Introduction
Thrombotic thrombocytopenic purpura (TTP) is a rare
blood disorder, which is acquired in the majority of cases. TTP is
characterized by thrombotic microangiopathy, which may lead to
microangiopathic hemolytic anemia and thrombocytopenia with or
without neurological symptoms, kidney damage and fever (1). This disease is further divided into
idiopathic TTP and secondary TTP, with the idiopathic type being
the most common form of acquired TTP. In acquired TTP, antibodies
against ADAMTS13 are detected in the patients' serum, leading to
deficient ADAMTS13 enzyme activity (generally <5%). ADAMTS13 is
a metalloproteinase that is responsible for cleaving large
multimers of von Willebrand factor (vWF) into smaller units. Due to
decreased ADAMTS13 activity in TTP, the plasma vWF multimers may
not be eliminated, and thus thrombosis occurs with platelet
accumulation (2).
The current international standard treatment for TTP
is plasma exchange (PEX), which reduces mortality by ≤90% (1). However, relapse is common in patients
only receiving PEX. Hence, patients with acquired TTP receive
additional immunosuppressive therapy, such as glucocorticoids,
which are able to suppress ADAMTS13 autoantibodies and reduce
pathogenic cytokine levels (3).
However, relapse rate of patients with TTP remains at 30–50%, and
the first relapse usually occurs within 1 year following treatment.
Relapse of TTP is the primary cause of mortality and thrombosis
syndrome. In order to reduce TTP recurrence, other
immunosuppressive agents such as cyclosporine A, vincristine,
cyclophosphamide, and rituximab (RTX) may be used.
RTX is a chimeric mouse-human monoclonal antibody
against the B-lymphocyte antigen, CD20, which is primarily detected
on the surface of B-cells. A standard dose of RTX (a commonly-used
dose in B-cell lymphoma is 375 mg/m2/week, continuously
for 4 weeks) was initially used for the treatment of autoimmune
diseases and immune TTP (a type of secondary TTP), and has resulted
in encouraging results (4,5). Zaja et al (6) conducted a prospective clinical trial
attempting to treat relapsed and newly-diagnosed acquired ITP
patients with a low-dose of RTX (100 mg weekly, for 4 weeks). The
authors concluded that low-dose RTX administration could achieve a
similar efficacy as treatment with the standard dose (6). With acquired TTP, abnormal
B-lymphocytes produce ADAMTS13 autoantibodies. The antibody was
first successfully used to treat non-Hodgkin lymphomas of B-cell
origin; however, there is evidence for its efficacy in the
treatment of other hematological or autoimmune diseases like
autoimmune hemolytic anaemia (7) or
chronic idiopathic thrombocytopenia (ITP). The treatment mechanism
of TTP by RTX is similar to that in ITP; the production of
autoantibodies and the mitigation of the activity of B lymphocytes
antigen-activated T lymphocytes. Fakhouri et al (8) and Scully et al (9) prospectively investigated the
administration of a standard dose of RTX (375
mg/m2/week, continuously for 4 weeks) for the treatment
of relapsed and newly-diagnosed TTP patients. They observed that
the majority of cases experienced increased ADAMTS13 activity and a
decreased number of ADAMTS13 antibodies, while the platelet count
in 68% of patients increased to >50×109/l before
commencing the second RTX infusion week (8,9).
Furthermore, a significant reduction in the relapse rate was
observed, and the majority of patients maintained a longer
remission following RTX therapy. Multiple clinical studies
concurrently demonstrated that a standard dose of RTX is effective
in the treatment of TTP (10–12).
Based on the successful application of standard-dose RTX for TTP
treatment and low-dose RTX for ITP treatment in previous studies,
the present study investigated the use of low-dose RTX for the
treatment of relapsed or refractory TTP. In the present study, 2
successfully treated cases of TTP were presented. Written informed
consent was obtained from the patients.
