Introduction
Acute ischemic stroke has been recognized as the
third leading cause of mortality (1). In vivo and in vitro
models of ischemic stroke have previously been used to investigate
the mechanisms underlying ischemic damage, and to analyze the
effectiveness of various therapeutic compounds for treating
ischemic stroke (2–6). Experimental transient cerebral
ischemia, which is typically induced by middle cerebral artery
occlusion in rats/mice, and bilateral common carotid artery
occlusion in gerbils, has previously been associated with selective
neuronal cell damage/death in vulnerable regions of the brain,
including the neocortex and hippocampus (7,8). In
addition, experimental transient cerebral ischemia-induced neuronal
cell damage has been associated with free radical-associated damage
and oxidative stress (7,9,10).
Therefore, regulating oxidative stress levels and the production of
antioxidants may be considered a potential strategy in the
prevention and treatment of transient cerebral ischemia-induced
neuronal cell damage/death (11,12).
Antiepileptic drugs act on one or more target
molecules in the brain, including ion channels, neurotransmitter
transporters and neurotransmitter metabolic enzymes, which are
involved in major physiological processes, and learning and memory
functions (13,14). Lacosamide
(R-2-acetamido-N-benzyl-3-methoxypropionamide), which was formerly
known as harkoseride, belongs to a group of functionalized amino
acids and is a novel antiepileptic drug (15,16).
Lacosamide was initially shown to exert activity in various animal
models of epilepsy (17,18), and has since been evaluated in phase
III clinical trials for the treatment of human patients with
epilepsy (19). As well as analgesic
properties, lacosamide has demonstrated therapeutic potential in
experimental animal models of neuropathic and inflammatory pain
(20–22). Furthermore, lacosamide has been
reported to alleviate neuropathic pain-like behaviors in the
central nervous system of a rat model of spinal cord injury
(16). It has previously been
suggested that antiepileptic drugs may affect various signaling
pathways associated with neuronal plasticity and survival, thus
suggesting a potential application for antiepileptic drugs in the
treatment of nonepileptic conditions (23).
Various drugs have been reported to show efficacy in
the treatment of symptoms other than what they were designed for.
Therefore, broadening the activity profile of existing clinical
drugs has emerged as a novel strategy in the drug developmental
process. In our previous study, we demonstrated that the
antipsychotic drug, risperidone, was able to exert neuroprotective
effects against transient cerebral ischemia-induced neuronal cell
death (12). To the best of our
knowledge, there is no study regarding the effects of lacosamide on
experimentally-induced ischemic stroke; therefore, the present
study aimed to analyze the protective effects of lacosamide against
neuronal cell damage/death in the hippocampus of a gerbil model of
transient cerebral ischemia. Gerbils have previously demonstrated
efficacy for investigating the effects of transient cerebral
ischemia (24–26).
Materials and methods
Experimental animals
A total number of 42 male Mongolian Gerbils (age, 6
months; weight, 65–75 g) were obtained from the Experimental Animal
Center of Kangwon National University (Chuncheon, South Korea) and
maintained in a controlled environment (temperature, 23°C;
humidity, 60%), under a 12 h light/12 h dark cycle, with ad
libitum access to food and water throughout the experimental
period. All experimental procedures were conducted in accordance
with the National Institutes of Health guidelines for the Care And
Use of Laboratory Animals (27), and
the experimental protocol was approved by the Institutional Animal
Care and Use Committee of Kangwon National University (Chuncheon,
South Korea; approval no. KW-130424-1). All of the experiments were
designed to minimize the number of gerbils used and their
suffering.
Induction of transient cerebral
ischemia
The gerbils were anesthetized using a mixture of
2.5% isoflurane (Baxter, Deerfield, IL, USA), 33% oxygen and 67%
nitrous oxide. The bilateral common carotid arteries were isolated
and occluded using non-traumatic aneurysm clips, after which gauze
soaked in 0.9% saline was placed onto the necks of the gerbils in
order to prevent dehydration of the arteries and the surrounding
tissue. Complete interruption of blood flow was confirmed by
observing the central artery in the retinae of the gerbils using an
ophthalmoscope. Following occlusion for 5 min, the aneurysm clips
were removed from the common carotid arteries. The body
temperatures under normothermic conditions (37±0.5°C) were
monitored using rectal temperature probes (TR-100; Fine Science
Tools, Foster City, CA, USA), and were maintained using a
thermometric blanket prior to, during and following the surgery,
until the gerbils recovered from the anesthesia. Thereafter,
animals were maintained in a thermal incubator (Mirae Medical
Industry, Seoul, South Korea), prior to sacrifice. The
sham-operated gerbils were subjected to an identical surgical
protocol; however the common carotid arteries were not
occluded.
Treatment with lacosamide
In order to investigate the protective effects of
lacosamide against ischemic damage 5 days post-surgery, the gerbils
were distributed into the following groups (n=7 at each time in
each group): i) Vehicle (saline)-treated-sham-operated-group
(sham-group); ii) vehicle-treated-ischemia-operated-group
(ischemia-group); iii) 10 mg/kg
lacosamide-pretreated-ischemia-operated-group; iv) 25 mg/kg
lacosamide-pretreated-ischemia-operated-group; v) 10 mg/kg
lacosamide-posttreated-ischemia-operated-group; and vi) 25 mg/kg
lacosamide-posttreated-ischemia-operated-group. Lacosamide (UCB
Pharma SA, Brussels, Belgium) was dissolved in saline and
intraperitoneally administered to the gerbils once daily for 3 days
prior to or following the ischemic surgery. The final gerbil to be
treated in the lacosamide-pretreated-ischemia-groups, and the first
in the lacosamide-posttreated-ischemia-groups, were treated at 30
min prior to and following surgery, respectively.
