Introduction
Severe acute pancreatitis (SAP) is a serious
systemic inflammatory disease with high mortality rate, which may
cause systemic inflammatory response syndrome (SIRS) and multiple
organ dysfunction syndrome (MODS) due to excessive inflammatory
reactions. However, the exact pathogenesis remains unclear
(1). Studies have found that nuclear
factor κB (NF-κB) has a high level of expression in SAP, and plays
an important role in regulating the inflammatory response (2,3). Tumor
necrosis factor (TNF)-α is an inflammatory mediator that is also
plays a critical role in the pathogenesis of acute pancreatitis by
driving the subsequent inflammatory response (4). Ulinastatin is a purified antiprotease
isolated from the fresh urine of healthy adults, which possesses
powerful anti-inflammatory effects and has been demonstrated to
exert significant therapeutic effects in several forms of acute
pancreatitis (5,6). However, the impact of ulinastatin
administered by peritoneal lavage on the expression levels of NF-κB
and TNF-α in multiple organs in SAP has not been studied. According
to our preliminary study (7),
peritoneal lavage with the addition of ulinastatin at a
concentration of 62.5 U/ml to the lavage fluid exerts the best
therapeutic effect. The present study investigated the expression
levels of NF-κB and TNF-α in multiple organs and thereby explored
the underlying mechanism of intraperitoneal lavage with ulinastatin
on a rat model of SAP. The results may provide convincing
theoretical evidence supporting the further experimental and
clinical application of ulinastatin as a peritoneal lavage.
Materials and methods
Experimental animals and ethics
A total of 100 healthy male Wistar rats weighing
300±15 g were obtained from the Experimental Animal Center of the
PLA General Hospital (Beijing, China). The experimental protocol
was approved by the Ethics Committee for Animal Research from the
PLA General Hospital. Ethical standards were adhered to and the
rats received humane care.
Reagents
Reagents were purchased as follows: Chloral hydrate
(Shanghai Yingxin Laboratory Equipment Co., Ltd., Shanghai, China);
sodium taurocholate (Shanghai Hufeng Biotechnology Co., Ltd.,
Shanghai, China); ulinastatin (Guangdong Tianpu Biochemical
Pharmaceutical Co., Ltd., Guangzhou, China); IL-1 and IL-6 assay
kits (Shanghai Hengyuan Biological Technology Co., Ltd., Shanghai,
China); and rat soluble NF-κB and TNF-α ELISA kits (Wuhan Hua Mei
Biological Engineering Co., Ltd., Wuhan, China).
Experimental groups
Rats were randomly divided into five groups: Group C
(n=20), sham-operated without induction of SAP, peritoneal lavage
or intravenous injection, but with insertion of a catheter; group
SAP (n=20), induction of SAP without peritoneal lavage or
intravenous injection, but with insertion of a catheter; group SL
(n=20), saline lavage for 3 h immediately after the induction of
SAP; group IU (n=20), intravenous ulinastatin at 2,500 U/100 g
immediately after the induction of SAP and with insertion of a
catheter, but without peritoneal lavage; group UL (n=20),
ulinastatin lavage at a concentration of 62.5 U/ml for 3 h
immediately after the induction of SAP.
Animal model
All rats fasted for 12 h and had no water for 4 h
prior to surgery. Rats were anesthetized with intraperitoneal
injections of 10% chloral hydrate (3 ml/kg). After making an
incision in the abdomen, clamping the opening and remote part of
the duodenal bile duct with injury-free metal clips, a syringe
needle was inserted into the opening of the duodenal bile duct.
Then, 5% sodium taurocholate (freshly prepared in saline solution,
0.6 ml) was retrogradely injected into the bile duct at constant
rate of 0.2 ml/min using an infusion pump. After 5 min, the needle
and metal clips were removed. This procedure produces a
consistently high mortality rate (>80% within 12 h) (8). Group C was sham-operated without
induction of SAP.
Prior to closure of the abdomen, a silicon catheter
(catheter A) with five lateral outlets was placed adjacent to the
pancreas and another silicon catheter (catheter B) with five
lateral outlets was placed in the pelvic cavity. All groups
accepted peritoneal catheter insertion.
