Introduction
Colon diverticula are outpouchings of the mucosa and
muscularis mucosa. Diverticula develop where the colon wall is weak
(1), the negative outcomes of which
are bleeding and acute diverticulitis (2,3).
Bleeding from diverticula accounts for 20–50% of lower
gastrointestinal bleeding cases (4);
this is usually self-limiting, but is occasionally fatal for
patients taking non-steroidal anti-inflammatory drugs and
anticoagulants (5–7). Acute diverticulitis is treated with
antibiotics (8), which can cause
complications, and surgery is required if diverticulum perforation
occurs (9). The risk of bleeding and
perforation may be reduced if the presence of diverticula is
identified in advance.
Studies on the risks of diverticula predominantly
focus on aging, genetic factors and dietary fiber. The odds ratio
of siblings with diverticula is 7.15 for monozygotic twins and 3.2
for dizygotic twins (10). Among the
cases of diverticula, 40% result from inherited factors and 60%
from environmental factors (10).
However, controversy remains over whether dietary fiber is one of
the causes (11,12). Crowe et al (11) concluded that increased dietary fiber
intake decreases the risk of diverticula, but Peery et al
(12) reported that fiber intake
does not affect the prevalence of diverticula. With regard to risk
factors, Song et al (13)
attributed aging, high-fat diets and high alcohol consumption as
factors increasing the risk of diverticula.
If laboratory data that are correlated with the
presence of diverticula were available, it would be possible to
develop strategies to decrease the risk of developing diverticula;
the present study therefore analyzed the laboratory data of
patients who underwent colonoscopy in order to determine variables
that predict the presence of diverticula.
Materials and methods
Patients
Patient records from between April 2011 and March
2014 were analyzed retrospectively. A total of 1,520 patients
underwent colonoscopy and were included in the analysis, including
758 men (mean age ± standard deviation, 68.9±10.9 years) and 762
women (68.7±10.8 years). The current study was approved by the
National Hospital Organization Shimshizu Hospital Ethics Committee
(Yotsukaido, Japan) and was not categorized as a clinical trial as
it was performed during routine clinical practice. Written informed
consent was waived since the present study was retrospective.
Patient anonymity was preserved.
Colonoscopy
A colonoscopy was performed for patients with
abdominal symptoms, anemia or positive fecal occult blood. A
colonoscopy was also performed for screening purposes. The
colonoscopy devices used were CF-Q260DL/I and PCF-Q260AL/I
(Olympus, Tokyo, Japan).
Blood variables
White blood cell count, platelet count, body mass
index, and levels of hemoglobin (Hb), C-reactive protein, total
protein, albumin, total bilirubin, alkaline phosphatase, aspartate
aminotransferase, alanine aminotransferase, γ-glutamyl
transpeptidase, lactate dehydrogenase, uric acid (UA), blood urea
nitrogen, creatinine, total cholesterol, triglyceride (TG),
high-density lipoprotein cholesterol, low-density lipoprotein
cholesterol, blood glucose, HbA1c, carcinoembryonic antigen and
carbohydrate antigen 19-9 were analyzed.
Statistical analysis
A one-way analysis of variance was performed to
analyze the association between the presence of diverticula and
each variable. A χ2 test was used to determine the
correlation between the presence of diverticula and age group,
gender and UA levels ≥5.1 mg/dl. A receiver operating
characteristic (ROC) analysis was performed to determine the
threshold values able to predict the presence of diverticula.
Specificity and sensitivity were automatically calculated using an
ROC program in the statistical software. P<0.05 was considered
to indicate statistical significance. JMP 10.0.2 software (SAS
Institute, Cary, NC, USA) was used for the statistical
analysis.