Case report
Case 1
A 38-year-old male presented at the First Affiliated
Hospital of Zhejiang University (Hangzhou, China) with petechia and
ecchymosis on the entire body in August 2011. Laboratory tests
revealed a normal hemoglobin level (14.5 g/dl), a reduced platelet
count (8×109/l), an elevated lactate dehydrogenase (LDH)
level (580 U/l) and erythrocyte count of 2,073/µl in the urine. An
ultrasound of the urinary system was normal and bone marrow smears
revealed megakaryocytic hyperplasia. The patient then developed
abnormal psychological symptoms; however, a computed tomography
(CT) scan of the head revealed no abnormalities. The hemoglobin
level was reduced to 9.7 g/dl, while the platelet count remained at
8×109/l. A further blood test indicated that the
ADAMTS13 activity was deficient, with the presence of circulating
ADAMTS13 inhibitor. Subsequent to excluding secondary causes, the
patient was diagnosed with TTP.
The patient received 9 sessions of PEX, along with
administration of oral cyclosporine A (CsA; 5 mg/kg, total 300 mg).
PEX and dexamethasone (10 mg/day) were immediately administered.
Due to plasma shortage, PEX was administered at least once every 2
days (a total of 6 PEXs). After 1 week, 150 mg CsA was administered
every 12 h, and the period of PEX was once a week when PLT
increased to normal (a total of 3 PEXs). Blood cell count was
evaluated twice a week. If blood cell count remained stable,
dexamethasone dose was gradually tapered (one or two tablets were
reduced per 2 weeks) until stop, and CsA was reduced by 50 mg/week
to maintenance therapy dose of 50 mg/day. CsA was discontinued at
relapse after 1 year and changed to dexamethasone and PEXs, and
RTX. The platelet count of the patient reached
>100×109/l, and the CsA administration was gradually
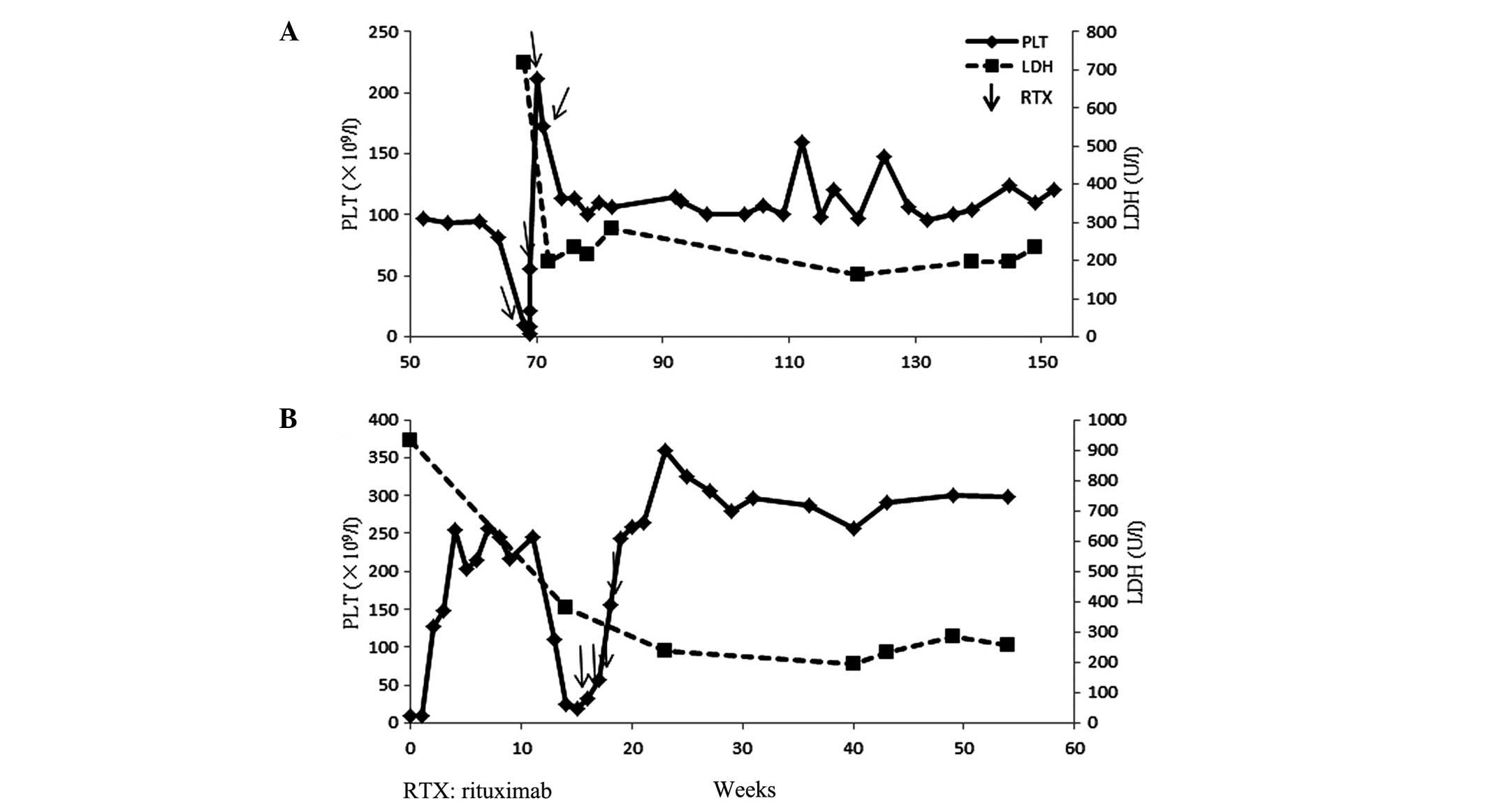
tapered until it was discontinued. On week 68 after first
admission, the patient presented with hematuria and skin petechia.