Peritoneal lavage
Intraperitoneal lavage was performed immediately
following the establishment of the SAP model in the rats of groups
SL and UL. Lavage fluid was warmed to 37°C and injected from
catheter A at 80 ml/h for 15 min and catheter B was blocked. Then,
catheter A was blocked and the fluid was allowed to flow out for 15
min from catheter B. Thus, each lavage procedure lasted 30 min, and
lavage was performed 6 times (3 h total lavage time). Volume input
and output were monitored. The lavage fluid consisted of saline
solution with or without the addition of ulinastatin at a
concentration of 62.5 U/ml, which our earlier study indicated to
exert the optimum therapeutic effect. Following the lavage
procedure, the catheters A and B were blocked and the rats were
kept in single cages, with free access to water and no solid
food.
Intravenous ulinastatin
To compare the effect of peritoneal lavage with that
of intravenously administered ulinastatin, group IU immediately
received intravenous ulinastatin 2,500 U/100 g (freshly prepared in
0.15 ml saline solution, which approximates the total dose in group
UL) from the caudal vein after SAP induction, but no lavage.
Observation indices
The survival time of each group (n=10 for each) was
recorded for 12 h and the survival rate at 3, 6, 9 and 12 h was
calculated. Rats surviving to 12 h were anesthetized and sacrificed
humanely. At 3 h after surgery, rats in each group (n=10 for each)
were also humanely sacrificed, and blood samples were collected for
the detection of inflammatory mediators (including IL-1 and IL-6),
and specimens of multiple organs (including pancreas, lung, liver
and kidney) were prepared for determination of the expression
levels of NF-κB and TNF-α.
All tissues were homogenized with cold saline at a
1:9 dilution using a PowerGen 1000 homogenizer (Thermo Fisher
Scientific, Waltham, MA, USA). All tissue specimens were rinsed in
cold saline to remove blood, dried with filter paper, cut into
small pieces using a disposable scalpel, and 0.4 g tissue
(pancreas, lung, live or kidney) was added to a 10-ml beaker. Next,
3.6 ml cold saline was added to the tissue and homogenization was
achieved by using the homogenizer for 1–2 min. Subsequently, 10%
homogenate was prepared using low temperature centrifugation at
1,000 × g for 10–15 min at 4°C and the supernatant was obtained for
determination.
Assays for serum IL-1, IL-6 content
and supernatant of homogenate NF-κB and TNF-α expression
levels
Samples for IL-1, IL-6, NF-κB and TNF-α were
aliquoted in portions and stored at −80°C for no longer than 2
months. Measurements were performed using the IL-1, IL-6, rat
soluble NF-κB and TNF-α ELISA kits and a standard ELISA reader
according to the manufacturers instructions.
Statistical analysis
Data are expressed as mean ± standard deviation for
normally distributed variables or median and interquartile range
for highly skewed variables. Statistical analyses were performed
using the SPSS software package, version 19.0 (SPSS, Inc., Chicago,
IL, USA).
In survival experiments, the survival rate after the
12 h observation period was conducted by the Kaplan-Meier or
Kruskal-Wallis H test. Analysis of variance was used for comparison
of normally distributed data. Multiple comparisons were subjected
to Kruskal-Wallis H test and Bonferroni correction test. The
Chi-square test was used to evaluate the equality of frequencies
for discrete variables. P<0.05 was considered to indicate a
statistically significant difference.
Results
Survival rate
All rats in group C were alive at 12 h (100%). At 6
h, group SAP had a survival rate of 20% and the survival rates in
groups SL (50%), IU (60%) and UL (100%) were all increased compared
with that in group SAP. However, at 9 h, only group UL (50%) had a
greatly increased survival rate compared with that in than groups
SAP (10%), SL (20%) and IU (20%). At 12 h, there was no difference
in survival rate among groups SAP (0%), SL (0%), IU (0%) and UL
(10%). The results are summarized in Table I.
 | Table I.Comparison of survival rate among the
five groups (each n=10). |
Table I.
Comparison of survival rate among the
five groups (each n=10).