Results
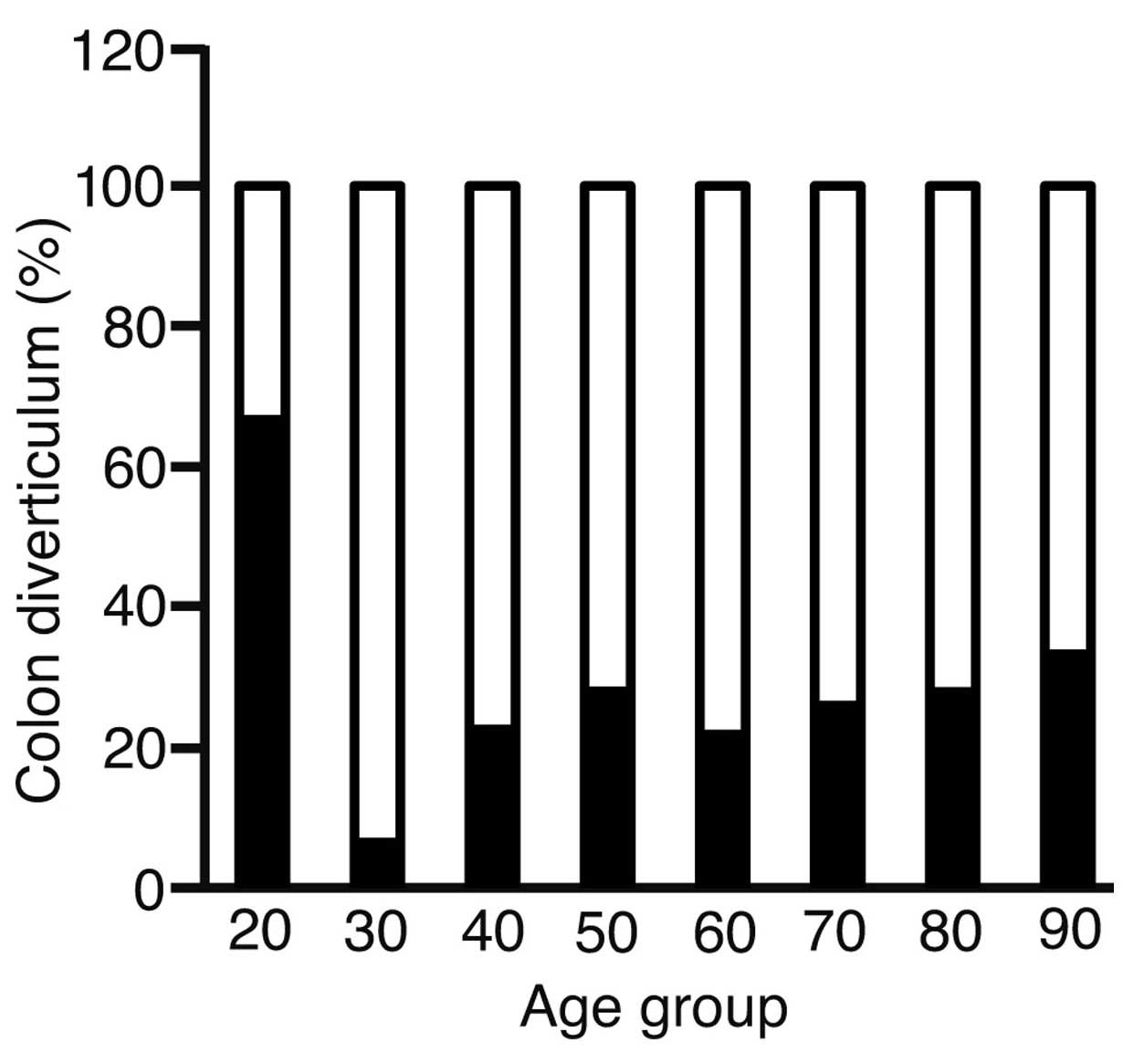
To analyze the correlation between age and the
presence of diverticula, the patients were divided into the
following age groups: Group 20 (20–29 years old, 3 patients); group
30 (30–39 years old, 31 patients); group 40 (40–49 years old, 93
patients); group 50 (50–59 years, 125 patients); group 60 (60–69
years old, 508 patients); group 70 (70–79 years old, 597 patients);
group 80 (80–89 years old; 154 patients) and group 90 (>90 years
old; 9 patients) (Fig. 1). The
incidence of diverticula increased with advancing age, but no
significant correlation was found by χ2 test
(P=0.0643).
The experimental variables were compared to
determine significant differences between patients with and without
diverticula (Table I). Hb levels
were lower in patients with diverticula compared with those without
diverticula (P=0.0027). UA and TG levels were also higher in
patients with diverticula (P=0.0066 and P=0.0136,
respectively).
 | Table I.Blood variable differences in patients
with and without colon diverticula. |
Table I.