Laboratory tests revealed an extremely low platelet count of
4×109/l, a hemoglobin level of 13.3 g/dl, a reticulocyte
count of 3.3% and an LDH level of 836 U/l. Repeated detection
demonstrated deficient ADAMTS13 activity and detectable levels of
anti-ADAMTS13 inhibitor. Therefore, a diagnosis of refractory TTP
was concluded. The patient was administered a low-dose of RTX, at
100 mg/week, continuously for 4 weeks. In the first week of RTX
treatment, the patient received 2 PEX sessions. Concurrently, the
patient received 40 mg/day methylprednisolone, which continued for
~3 months following the initiation of the RTX treatment, and the
dose was gradually tapered until discontinuation. The treatment was
well-tolerated with no side-effects. Following the first week of
RTX treatment, the patient's platelet count increased to
220×109/l and the LDH level returned to the normal
levels. During the follow-up, repeated detection demonstrated 100%
ADAMTS13 activity and undetectable levels of anti-ADAMTS13
antibodies. The patient remained asymptomatic with a normal
platelet count in August 2015 (Fig.
1A).
Case 2
A 34-year-old female presented at the First
Affiliated Hospital of Zhejiang University with a sudden headache,
nausea and vomiting associated with fever and an altered mental
status in May 2012. A central nervous system examination was
unremarkable and a CT scan of the head revealed no abnormalities.
Laboratory tests demonstrated the following: A reduced platelet
count compared with normal values (6×109/l); reduced
hemoglobin level (8 g/dl); an elevated LDH level (932 U/l); total
bilirubin, 2.4 mg/dl; direct bilirubin, 1.2 mg/dl; plasma free
hemoglobin, 1.55 mg/dl; and erythrocyte count in urine, 63.8/µl.
Bone marrow smears revealed erythroid hyperplasia. A peripheral
blood smear showed poikilocytosis and evident erythrocyte debris.
Further detection revealed deficient ADAMTS13 activity, detectable
anti-ADAMTS13 inhibitor levels and a reticulocyte count of 22.5%.
Antinuclear antibody titers, immunoglobulin, thyroid function,
tumor markers (AFP, CEA, CA199, CA153 and CA155) and a Coombs' test
were negative, and thus a diagnosis of ITP was concluded. After the
patient was treated with 2 PEX sessions, the hemoglobin level
increased to 7.4 g/dl, with a reticulocyte count of 16.3% and a
platelet count of 128×109/l. A prednisone dose of 30
mg/day was orally administered for ~3 months and then gradually
tapered until discontinuation. On week 14, the patient's platelet
count decreased again to 25×109/l. A plasma transfusion
and PEX were administered and the prednisone dose was adjusted to
60 mg/day, but the platelet count did not improve significantly. On
week 15, the patient was administered a low intravenous dose of
RTX, at 100 mg/week, continuously for 4 weeks. The treatment was
well-tolerated without any side-effects. The platelet count
increased to the normal level following the second week of RTX
treatment. The patient recovered (hemoglobin level, 11.2 g/dl;
platelet count, 235×109/l) in June 2014. At week 49
following treatment, the patient became pregnant and successfully
delivered a healthy child, without any hematological abnormalities.