Spontaneous motor activity (SMA)
analysis
In order to investigate the effects of lacosamide on
ischemia-induced hyperactivity, the SMA of the gerbils was measured
1 day following ischemia-reperfusion. The gerbils, which were not
exposed to the open field prior to ischemia, were individually
placed in a Plexiglas cage (25×20×12 cm) located within a
soundproof chamber. The locomotor activity was recorded using the
Photobeam Activity System-Home Cage (San Diego Instruments, San
Diego, CA, USA), according to our previous study, with
modifications (28). The cage was
fitted with two parallel horizontal infrared beams 2 cm off the
floor. Movement was detected via the interruption of an array of 32
infrared beams produced by photocells. The SMA of all the gerbils
was monitored simultaneously for 60 min, and locomotor activity
data were acquired using an AMB analyzer (IPC Electronics, Cumbria,
UK). Data collection was initiated 15 min following habituation in
the Plexiglas cage. The results were evaluated in terms of the
distance (meters) traveled in the 60 min test period.
Tissue processing for histology
For histological analysis, the gerbils were
anesthetized using sodium pentobarbital (30 mg/kg; JW Pharm. Co.,
Ltd., Seoul, South Korea), after which they were treated with 0.1 M
phosphate-buffered saline (PBS; pH 7.4), and then 4%
paraformaldehyde in 0.1 M phosphate-buffer (pH 7.4), via a
transcardial perfusion. Subsequently, the brains were removed and
postfixed in paraformaldehyde for 6 h, after which the brain
tissues were cryoprotected via infiltration with 30% sucrose
overnight. Thereafter, frozen tissues were serially sectioned using
a cryostat (Leica Microsystems GmbH, Wetzlar, Germany) into 30 µm
coronal sections, which were subsequently distributed into 6-well
plates containing PBS.
Staining with Neuronal Nuclei
(NeuN)
In order to examine the neuronal damage in the
hippocampus following ischemia-reperfusion, NeuN (a marker for
neurons) immunohistochemistry was conducted, as outlined in
previous studies (29,30). Briefly, the tissue sections were
treated with 0.3% hydrogen peroxide in PBS for 30 min, followed by
10% normal horse or normal rabbit serum (Vector Laboratories, Inc.,
Burlingame, CA, USA) in 0.05 M PBS for 30 min. Subsequently, the
tissue sections were incubated with diluted mouse anti-NeuN (a
neuron-specific soluble nuclear antigen; cat. no., MAB377;
dilution, 1:1,000; Chemicon International, Inc., Temecula, CA, USA)
overnight at 4°C. Thereafter, the tissues were exposed to
streptavidin peroxidase-conjugated biotinylated goat anti-mouse
immunoglobulin G (cat. no., BA-9200; dilution, 1:250; Vector
Laboratories, Inc.), after which they were visualized using
3,3′-diaminobenzidine (Sigma-Aldrich, St. Louis, MO, USA) in 0.1 M
Tris HCl buffer, and mounted onto gelatin-coated slides. Following
dehydration, the tissue sections were mounted with Canada balsam
(Kato Chemical, Co., Tokyo, Japan).
In order to quantitatively analyze NeuN
immunoreactivity, digital images of the hippocampus were captured
using an AxioM1 light microscope (Carl Zeiss AG, Oberkochen,
Germany), equipped with a digital camera (Axiocam; Carl Zeiss AG)
connected to a PC monitor. NeuN immunoreactive neurons were counted
in a 250×250 µm square applied at the approximate center of the
cornu ammonis (CA)-1 region using the Optimas 6.5 Image Analyzing
software (CyberMetrics, Co., Scottsdale, AZ, USA). The studied
tissue sections were selected with 300 µm intervals, according to
anatomical landmarks corresponding to AP −1.4 to −1.9 mm of the
gerbil brain atlas (31). Cell
counts were obtained by averaging the counts from each gerbil.
Immunohistochemical glial fibrillary
acidic protein (GFAP) and ionized calcium-binding adapter
molecule-1 (Iba-1) staining
In order to investigate glial activation and
oxidative stress in the ischemic hippocampus of the gerbils
following transient cerebral ischemia, immunohistochemical staining
using rabbit anti-GFAP (cat. no., AB5804; dilution, 1:1,000;
Chemicon International, Inc.) for astrocytes and rabbit anti-Iba
(cat. no., 019–19741; dilution, 1:1,000, Wako Pure Chemical
Industries, Ltd., Osaka, Japan) for microglia, was conducted, as
outlined previously (32). In
addition, a negative control test was conducted using pre-immune
serum as a substitute for primary antibody, in order to confirm the
specificity of the immunostaining procedure. The negative control
test resulted in the absence of immunoreactivity in all
structures.
A total of 15 sections per gerbil were selected to
quantitatively analyze GFAP and Iba-1 immunoreactivity. GFAP and
Iba-1 immunoreactivity were graded as follows: Digital images of
the hippocampal CA1 region were captured using an AxioM1 light
microscope (Carl Zeiss AG), equipped with a digital camera
(Axiocam; Carl Zeiss AG) connected to a PC monitor.