|
| Survival rate
(%) |
|---|
|
|
|
|---|
| Time point | C | SAP | SL | IU | UL |
|---|
| 3 h | 10/10 (100) | 9/10 (90) | 10/10 (100) | 10/10 (100) | 10/10 (100) |
| 6 h | 10/10 (100) | 2/10
(20)a | 5/10
(50)a | 6/10
(60)a,b | 10/10
(100)b |
| 9 h | 10/10 (100) | 1/10
(10)a | 2/10
(20)a | 2/10
(20)a | 5/10
(50)b |
| 12 h | 10/10 (100) | 0/10 (0)a | 0/10 (0)a | 0/10 (0)a | 1/10
(10)a |
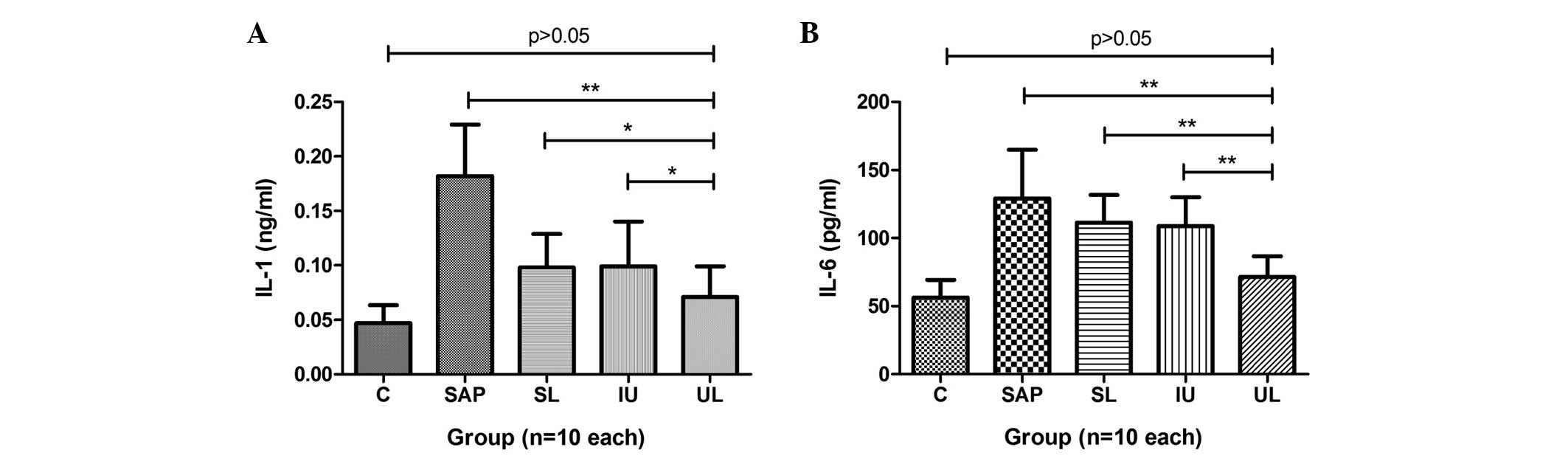
Inflammatory mediators
The plasma levels of IL-1 and IL-6 in the rats of
group SAP were significantly increased compared with those in group
C. The levels of IL-1 in groups SL, IU and UL were significantly
reduced compared with those in group SAP. The lowest levels of IL-1
were observed in group UL (Fig. 1).
Compared with the IL-6 levels in group SAP, those in groups IU and
UL were significantly reduced, and the strongest effect was
observed in group UL, but group SL did not exhibit a statistically
significant difference (Fig. 1). The
results are summarized in Table
II.
 | Table II.Effect of the different treatments on
inflammatory mediators. |
Table II.
Effect of the different treatments on
inflammatory mediators.
| Mediator | C | SAP | SL | IU | UL |
|---|
| IL-1 (ng/ml) | 0.047±0.016 |
0.182±0.047a |
0.102±0.027a,b |
0.105±0.039a,b |
0.071±0.028b |
| IL-6 (pg/ml) | 56.19±13.08 |
129.15±35.85a |
111.35±20.38a |
108.73±34.96a,c |
71.34±15.28b |
Expression levels of TNF-α in multiple
organs
The expression levels of TNF-α in the pancreas,
lung, liver and kidney of rats in group SAP were significantly
increased compared with those in group C. In groups SL, IU and UL,
the expression levels of TNF-α in the pancreas were significantly
reduced compared with those in group SAP, and groups SL and UL
exhibited greater reductions in TNF-α levels than group IU
(Fig. 2A). The expression levels of
TNF-α in the lungs of rats in groups SL, IU and UL were
significantly reduced compared with those in group SAP, and the
TNF-α levels in group UL were the lowest among these three groups
(Fig. 2B). The expression levels of
TNF-α in the livers of the rats in groups SL, IU and UL were
significantly reduced compared with those in group SAP. The effects
in groups UL and SL were comparable and stronger than those in
group IU (Fig. 2C). The expression
levels of TNF-α in the kidneys of the rats in groups SL, IU and UL
were significantly reduced compared with those in group SAP, and
were reduced to the greatest extent in group UL (Fig. 2D). The results are summarized in
Table III.