The patient was in good condition at the 23-month follow-up.
Regular testing demonstrated that her platelet count, LDH level and
serum ADAMTS13 activity were maintained within the normal levels
(Fig. 1B). Regular testing
demonstrated that the patient's platelet count, LDH level and serum
ADAMTS13 activity remained within the normal levels in August
2015.
Discussion
In the majority of cases, TTP is caused by
auto-antibodies that inhibit the vWF multimer-cleaving enzyme,
ADAMTS13. Prospective studies have demonstrated that a standard
dose of RTX is effective for the treatment of immune TTP, if
patients failed to respond to daily PEX and steroids, as well as
for the treatment of relapsed acute ITP (8,9). The
British Committee for Standards in Heamatology published guidelines
regarding the diagnosis and management of TTP and recommended that
patients with refractory or relapsing immune-mediated TTP should be
administered RTX, typically at a dose of 375 mg/m2
weekly for 4 weeks (13). However,
to date, clinicians have limited experience using low-dose RTX for
the treatment of patients with acquired TTP.
The 2 refractory and relapsed TTP cases presented in
the current study were treated with low-dose RTX using a dose of
100 mg per week for 4 consecutive weeks. The 2 patients obtained
favorable outcomes and achieved a sustained, long-term remission.
As of this report, 1 patient achieved permanent remission for 23
months, while the other patient, who was refractory to PEX,
steroids and CsA, was also in remission for 19 months. Recently,
Pequeño-Luévano et al (14)
reported the use of low-dose RTX (100 mg/day, continuously for 7
days) as a first-line therapy at the same time as PEX treatment in
3 ITP cases, and as a salvage therapy for a relapsing case. With
this treatment, all 4 patients achieved complete remission, were
asymptomatic as of the report and had achieved a complete response
duration of 8–22 months (14).
Coincidentally, similar to the observations of the previous study,
the present case report illustrated that low-dose RTX treatment may
be an effective alternative for certain acute acquired TTP cases,
particularly for patients with relapsed and refractory disease.
The mechanism though which RTX functions in the
treatment of TTP is similar to its function in ITP. It works mainly
by eliminating activated CD20+ B-lymphocytes, increasing
the number of regulatory T (Treg) cells, and improving the function
of the Treg cells. Regarding the effectiveness of low-dose RTX,
possible mechanisms may involve the small amount of abnormally
activated B-lymphocytes in TTP, as opposed to clonal B-lymphocytes
in malignant lymphoma; thus, a lower RTX dose may eliminate the
abnormally activated B-lymphocytes. In addition, certain studies
have demonstrated that after the first week of RTX treatment,
peripheral blood CD20+ cells had almost disappeared in
ITP patients (15,16). Therefore, for autoimmune diseases,
the current authors hypothesized that RTX may not require a dose as
large as that used in B-cell lymphoma. Furthermore, the successful
treatment of ITP patients involving low-dose RTX also demonstrates
the effectiveness of low-dose RTX for autoimmune diseases. Under
the premise of ensuring efficacy, the lower the RTX dose, the lower
the side-effect rate will be.
In conclusion, the present study described the
successful treatment of 2 cases using low-dose RTX for relapsed and
refractory TTP, and the results in the two cases were independent
of the PEX treatment. However, numerous questions remain to be
answered, including which RTX dose is the most suitable for the
treatment of TTP. Furthermore, the frequency and timing of RTX
remain to be investigated. Therefore, further prospective clinical
investigation is required on the use of low-dose RTX for the
treatment of TTP.
References
|
1
|
Rock GA, Shumak KH, Buskard NA, Blanchette
VS, Kelton JG, Nair RC and Spasoff RA: Comparison of plasma
exchange with plasma infusion in the treatment of thrombotic
thrombocytopenic purpura. Canadian Apheresis Study Group. N Engl J
Med. 325:393–397. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tsai HM: Current concepts in thrombotic
thrombocytopenic purpura. Annu Rev Med. 57:419–436. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Allford SL, Hunt BJ, Rose P and Machin SJ:
Haemostasis and Thrombosis Task Force, British Committee for
Standards in Haemotology: Guidelines on the diagnosis and
management of the thrombotic microangiopathic haemolytic anaemias.