Semi-quantification of the immunostaining intensities of GFAP and
Iba-1 was conducted using MetaMorph 4.01 digital image analysis
software (Universal Imaging, Bedford Hills, NY, USA). The mean
intensity of GFAP and Iba-1 immunostaining in each immunoreactive
structure was measured using a 0–255 gray scale system (white to
dark signal corresponded to 255 to 0). Using this approach, the
level of immunoreactivity was scaled as one of the following: -, ±,
+ or ++, representing no staining (gray scale value, ≥200), weakly
positive (gray scale value, 150–199), moderately positive (gray
scale value, 100–149), or strongly positive (gray scale value, ≤99)
staining, respectively.
Statistical analysis
The data are presented as the mean ± standard error
of the mean. Statistical differences were analyzed using one-way
analysis of variance, followed by post-hoc Bonferroni's multiple
comparison test with SPSS 17.0 software (SPSS Inc., Chicago, IL,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
SMA analysis
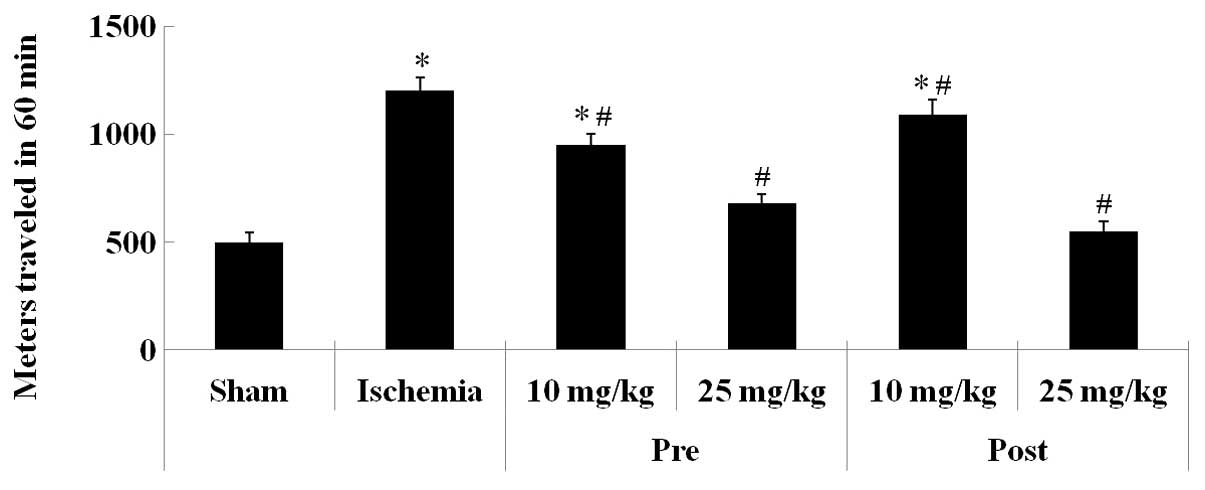
The SMA of the gerbils was examined 1 day following
ischemia-reperfusion. The SMA of the gerbils in the ischemia-group
was significantly increased 1 day following ischemia-reperfusion,
as compared with the sham-group gerbils (P<0.05; Fig. 1). Furthermore, the SMA of the gerbils
in the lacosamide-pretreated-ischemia-groups (10 and 25 mg/kg) was
significantly decreased, as compared with the ischemia-group
gerbils, although the reduction in SMA was more significant for the
gerbils pretreated with 25 mg/kg lacosamide, as compared with the
10 mg/kg lacosamide pretreated group (P<0.05; Fig. 1). In addition, the alterations in the
SMA of the lacosamide-posttreated-ischemia-group gerbils were
consistent with those observed for the
lacosamide-pretreated-ischemia-groups.
Neuroprotective effects
NeuN-positive neurons were predominantly observed in
the stratum pyramidale (SP) of the hippocampal CA1 region of the
sham-group gerbils (Fig. 2A and B),
whereas very few NeuN-positive neurons were detected in the SP of
the ischemia-group gerbils (~8% of the sham-group; Fig. 2C and D), 5 days following
ischemia-reperfusion.