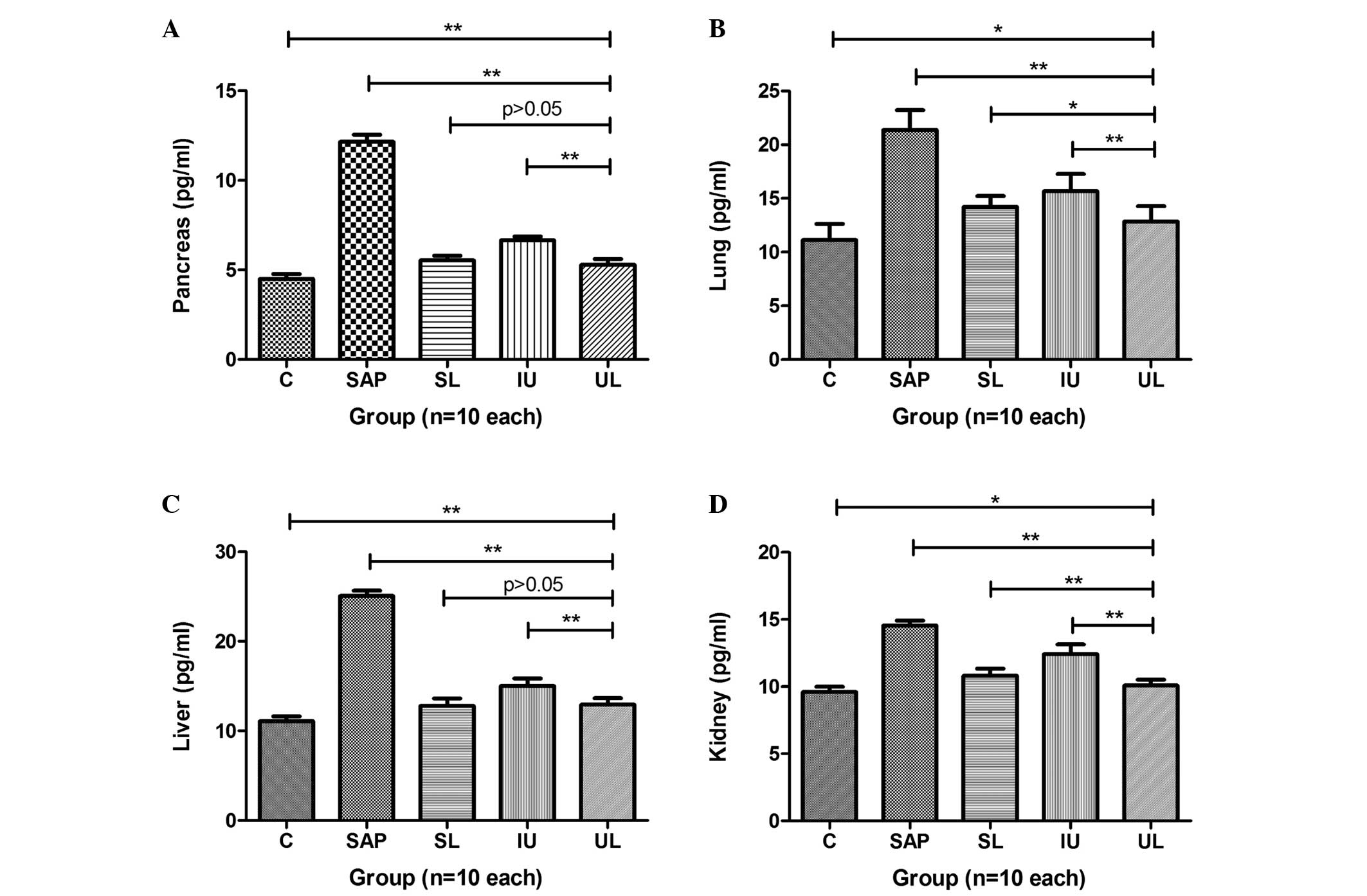 | Figure 2.Comparison of different treatments on
the expression levels of TNF-α in the (A) pancreas, (B) lung, (C)
liver and (D) kidney. The expression levels of TNF-α in multiple
organs were greatly increased in group SAP compared with those in
group C. The treatments administered to groups SL, IU and UL all
significantly decreased the expression levels of TNF-α in multiple
organs compared with those in group SAP and group UL exhibited the
greatest reduction in expression levels. *P<0.05, **P<0.01.