Br J Haematol. 120:556–573. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zaja F, Baccarani M, Mazza P, Bocchia M,
Gugliotta L, Zaccaria A, Vianelli N, Defina M, Tieghi A, Amadori S,
et al: Dexamethasone plus rituximab yields higher sustained
response rates than dexamethasone monotherapy in adults with
primary immune thrombocytopenia. Blood. 115:2755–2762. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Patel VL, Mahévas M, Lee SY, Stasi R,
Cunningham-Rundles S, Godeau B, Kanter J, Neufeld E, Taube T,
Ramenghi U, et al: Outcomes 5 years after response to rituximab
therapy in children and adults with immune thrombocytopenia. Blood.
119:5989–5995. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zaja F, Vianelli N, Volpetti S, Battista
ML, Defina M, Palmieri S, Bocchia M, Medeot M, De Luca S, Ferrara
F, et al: Low-dose rituximab in adult patients with primary immune
thrombocytopenia. Eur J Haematol. 85:329–334. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zecca M, De Stephano P, Nobili B and
Locatelli F: Anti-CD20 monoclonal antibody for the treatment of
severe, immune-mediated, pure red cell aplasia and hemolytic
anaemia. Blood. 97:3995–39972001. View Article : Google Scholar
|
|
8
|
Fakhouri F, Vernant JP, Veyradier A, Wolf
M, Kaplanski G, Binaut R, Rieger M, Scheiflinger F, Poullin P,
Deroure B, et al: Efficiency of curative and prophylactic treatment
with rituximab in ADAMTS13-deficient thrombotic thrombocytopenic
purpura: A study of 11 cases. Blood. 106:1932–1937. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Scully M, McDonald V, Cavenagh J, Hunt BJ,
Longair I, Cohen H and Machin SJ: A phase 2 study of the safety and
efficacy of rituximab with plasma exchange in acute acquired
thrombotic thrombocytopenic purpura. Blood. 118:1746–1753. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Froissart A, Buffet M, Veyradier A,
Poullin P, Provôt F, Malot S, Schwarzinger M, Galicier L, Vanhille
P, Vernant JP, et al: Efficacy and safety of first-line rituximab
in severe, acquired thrombotic thrombocytopenic purpura with a
suboptimal response to plasma exchange. Experience of the French
thrombotic microangiopathies reference center. Crit Care Med.
40:104–111. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Iioka F, Shimomura D, Ishii T, Maesako Y,
Ohgoe K, Nakamura F, Matsuo S and Ohno H: Short- and long-term
effects of rituximab for the treatment of thrombotic
thrombocytopenic purpura: Four case reports. Int J Hematol.
96:506–512. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
de la Rubia JD, Moscardó F, Gómez MJ,
Guardia R, Rodríguez P, Sebrango A, Zamora C, Debén G, Goterris R,
López R, et al: Efficacy and safety of rituximab in adult patients
with idiopathic relapsing or refractory thrombotic thrombocytopenic
purpura: Results of a Spanish multicenter study. Transfus Apher
Sci. 43:299–303. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Scully M, Hunt BJ, Benjamin S, Liesner R,
Rose P, Peyvandi F, Cheung B and Machin SJ: British Committee for
Standards in Haematology: Guidelines on the diagnosis and
management of thrombotic thrombocytopenic purpura and other
thrombotic microangiopathies. Br J Haematol. 158:323–335. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pequeño-Luévano M, Villarreal-Martínez L,
Jaime-Pérez JC, Gómez-de-León A, Cantú-Rodríguez OG, González-Llano
O and Gómez-Almaguer D: Low-dose rituximab for the treatment of
acute thrombotic thrombocytopenic purpura: Report of four cases.
Hematology. 18:233–236. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Taube T, Schmid H, Reinhard H, von
Stackelberg A and Overberg US: Effect of a single dose of rituximab
in chronic immune thrombocytopenic purpura in childhood.
Haematologica. 90:281–283. 2005.PubMed/NCBI
|
|
16
|
Zaja F, Battista ML, Pirrotta MT, Palmieri
S, Montagna M, Vianelli N, Marin L, Cavallin M, Bocchia M, Defina
M, et al: Lower dose rituximab is active in adults patients with
idiopathic thrombocytopenic purpura. Haematologica. 93:930–933.
2008. View Article : Google Scholar : PubMed/NCBI
|