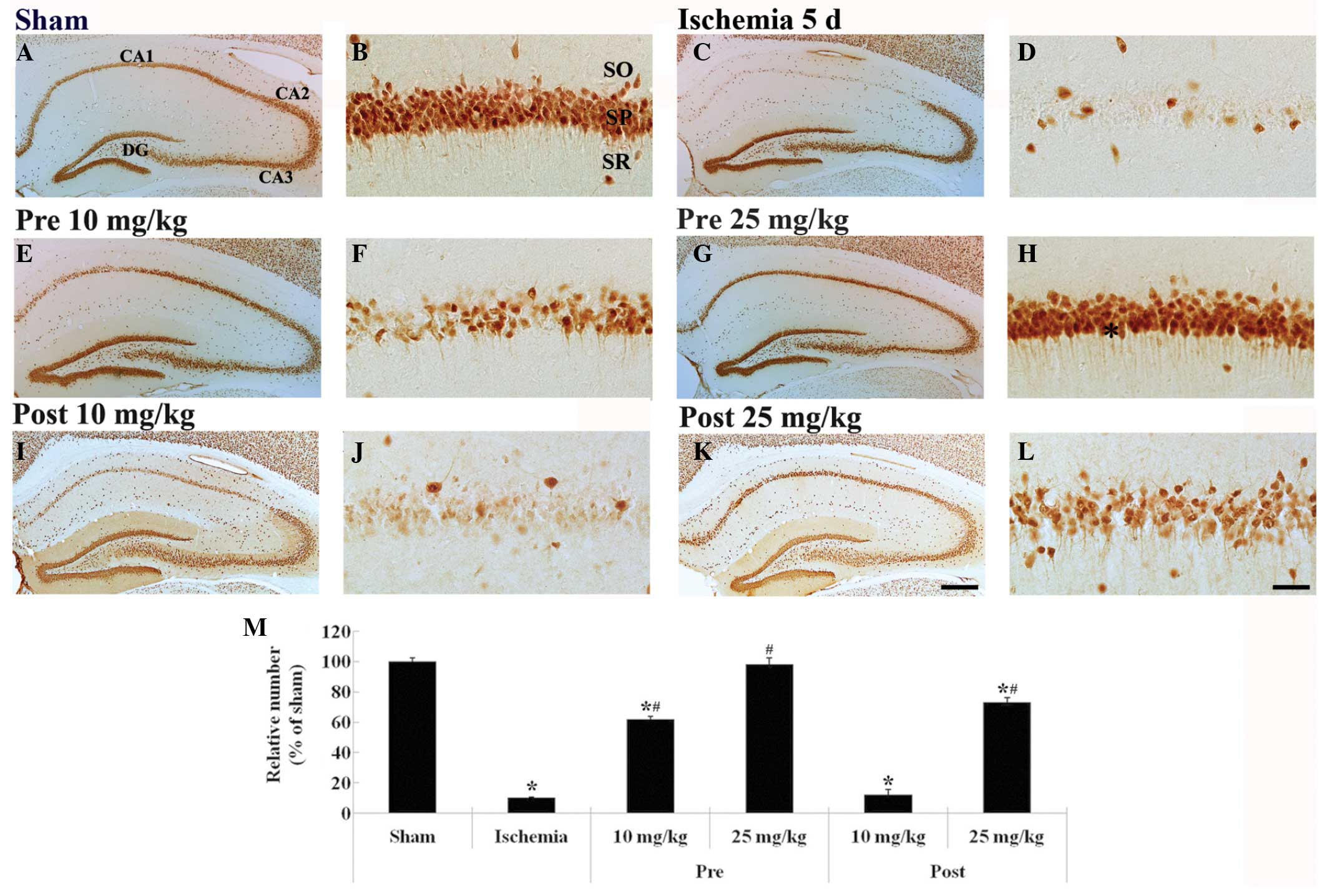 | Figure 2.NeuN immunohistochemistry in the
hippocampus of the (A and B) sham-, (C and D) ischemia-, (E-H)
lacosamide-pretreated-ischemia- and (I-L)
lacosamide-posttreated-ischemia-groups (n=7 gerbils/group) 5 days
following ischemia-reperfusion. In the 25 mg/kg
lacosamide-pretreated-ischemia-group, the distribution pattern of
NeuN-positive neurons in the SP (asterisk) resembled that in the
sham-group (Scale bar=400 µm for A, C, E, G, I and K; scale bar=50
µm for B, D, F, H, J and L). (M) Relative percentage of
NeuN-immunoreactive neurons in the hippocampal CA1 region of the
various groups. Data are presented as the mean ± standard error of
the mean. *P<0.05, vs. the sham-group; #P<0.05,
vs. the ischemia-group. NeuN, neuronal nuclei; CA, cornu ammonis;
DG, dentate gyrus; SO, stratum oriens; SP, stratum pyramidale; SR,
stratum radiatum. |
The mean number of NeuN-positive neurons was
significantly increased (~60% of the sham-group) in the 10 mg/kg
lacosamide-pretreated-ischemia-group, as compared with the
ischemia-group (P<0.05; Fig. 2E and
F). Conversely, the mean number of NeuN-positive neurons in the
SP of the 25 mg/kg lacosamide-pretreated-ischemia-group resembled
that in the sham-group (P>0.05; Fig.
2G, H and M).
The mean number of NeuN-positive neurons in the 10
mg/kg lacosamide-posttreated-ischemia-group resembled (~9% of the
sham-group) that in the ischemia-group (P>0.05; Fig. 2I, J and M). Conversely, in the 25
mg/kg lacosamide-posttreated-ischemia-group, a high number of
NeuN-positive neurons (~75% of the sham-group) was detected in the
SP 5 days following ischemia-reperfusion (P>0.05; Fig. 2K–M).
Glial activation
Astrocytes
GFAP-immunoreactive astrocytes in the sham-group
gerbils were at resting potential, corresponding to a small body
with thread-like processes, and were detected in all the layers of
the CA1 hippocampal region (Fig.
3Aa). Conversely, GFAP-immunoreactive astrocytes in the
ischemia-group were activated, and contained a bulky cytoplasm.
Furthermore, GFAP immunoreactivity was markedly increased in all
layers of the CA1 region of the ischemia-group rats (Table I and Fig.
3Ab).