C, control; SAP, severe acute pancreatitis; SL, saline lavage; IU,
intravenous ulinastatin; UL, ulinastatin lavage; TNF-α, tumor
necrosis factor-α. |
 | Table III.Effect of different treatment on the
expression levels of TNF-α in multiple organs at 3 h (pg/ml). |
Table III.
Effect of different treatment on the
expression levels of TNF-α in multiple organs at 3 h (pg/ml).
| Organ | C | SAP | SL | IU | UL |
|---|
| Pancreas |
4.497±0.275 |
12.153±0.398a |
5.535±0.260a,b |
6.651±0.213a,b |
5.287±0.334a,b |
| Lung | 11.126±1.513 |
21.383±1.834a |
14.219±1.002a,b |
15.670±1.614a,b |
12.870±1.412b,c |
| Liver | 11.079±0.566 |
25.054±0.605a |
12.797±0.842a,b |
15.009±0.866a,b |
12.929±0.745a,b |
| Kidney |
9.587±0.405 |
14.566±0.355a |
10.802±0.538a,b |
12.403±0.731b,c |
10.082±0.436a,b |
Expression levels of NF-κB in multiple
organs
The expression levels of NF-κB in the pancreas,
lung, liver and kidney of rats in group SAP were significantly
greater than those in group C. In groups SL and UL, the expression
levels of NF-κB in the pancreas were significantly reduced compared
with those in group SAP, but those of group IU were not. The
effects observed in group SL were almost the same as those in group
UL (Fig. 3A). The expression levels
of NF-κB in the lungs of rats in groups SL, IU and UL were
significantly reduced compared with those in group SAP, with group
UL exhibited the greatest reduction (Fig. 3B). The expression levels of NF-κB in
the livers of the rats in groups SL, IU and UL were significantly
reduced compared with those in group SAP. The effects observed in
groups UL and SL were greater than those in group IU (Fig. 3C). The expression levels of NF-κB in
the kidneys of rats in groups SL, IU and UL were significantly
reduced compared with those in group SAP. The reduction in group UL
was greater than that in groups SL and IU (Fig. 3D). The results are summarized in
Table IV.
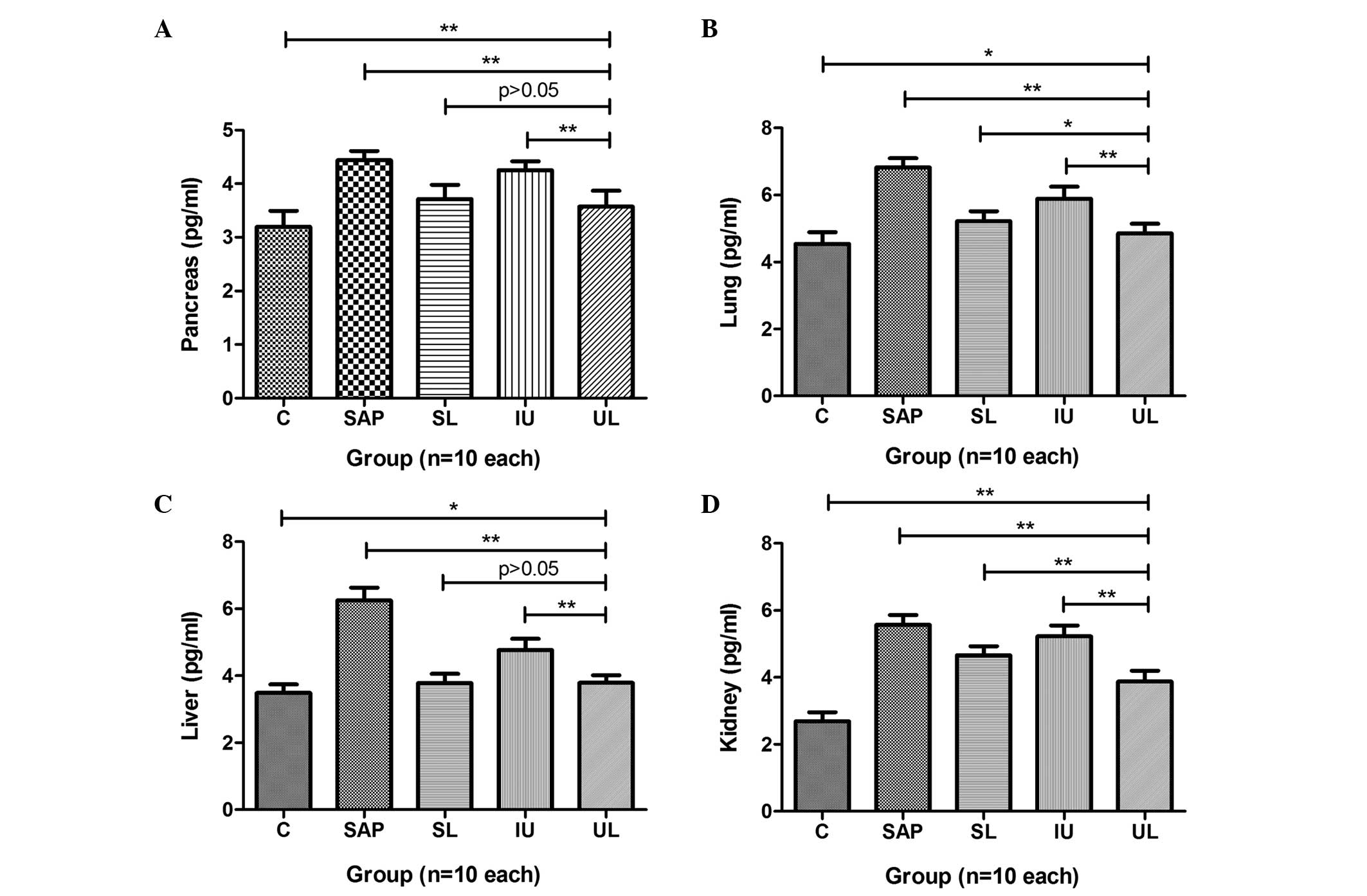 | Figure 3.Comparison of different treatments on
the expression levels of NF-κB in the (A) pancreas, (B) lung, (C)
