufficient agreement (BAr>0.2). However, the
beat-to-beat minor differences had no significant impact on the
overall characteristics of PRV, and thus the other indices during
apnea had an acceptable agreement between PRV and HRV.
The results from healthy subjects during apnea
differed from patients with sleep apnea. A previous study stated
that the short-term (2 min) variability indices (rMSSD and HF), the
long-term variability index (SDNN) and the index associated with
sympathovagal balance (LF/HF) between PRV and HRV showed
significant differences during the obstructive sleep apnea events
(18). Patients with sleep apnea
usually develop circulatory disorders, such as arrhythmia,
hypertension and heart failure (19), which may alter the PPG pulse
characteristics (2). Thus, the
pathological changes may lead to the different results.
Nevertheless, the results from healthy subjects provide a reference
for using PPG in sleep apnea and other diseases.
In conclusion, the results demonstrated that HR
indices (mean and HR values), long-term variability indices (SDNN,
SD2 and LF) and two proportional indices reflecting sympathovagal
balance of PRV (LF and HF) offer stable reliability regardless of
respiratory mode. However, the agreement between PRV and HRV in
terms of short-term variability (rMSSD, SD1 and HF) and LF/HF is
susceptible to respiratory changes. Additionally, compared with
spontaneous respiratory conditions, HRV presented significant and
reversible changes under paced respiratory and apnea conditions.
Such opposite changes in HRV reflect the inverse autonomic nervous
responses to different respiratory ways. Therefore, a more general
conclusion may be obtained on the accuracy of PRV for healthy
subjects. When long-term variability and sympathovagal balance
increase significantly, the agreement for short-term variability
indices between PRV and HRV would become insufficient, while the
significant decrease of long-term variability and sympathovagal
balance may result in a marginal inaccuracy of LF/HF. The results
of this study presented the distinctive characteristics of PRV
indices in different autonomic nervous states, which should be
considered when applying PPG as an alternative approach of ECG to
obtain HRV indices for the evaluation of autonomic function.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (no. 81101117), the Natural
Science Foundation of Shaanxi Province (no. 2012JM4030), the
Shaanxi Province Foundation for Returnees (no. SLZ2009008), the
Fundamental Research Funds for the Central Universities (nos.
XJJ20100170, XJJ2012129 and 2012JDHZ49) and the China Postdoctoral
Science Fund (no. 2012M521779).
References
|
1
|
Kemp AH and Quintana DS: The relationship
between mental and physical health: Insights from the study of
heart rate variability. Int J Psychophysiol. 89:288–296. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Allen J: Photoplethysmography and its
application in clinical physiological measurement. Physiol Meas.
28:R1–R39. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wong JS, Lu WA, Wu KT, Liu M, Chen GY and
Kuo CD: A comparative study of pulse rate variability and heart
rate variability in healthy subjects. J Clin Monit Comput.
26:107–114. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gil E, Orini M, Bailón R, Vergara JM,
Mainardi L and Laguna P: Photoplethysmography pulse rate
variability as a surrogate measurement of heart rate variability
during non-stationary conditions. Physiol Meas. 31:1271–1290. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schäfer A and Vagedes J: How accurate is
pulse rate variability as an estimate of heart rate variability? A
review on studies comparing photoplethysmographic technology with
an electrocardiogram. Int J Cardiol. 166:15–29. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Heart rate variability: Standards of
measurement, physiological interpretation and clinical use. Task
force of the European Society of Cardiology and the North American
Society of Pacing and Electrophysiology. Circulation. 93:1043–1065.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bland JM and Altman DG: Statistical
methods for assessing agreement between two methods of clinical
measurement. Lancet. 1:307–310. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lu G, Yang F, Taylor JA and Stein JF: A
comparison of photoplethysmography and ECG recording to analyse
heart rate variability in healthy subjects. J Med Eng Technol.
33:634–641. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Giardino ND, Lehrer PM and Edelberg R:
Comparison of finger plethysmograph to ECG in the measurement of
heart rate variability. Psychophysiology. 39:246–253. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Charlot K, Cornolo J, Brugniaux JV,
Richalet JP and Pichon A: Interchangeability between heart rate and
photoplethysmography variabilities during sympathetic stimulations.
Physiol Meas. 30:1357–1369. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pinna GD, Maestri R, La Rovere MT, Gobbi E
and Fanfulla F: Effect of paced breathing on ventilatory and
cardiovascular variability parameters during short-term
investigations of autonomic function. Am J Physiol Heart Circ
Physiol. 290:H424–H433. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Driscoll D and Dicicco G: The effects of
metronome breathing on the variability of autonomic activity
measurements. J Manipulative Physiol Ther. 23:610–614. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stark R, Schienle A, Walter B and Vaitl D:
Effects of paced respiration on heart period and heart period
variability. Psychophysiology. 37:302–309. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Foo JY and Lim CS: Pulse transit time as
an indirect marker for variations in cardiovascular related
reactivity. Technol Health Care. 14:97–108. 2006.PubMed/NCBI
|
|
15
|
Hayano J, Barros AK, Kamiya A, Ohte N and
Yasuma F: Assessment of pulse rate variability by the method of
pulse frequency demodulation. Biomed Eng Online. 4:622005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shi P, Hu S and Zhu Y: A preliminary
attempt to understand compatibility of photoplethysmographic pulse
rate variability with electrocardiogramic heart rate variability. J
Med Biol Eng. 28:173–180. 2008.
|
|
17
|
Malliani A, Pagani M, Lombardi F and
Cerutti S: Cardiovascular neural regulation explored in the
frequency domain. Circulation. 84:482–492. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Khandoker AH, Karmakar CK and Palaniswami
M: Comparison of pulse rate variability with heart rate variability
during obstructive sleep apnea. Med Eng Phys. 33:204–209. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Leung RS: Sleep-disordered breathing:
Autonomic mechanisms and arrhythmias. Prog Cardiovasc Dis.
51:324–338. 2009. View Article : Google Scholar : PubMed/NCBI
|