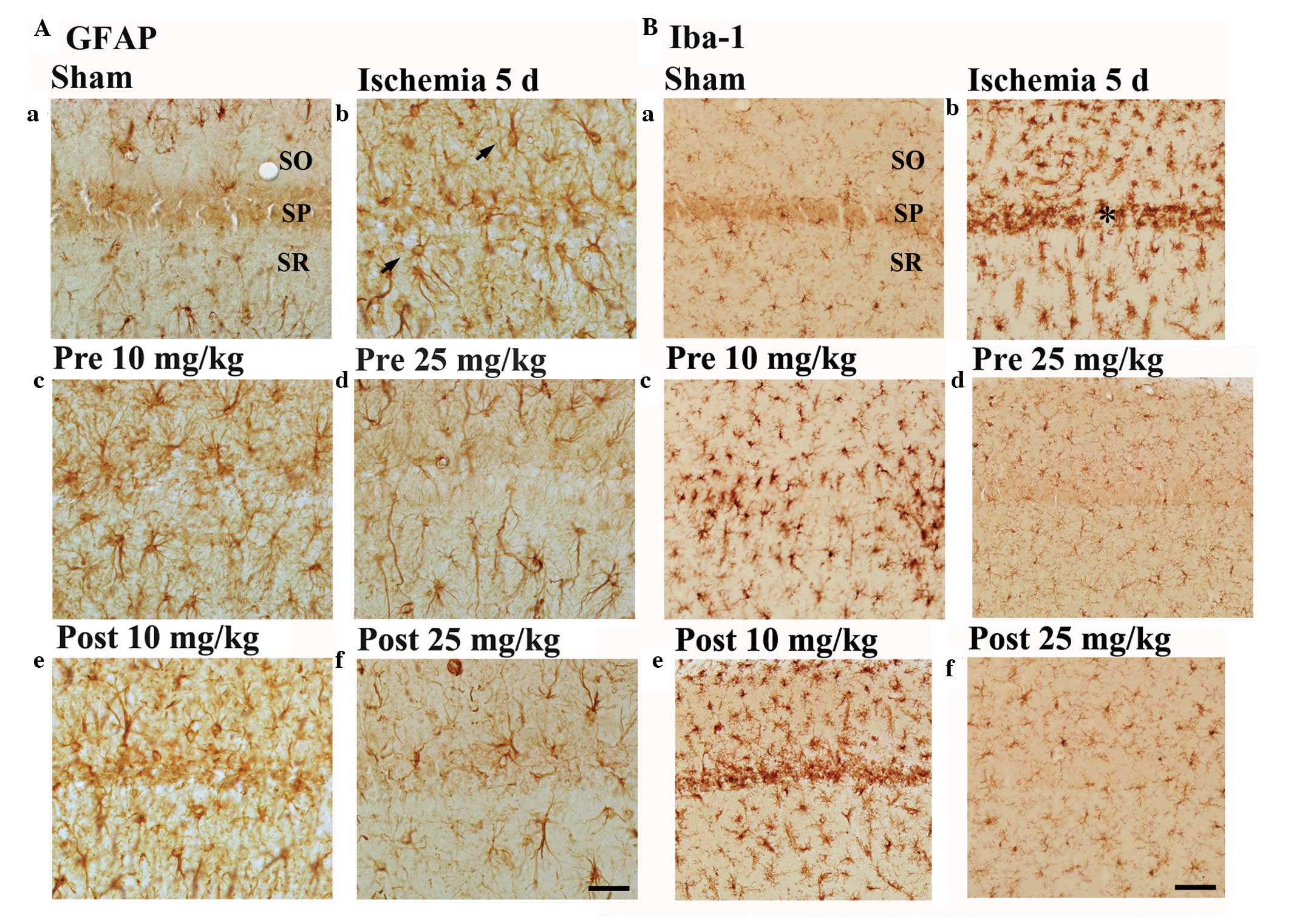 | Figure 3.(A) GFAP immunohistochemistry in the
CA1 region of the (a) sham-, (b) ischemia-, (c and d)
lacosamide-pretreated-ischemia- and (e and f)
lacosamide-posttreated-ischemia- groups 5 days following
ischemia-reperfusion. GFAP-immunoreactive astrocytes (arrows) were
markedly activated in the ischemia-group gerbils. In the 10 mg/kg
lacosamide-treated-ischemia-groups, the distribution pattern of
GFAP-immunoreactive astrocytes resembled that in the
ischemia-group. Conversely, in the 25 mg/kg
lacosamide-treated-ischemia-groups, the activation of
GFAP-immunoreactive astrocytes was markedly reduced, as compared
with that in the ischemia group. (B) Iba-1 immunohistochemistry in
the CA1 region of the (a) sham-, (b) ischemia-, (c and d)
lacosamide-pretreated-ischemia- and (e and f)
lacosamide-posttreated-ischemia- groups 5 days following
ischemia-reperfusion. In the ischemia-group, Iba-1-immunoreactive
microglia in the CA1 region were activated and aggregated in the SP
(asterisk). In the 25 mg/kg lacosamide-treated-ischemia-groups, the
pattern of Iba-1-immunoreactive microglia resembled that in the
sham group. Scale bar=50 µm. CA, cornu ammonis; SO, stratum oriens;
SP, stratum pyramidale; SR, stratum radiatum; GFAP, glial
fibrillary acidic protein; Iba-1, ionized calcium-binding adapter
molecule. |
 | Table I.Semi-quantification of the
immunostaining intensity of GFAP in the CA1 region of the sham-,
ischemia- and lacosamide-treated-groups, 5 days following
ischemia-reperfusion. |
Table I.