liver and (D) kidney. The expression levels of NF-κB in multiple
organs were greatly increased in group SAP compared with those in
group C. Compared with the NF-κB levels in group SAP, those in
groups SL, IU and UL were significantly decreased and group UL
exhibited the greatest reduction in NF-κB levels. *P<0.05,
**P<0.01. C, control; SAP, severe acute pancreatitis; SL, saline
lavage; IU, intravenous ulinastatin; UL, ulinastatin lavage; NF-κB,
nuclear factor-κB. |
 | Table IV.Effect of different treatments on the
expression levels of NF-κB in multiple organs at 3 h (pg/ml). |
Table IV.
Effect of different treatments on the
expression levels of NF-κB in multiple organs at 3 h (pg/ml).
| Organ | C | SAP | SL | IU | UL |
|---|
| Pancreas | 3.198±0.295 |
4.424±0.153a |
3.716±0.266a,b |
4.254±0.166a |
3.574±0.297a,b |
| Lung | 4.543±0.349 |
6.821±0.276a |
5.215±0.300a,b |
5.890±0.359a,b |
4.856±0.285b,c |
| Liver | 3.489±0.257 |
6.250±0.384a |
3.781±0.276b,c |
4.766±0.334a,b |
3.793±0.223b,c |
| Kidney | 2.690±0.277 |
5.568±0.297a |
4.665±0.267a,b |
5.225±0.325a,d |
3.878±0.329a,b |
Discussion
SAP is a systemic disease with a high mortality
rate, which may induce SIRS and MODS due to the excessive
inflammatory reactions that play an important role in the
pathogenesis of SAP. It has been reported that pancreatic enzymes
released into the peritoneal exudate could aggravate the
inflammatory condition by damaging multiple organs (1,9).
Therefore, peritoneal lavage and antiprotease therapy may bring an
improved therapeutic effect through being applied locally to the
peritoneal cavity (10). The
glycoprotein ulinastatin is widely used to treat patients with SAP
via the intravenous route (11,12); one
of its therapeutic functions is regulation of the inflammatory
response. Ulinastatin may provide a greater therapeutic effect for
SAP if administered by peritoneal lavage as only a small amount of
intravenously administered ulinastatin is able to reach the
pancreas and other organs because of microcirculatory dysfunction
(13).