Semi-quantification of the
immunostaining intensity of GFAP in the CA1 region of the sham-,
ischemia- and lacosamide-treated-groups, 5 days following
ischemia-reperfusion.
| Group | CA1 subregion | Immunostaining
intensity |
|---|
| Sham | SO | + |
|
| SP | ± |
|
| SR | + |
| Ischemia | SO | ++ |
|
| SP | +++ |
|
| SR | ++ |
| Pretreatment |
|
|
| 10
mg/kg | SO | ++ |
|
| SP | + |
|
| SR | ++ |
| 25
mg/kg | SO | + |
|
| SP | ± |
|
| SR | + |
| Posttreatment |
|
|
| 10
mg/kg | SO | ++ |
|
| SP | ++ |
|
| SR | ++ |
| 25
mg/kg | SO | ++ |
|
| SP | + |
|
| SR | ++ |
GFAP-immunoreactive astrocytes were activated in the
10 mg/kg lacosamide-pretreated-ischemia-group gerbils, although to
a lesser extent than in the ischemia-group gerbils (Table I and Fig.
3Ac). Conversely, GFAP-immunoreactive astrocytes were not
markedly activated in the 25 mg/kg
lacosamide-pretreated-ischemia-group, as compared with in the 10
mg/kg lacosamide-pretreated-ischemia-group (Table I and Fig.
3Ad). The morphology of GFAP-immunoreactive astrocytes in the
10 mg/kg lacosamide-posttreated-ischemia-group resembled those in
the ischemia-group (Table I and
Fig. 3Ae), whereas
GFAP-immunoreactive astrocytes in the 25 mg/kg
lacosamide-posttreated-ischemia-group were not markedly activated,
as compared with the 10 mg/kg lacosamide-posttreated-ischemia-group
(Table I and Fig. 3Af).
Microglia
Iba-1-immunoreactive microglia were distributed
throughout all layers of the CA1 region of the sham-group gerbils,
and were observed in a rest form that was ramified in appearance
(Fig. 3Ba). Conversely, the
Iba-1-immunoreactive microglia in the ischemia-group were activated
(hypertrophied in appearance) and aggregated in the SP layer of the
CA1 region. In addition, the Iba-1 immunoreactivity of the
ischemia-group microglia was markedly increased (Table II and Fig. 3Bb), as compared with the
sham-group.
 | Table II.Semi-quantification of the
immunostaining intensity of Iba-1 in the CA1 region of the sham-,
ischemia- and lacosamide-treated groups, 5 days following
ischemia-reperfusion. |
Table II.
Semi-quantification of the
immunostaining intensity of Iba-1 in the CA1 region of the sham-,
ischemia- and lacosamide-treated groups, 5 days following
ischemia-reperfusion.
| Group | CA1 subregion | Immunostaining
intensity |
|---|
| Sham | SO | ± |
|
| SP | ± |
|
| SR | ± |
| Ischemia | SO | ++ |
|
| SP | ++ |
|
| SR | ++ |
| Pre-treatment |
|
|
| 10
mg/kg | SO | ++ |
|
| SP | + |
|
| SR | ++ |
| 25
mg/kg | SO | ± |
|
| SP | ± |
|
| SR | ± |
| Post-treatment |
|
|
| 10
mg/kg | SO | ++ |
|
| SP | ++ |
|
| SR | ++ |
| 25
mg/kg | SO | + |
|
| SP | ± |
|
| SR | + |
Iba-1-immunoreactive microglia in the 10 mg/kg
lacosamide-pretreated-ischemia-group were activated and exhibited
strongly positive Iba-1 immunoreactivity; however, they did not
appear to be aggregated in the SP layer of the CA1 region (Table II and Fig. 3Bc). Conversely, the distribution
pattern of Iba-1 immunoreactive microglia in the 25 mg/kg
lacosamide-pretreated-ischemia-group resembled that in the
sham-group (Table II and Fig. 3Bd). Iba-1-immunoreactive microglia in
the 10 mg/kg lacosamide-posttreated-ischemia-group were aggregated
in the SP, similar to those in the ischemia-group (Table II and Fig. 3Be); whereas, the distribution pattern
of Iba-1-immunoreactive microglia in the 25 mg/kg
lacosamide-posttreated-ischemia-group resembled that in the
sham-group, although their Iba-1-immunoreactivity was increased
(Table II and Fig. 3Df), as compared with the sham
group.
Discussion
The results of the present study suggested that
lacosamide may exert additional beneficial therapeutic effects
beyond its use as an antiepileptic drug. Lacosamide effectively
protected against ischemia-induced neuronal cell damage in the
hippocampal CA1 region of a gerbil model of transient cerebral
ischemia. Pretreatment of the gerbils with 10 or 25 mg/kg
lacosamide protected against ischemia-induced neuronal cell damage,
whereas posttreatment with 25 mg/kg lacosamide protected against
ischemic damage in the gerbil hippocampal CA1 region 5 days
following ischemia-reperfusion.
To the best of our knowledge, the present study is
the first to investigate the protective effects of lacosamide
against ischemia-induced neuronal cell damage/death; however, Licko
et al (33) previously
reported that the long-term treatment of a rat model of electrical
status epilepticus with lacosamide attenuated neuronal cell loss
and alterations in hippocampal neurogenesis. The present study
demonstrated that neuroprotective strategies may be effective in
the treatment of patients with ischemic insults, and found that
novel antiepileptic drugs may exert additional activities that
protect against ischemia-induced neuronal cell damage.
In the present study, treatment with lacosamide
attenuated the ischemia-induced activation of astrocytes and
microglia in the gerbil ischemic hippocampal CA1 region in a
dose-dependent manner. Previous studies have detected an
association between ischemic stroke and glial cells: Glial cells
were activated by an ischemic stroke and were shown to be
associated with ischemia-induced neuronal cell death via the
release of inflammatory cytokines/mediators (34,35).
Furthermore, the production of inflammatory cytokines/mediators has
previously been associated with inflammation and neuropathic pain
(36,37), and lacosamide has been shown to
effectively inhibit pain, with minor adverse side effects, in an
animal model of inflammation and neuropathic pain (33). In addition, lacosamide demonstrated
antihyperalgesic activity in a rat model of tumor necrosis
factor-α-induced muscle pain (38).
Furthermore, lacosamide has been reported to decrease the
activation of microglia and upregulation of glial migration
factors, and delayed the downregulation of an interleukin-6
cytokine receptor subunit (39).
Therefore, in the present study, the attenuation of
ischemia-induced microglial activation in the hippocampal CA1
region of lacosamide-treated gerbils may have been associated with
the neuroprotective effects of lacosamide against transient
cerebral ischemic damage.
In conclusion, the results of the present study
suggested that lacosamide was able to protect against
ischemia-induced neuronal cell death/damage, and that this
neuroprotective activity may have been associated with attenuation
of glial cell activation.
Acknowledgements
The authors of the present study would like to thank
Mr. Seung Uk Lee from the Kangwon National University, South Korea
for his technical help. The present study was supported by the
National Research Foundation of Korea (NRF) funded by the Ministry
of Education, Science and Technology (grant no. 2010-0010580), the
Basic Science Research Program through the NRF funded by the
Ministry of Science, ICT & Future Planning (grant no.
NRF-2014R1A2A2A01005307), and the Basic Science Research Program
through the NRF funded by the Ministry of Education (grant no.
NRF-2014R1A1A2057092).
References
|
1
|
Singh J and Nguyen TN: Endovascular and
neurosurgical management of acute ischemic stroke. Emerg Med Clin
North Am. 30:695–712. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Domoráková I, Mechírová E, Danková M,
Danielisová V and Burda J: Effect of antioxidant treatment in
global ischemia and ischemic postconditioning in the rat
hippocampus. Cell Mol Neurobiol. 29:837–844. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huang X, Li Q, Li H and Guo L:
Neuroprotective and antioxidative effect of cactus polysaccharides
in vivo and in vitro. Cell Mol Neurobiol. 29:1211–1221. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mousavi SH, Tayarani-Najaran Z, Asghari M
and Sadeghnia HR: Protective effect of Nigella sativa extract and
thymoquinone on serum/glucose deprivation-induced PC12 cells death.
Cell Mol Neurobiol. 30:591–598. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nagy D, Marosi M, Kis Z, Farkas T, Rakos
G, Vecsei L, Teichberg VI and Toldi J: Oxaloacetate decreases the
infarct size and attenuates the reduction in evoked responses after
photothrombotic focal ischemia in the rat cortex. Cell Mol
Neurobiol. 29:827–835. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Oliveira IJ, Molz S, Souza DO and Tasca
CI: Neuroprotective effect of GMP in hippocampal slices submitted
to an in vitro model of ischemia. Cell Mol Neurobiol. 22:335–344.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kataoka T, Etani R, Takata Y, Nishiyama Y,
Kawabe A, Kumashiro M, Taguchi T and Yamaoka K: Radon inhalation
protects against transient global cerebral ischemic injury in
gerbils. Inflammation. 37:1675–1682. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sakai N, Yanai K, Ryu JH, Nagasawa H,
Hasegawa T, Sasaki T, Kogure K and Watanabe T: Behavioral studies
on rats with transient cerebral ischemia induced by occlusion of
the middle cerebral artery. Behav Brain Res. 77:181–188. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Candelario-Jalil E, Alvarez D, Merino N
and León OS: Delayed treatment with nimesulide reduces measures of
oxidative stress following global ischemic brain injury in gerbils.
Neurosci Res. 47:245–253. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Oztanir MN, Ciftci O, Cetin A and Aladag
MA: Hesperidin attenuates oxidative and neuronal damage caused by
global cerebral ischemia/reperfusion in a C57BL/J6 mouse model.
Neurol Sci. 35:1393–1399. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pei H, Cao D, Guo Z, Liu G, Guo Y and Lu
C: Bone morphogenetic protein-7 ameliorates cerebral ischemia and
reperfusion injury via inhibiting oxidative stress and neuronal
apoptosis. Int J Mol Sci. 14:23441–23453. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yan BC, Park JH, Ahn JH, Kim IH, Park OK,
Lee JC, Yoo KY, Choi JH, Lee CH, Hwang IK, et al: Neuroprotection
of posttreatment with risperidone, an atypical antipsychotic drug,
in rat and gerbil models of ischemic stroke and the maintenance of
antioxidants in a gerbil model of ischemic stroke. J Neurosci Res.
92:795–807. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen J, Cai F, Cao J, Zhang X and Li S:
Long-term antiepileptic drug administration during early life
inhibits hippocampal neurogenesis in the developing brain. J
Neurosci Res. 87:2898–2907. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rogawski MA and Löscher W: The
neurobiology of antiepileptic drugs. Nat Rev Neurosci. 5:553–564.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Geis C, Beyreuther BK, Stöhr T and Sommer
C: Lacosamide has protective disease modifying properties in
experimental vincristine neuropathy. Neuropharmacology. 61:600–607.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hao JX, Stöhr T, Selve N,
Wiesenfeld-Hallin Z and Xu XJ: Lacosamide, a new anti-epileptic,
alleviates neuropathic pain-like behaviors in rat models of spinal
cord or trigeminal nerve injury. Eur J Pharmacol. 553:135–140.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wasterlain CG, Stöhr T and Matagne A: The
acute and chronic effects of the novel anticonvulsant lacosamide in
an experimental model of status epilepticus. Epilepsy Res.