Studies of SAP treatment by ulinastatin-containing
peritoneal lavage have rarely been reported. However, on the basis
of our previous study, peritoneal lavage with the addition of 62.5
U/ml ulinastatin to the lavage fluid provides the optimum effect in
the treatment of SAP. In the survival experiment conducted in the
present study, group UL exhibited the greatest median survival rate
among the four treatment groups at 6 and 9 h. Ulinastatin
administered intravenously or by peritoneal lavage improved the
survival rate; however, peritoneal lavage with ulinastatin provided
the greatest effect.
IL-1 and IL-6 are significant proinflammatory
cytokines that are present at elevated levels in the pathogenesis
of acute pancreatitis and play a critical role in mediating the
systemic inflammatory response (14,15). It
has been reported that the levels of proinflammatory cytokines
correlate with the severity of acute pancreatitis (16). The present study demonstrated that
the serum levels of IL-1 and IL-6 in group SAP were significantly
elevated compared with those in group C. Following treatment, the
levels of IL-1 and IL-6 in group UL were much lower than those in
group SAP and were also lower than those in groups SL and IU. This
indicated that the administration of ulinastatin by peritoneal
lavage most effectively lowered the levels of inflammatory
mediators in the serum, alleviated the severe systemic inflammatory
response and inhibited the progress of SAP by inhibiting the
release of inflammatory mediators.
TNF-α is considered to be one of the major factors
associated with the multiple tissue or organ damage caused by a
systemic inflammatory response and could be a therapeutic target
for therapeutic applications in SAP (17). The results of the present study
showed that the levels of TNF-α in multiple organs of group SAP
were greater than those in group C. After treatment, the expression
levels of TNF-α in the pancreas, lung, liver, and kidney of group
UL were significantly lower than those in groups SL and IU. This
indicated that saline lavage with ulinastatin protected multiple
organs from further damage through inhibiting TNF-α to attenuate
the systemic inflammatory response. The results for the serum
levels of IL-1 and IL-6 in the present study are consistent with
this.
A number of studies have demonstrated that the
activation of NF-κB plays a significant role in the onset of SAP,
due to its ability to regulate the expression of inflammatory
mediators (16,18). The activation of NF-κB has been shown
to increase the expression of TNF-α (19). In the present study, following the
induction of SAP, it was observed that NF-κB was activated
significantly in multiple organs of the rats in group SAP compared
with group C; the expression of TNF-α in the various organs from
group SAP also increased markedly. Peritoneal lavage with
ulinastatin significantly reduced the expression of NF-κB in
multiple organs; among the groups SL, IU and UL, the greatest
effects were observed in group UL. The trend of changes in TNF-α in
multiple organs was similar to that of NF-κB among groups SAP, SL,
IU and UL. Therefore, it can be inferred that peritoneal lavage
with ulinastatin alleviates the inflammatory response and
progression of SAP by inhibiting the activation of NF-κB. This may
lower the expression of TNF-α, thereby reducing the release of IL-1
and IL-6.
In conclusion, this study provides evidence that
peritoneal lavage with ulinastatin may effectively improve the
prognosis of SAP by mitigating the extent of inflammation,
specifically by inhibiting NF-κB activation, lowering the
expression of TNF-α and reducing the levels of IL-1 and IL-6. The
results in this study may provide a basis for further experimental
or clinical research into SAP.
Acknowledgements
The authors acknowledge the financial support
provided by the Innovative Doctor Project of the PLA General
Hospital and the Welfare Industry Research Program of the Ministry
of Health: The establishment of new diagnostic criteria of severe
pneumonia and acute lung injury and the optimization scheme of
clinical treatment (No. 201302017) and the ‘Twelfth Five Year Plan’
in rural areas of China National Science and Technology Planning
Issues (No. 2012BAJ18B02-03). The authors are also grateful to
Professor Chong-Hui Li (Department of Hepatobiliary Surgery, PLA
General Hospital) for her considerable recommendations.
References
|
1
|
Balldin G, Borgström A, Genell S and
Ohlsson K: The effect of peritoneal lavage and aprotinin in the
treatment of severe acute pancreatitis. Res Exp Med (Berl).
183:203–213. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yu G, Wan R, Yin G, Xiong J, Hu Y, Xing M,
Cang X, Fan Y, Xiao W, Qiu L, et al: Diosmetin ameliorates the
severity of cerulein-induced acute pancreatitis in mice by
inhibiting the activation of the nuclear factor-κB. Int J Clin Exp
Pathol. 7:2133–2142. 2014.PubMed/NCBI
|
|
3
|
Xiao WQ, Yin GJ, Fan YT, Qiu L, Cang XF,
Yu G, Hu YL, Xing M, de Wu Q, Wang XP, et al: Catalpol ameliorates
sodium taurocholate-induced acute pancreatitis in rats via
inhibiting activation of nuclear factor kappa B. Int J Mol Sci.