94:10–17. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shandra A, Shandra P, Kaschenko O, Matagne
A and Stöhr T: Synergism of lacosamide with established
antiepileptic drugs in the 6-Hz seizure model in mice. Epilepsia.
54:1167–1175. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hovinga CA: SPM-927 (Schwarz Pharma).
IDrugs. 6:479–485. 2003.PubMed/NCBI
|
|
20
|
Beyreuther B, Callizot N and Stöhr T:
Antinociceptive efficacy of lacosamide in a rat model for painful
diabetic neuropathy. Eur J Pharmacol. 539:64–70. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Beyreuther B, Callizot N and Stöhr T:
Antinociceptive efficacy of lacosamide in the monosodium
iodoacetate rat model for osteoarthritis pain. Arthritis Res Ther.
9:R142007. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Stöhr T, Krause E and Selve N: Lacosamide
displays potent antinociceptive effects in animal models for
inflammatory pain. Eur J Pain. 10:241–249. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rogawski MA and Löscher W: The
neurobiology of antiepileptic drugs for the treatment of
nonepileptic conditions. Nat Med. 10:685–692. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu YR, Lei RY, Wang CE, Zhang BA, Lu H,
Zhu HC and Zhang GB: Effects of catalpol on ATPase and amino acids
in gerbils with cerebral ischemia/reperfusion injury. Neurol Sci.
35:1229–1233. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu YR, Li PW, Suo JJ, Sun Y, Zhang BA, Lu
H, Zhu HC and Zhang GB: Catalpol provides protective effects
against cerebral ischaemia/reperfusion injury in gerbils. J Pharm
Pharmacol. 66:1265–1270. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang W, Wang T, Feng WY, Wang ZY, Cheng MS
and Wang YJ: Ecdysterone protects gerbil brain from temporal global
cerebral ischemia/reperfusion injury via preventing neuron
apoptosis and deactivating astrocytes and microglia cells. Neurosci
Res. 81(82): 21–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Institute of Laboratory Animal Research.
2202011.
|
|
28
|
Yan BC, Choi JH, Yoo KY, Lee CH, Hwang IK,
You SG, Kang IJ, Kim JD, Kim DJ, Kim YM and Won MH: Leptin's
neuroprotective action in experimental transient ischemic damage of
the gerbil hippocampus is linked to altered leptin receptor
immunoreactivity. J Neurol Sci. 303:100–108. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Park JH, Joo HS, Yoo KY, Shin BN, Kim IH,
Lee CH, Choi JH, Byun K, Lee B, Lim SS, et al: Extract from
Terminalia chebula seeds protect against experimental ischemic
neuronal damage via maintaining SODs and BDNF levels. Neurochem
Res. 36:2043–2050. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schmued LC and Hopkins KJ: Fluoro-Jade B:
A high affinity fluorescent marker for the localization of neuronal
degeneration. Brain Res. 874:123–130. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Loskota WJ, Lomax P and Verity MA: A
stereotaxic atlas of the Mongolian gerbil brain (Meriones
unguiculatus). Ann Arbor Science (Ann Arbor, MI). 70–81. 1974.
|
|
32
|
Park JH, Joo HS, Yoo KY, Shin BN, Kim IH,
Lee CH, Choi JH, Byun K, Lee B, Lim SS, et al: Extract from
Terminalia chebula seeds protect against experimental ischemic
neuronal damage via maintaining SODs and BDNF levels. Neurochem
Res. 36:2043–2050. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Licko T, Seeger N, Zellinger C, Russmann
V, Matagne A and Potschka H: Lacosamide treatment following status
epilepticus attenuates neuronal cell loss and alterations in
hippocampal neurogenesis in a rat electrical status epilepticus
model. Epilepsia. 54:1176–1185. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bell MT, Puskas F, Agoston VA, Cleveland
JC Jr, Freeman KA, Gamboni F, Herson PS, Meng X, Smith PD, Weyant
MJ, et al: Toll-like receptor 4-dependent microglial activation
mediates spinal cord ischemia-reperfusion injury. Circulation.
128(Suppl 1): S152–S156. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Choudhury Roy G, Ryou MG, Poteet E, Wen Y,
He R, Sun F, Yuan F, Jin K and Yang SH: Involvement of p38 MAPK in
reactive astrogliosis induced by ischemic stroke. Brain Res.
1551:45–58. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Day YJ, Liou JT, Lee CM, Lin YC, Mao CC,
Chou AH, Liao CC and Lee HC: Lack of interleukin-17 leads to a
modulated micro-environment and amelioration of mechanical
hypersensitivity after peripheral nerve injury in mice. Pain.
155:1293–1302. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yan X, Yadav R, Gao M and Weng HR:
Interleukin-1 beta enhances endocytosis of glial glutamate
transporters in the spinal dorsal horn through activating protein
kinase C. Glia. 62:1093–1109. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Beyreuther BK, Geis C, Stöhr T and Sommer
C: Antihyperalgesic efficacy of lacosamide in a rat model for
muscle pain induced by TNF. Neuropharmacology. 52:1312–1317. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang B, Dawson H, Wang H, Kernagis D,
Kolls BJ, Yao L and Laskowitz DT: Lacosamide improves outcome in a
murine model of traumatic brain injury. Neurocrit Care. 19:125–134.
2013. View Article : Google Scholar : PubMed/NCBI
|