15:11957–11972. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Malleo G, Mazzon E, Siriwardena AK and
Cuzzocrea S: TNF-alpha as a therapeutic target in acute
pancreatitis - lessons from experimental models.
ScientificWorldJournal. 7:431–448. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li F, Zhang H, Xu KY, Wei Q and Zhou GX:
Role of the chemokine fractalkine in a rat model of acute
necrotizing pancreatitis and the interventional effect of
ulinastatin. Arch Iran Med. 16:83–87. 2013.PubMed/NCBI
|
|
6
|
Inoue K, Takano H, Shimada A, et al:
Urinary trypsin inhibitor protects against systemic inflammation
induced by lipopolysaccharide. Mol Pharmacol. 67:673–680. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Feng C, Su X, Chen LI, Zhou X, Li B, Wang
LL, Lv FQ and Li TS: Ulinastatin enhances the therapeutic effect of
intraperitoneal lavage on severe acute pancreatitis in rats. Exp
Ther Med. 9:1651–1655. 2015.PubMed/NCBI
|
|
8
|
Leonhardt U, Seidensticker F, Fussek M,
Stockmann F and Creutzfeldt W: Camostate (FOY-305) improves the
therapeutic effect of peritoneal lavage on taurocholate induced
pancreatitis. Gut. 31:934–937. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bhatia M, Wong FL, Cao Y, et al:
Pathophysiology of acute pancreatitis. Pancreatology. 5:132–144.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ohlsson K and Tegner H: Experimental
pancreatitis in the dog. Demonstration of trypsin in ascitic fluid,
lymph and plasma. Scand J Gastroenterol. 8:129–133. 1973.PubMed/NCBI
|
|
11
|
Wang G, Wen J, Wilbur RR, Wen P, Zhou SF
and Xiao X: The effect of somatostatin, ulinastatin and Salvia
miltiorrhiza on severe acute pancreatitis treatment. Am J Med Sci.
346:371–376. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wallner G, Solecki M, Ziemiakowicz R, Ćwik
G, Dyndor P and Maciejewski R: Morphological changes of the
pancreas in course of acute pancreatitis during treatment with
Ulinastatin. Pol Przegl Chir. 85:114–122. 2013.PubMed/NCBI
|
|
13
|
Matsukawa H, Hara A, Ito T, et al:
Continuous arterial infusion of protease inhibitor with
supplementary therapy for the patients with severe acute
pancreatitis - clinical effect of arterial injection of
ulinastatin. Nihon Shokakibyo Gakkai Zasshi. 95:1229–1234. 1998.(In
Japanese). PubMed/NCBI
|
|
14
|
Fisic E, Poropat G, Bilic-Zulle L, Licul
V, Milic S and Stimac D: The role of IL-6, 8 and 10, sTNFr, CRP and
pancreatic elastase in the prediction of systemic complications in
patients with acute pancreatitis. Gastroenterol Res Pract.
2013:2826452013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Papachristou GI: Prediction of severe
acute pancreatitis: Current knowledge and novel insights. World J
Gastroenterol. 14:6273–6275. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang XP, Zhang L, Chen LJ, et al:
Influence of dexamethasone on inflammatory mediators and NF-kappaB
expression in multiple organs of rats with severe acute
pancreatitis. World J Gastroenterol. 13:548–556. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schäfer C, Tietz AB and Göke B:
Pathophysiology of acute experimental pancreatitis: lessons from
genetically engineered animal models and new molecular approaches.
Digestion. 71:162–172. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
O'Reilly DA, Roberts JR, Cartmell MT,
Demaine AG and Kingsnorth AN: Heat shock factor-1 and nuclear
factor-kappaB are systemically activated in human acute
pancreatitis. JOP. 7:174–184. 2006.PubMed/NCBI
|
|
19
|
Algül H, Tando Y, Schneider G, Weidenbach
H, Adler G and Schmid RM: Acute experimental pancreatitis and
NF-kappaB/Rel activation. Pancreatology. 2:503–509. 2002.
View Article : Google Scholar : PubMed/NCBI
|