Blood variable differences in patients
with and without colon diverticula.
| Variable | Total number of
patients | Patients without
colon diverticula, n | Variable level, mean
± SD | Patients with colon
diverticula, n | Variable level, mean
± SD | P-value |
|---|
| WBC, µl | 743 | 563 | 6.06±2.06 | 180 | 6.22±2.08 | 0.3514 |
| Hb, g/dl | 742 | 63 | 12.8±0.22 | 179 | 1.24±0.20 | 0.0027 |
| CRP, mg/dl | 392 | 303 | 0.95±3.11 | 89 | 0.69±1.63 | 0.4452 |
| Plt,
104/µl | 734 | 554 | 2.23±0.73 | 180 | 2.20±0.69 | 0.6652 |
| TP, g/dl | 492 | 370 | 6.89±0.68 | 122 | 6.77±0.89 | 0.1268 |
| Alb, g/dl | 350 | 264 | 3.99±0.04 | 86 | 4.02±0.06 | 0.5985 |
| T-Bil, mg/dl | 499 | 378 | 0.76±0.42 | 121 | 0.75±0.33 | 0.9497 |
| ALP, IU/l | 255 | 196 | 2.38±1.02 | 59 | 2.23±0.59 | 0.2807 |
| AST, IU/l | 677 | 511 | 2.46±1.33 | 166 | 2.66±3.30 | 0.2617 |
| ALT, IU/l | 713 | 545 | 2.16±1.35 | 168 | 2.53±3.87 | 0.0591 |
| γ-GTP, IU/l | 290 | 225 | 0.51±2.24 | 65 | 0.45±0.56 | 0.8382 |
| LDH, IU/l | 379 | 297 | 2.06±1.04 | 82 | 1.97±0.49 | 0.4360 |
| UA, mg/d) | 282 | 213 | 5.13±1.46 | 69 | 5.66±1.28 | 0.0066 |
| BUN, mg/dl | 469 | 353 | 1.61±1.46 | 116 | 1.52±0.50 | 0.5124 |
| Cre, mg/dl | 713 | 545 | 0.84±0.39 | 168 | 0.86±0.26 | 0.6060 |
| T-Chol, mg/dl | 286 | 222 | 2.01±0.38 | 64 | 1.99±0.41 | 0.6999 |
| TG, mg/dl | 259 | 198 | 1.24±0.81 | 61 | 1.53±0.77 | 0.0136 |
| HDL, mg/dl | 197 | 145 | 5.97±1.75 | 52 | 5.68±1.59 | 0.2982 |
| LDL, mg/dl | 272 | 204 | 1.17±0.28 | 68 | 1.21±0.29 | 0.3834 |
| BG, mg/dl | 375 | 294 | 1.19±0.40 | 81 | 1.24±0.50 | 0.3211 |
| HbA1c, % | 180 | 139 | 6.17±0.94 | 41 | 6.27±1.35 | 0.5805 |
| BMI,
kg/m2 | 273 | 212 | 2.25±0.36 | 61 | 2.29±0.40 | 0.4734 |
| CEA, ng/ml | 208 | 167 | 1.61±7.68 | 41 | 9.86±5.61 | 0.0669 |
| CA19-9, U/ml | 205 | 165 | 0.22±0.63 | 40 | 0.66±3.20 | 0.0984 |
Table II reports the
χ2 test results comparing the prevalence of diverticula
between male and female patients. Diverticula were more frequent in
male patients than in female patients (P=0.0001). The mean ages of
the male and female patients with diverticula were 69.2±11.0 and
68.0±10.2 years, respectively.
 | Table II.Association between gender and the
presence of colon diverticula. |
Table II.
Association between gender and the
presence of colon diverticula.
|
| Colon diverticula,
n |
|
|---|
|
|
|
|
|---|
| Gender | Absent | Present | Total, n |
|---|
| Male |
543 | 219 |
762 |
| Female |
605 | 153 |
758 |
| Total | 1148 | 372 | 1520 |
Table I demonstrates
that UA levels were significantly higher in patients with
diverticula compared with those without diverticula. To confirm
these results, the patients were divided into two groups according
to UA level, as follows: Patients with UA levels ≥5.1 mg/dl and
those with UA levels <5.1 mg/dl. In the patient sample of the
current study, 5.1 mg/dl was the median UA level. The prevalence of
diverticula was compared between the two groups (Table III); this was significantly higher
in patients with UA levels ≥5.1 mg/dl than in those with UA levels
<5.1 mg/dl (P=0.0004; χ2 test). Diverticula were
markedly more frequent in patients with UA levels ≥5.1 mg/dl.
 | Table III.Association between uric acid level
and the presence of colon diverticula. |
Table III.
Association between uric acid level
and the presence of colon diverticula.
|
| Colon diverticula,
n |
|
|---|
|
|
|
|
|---|
| Uric acid
level | Absent | Present | Total, n |
|---|
| High (≥5.1
mg/dl) | 99 | 49 | 148 |
| Low (<5.1
mg/dl) | 114 | 20 | 134 |
| Total | 213 | 69 | 282 |
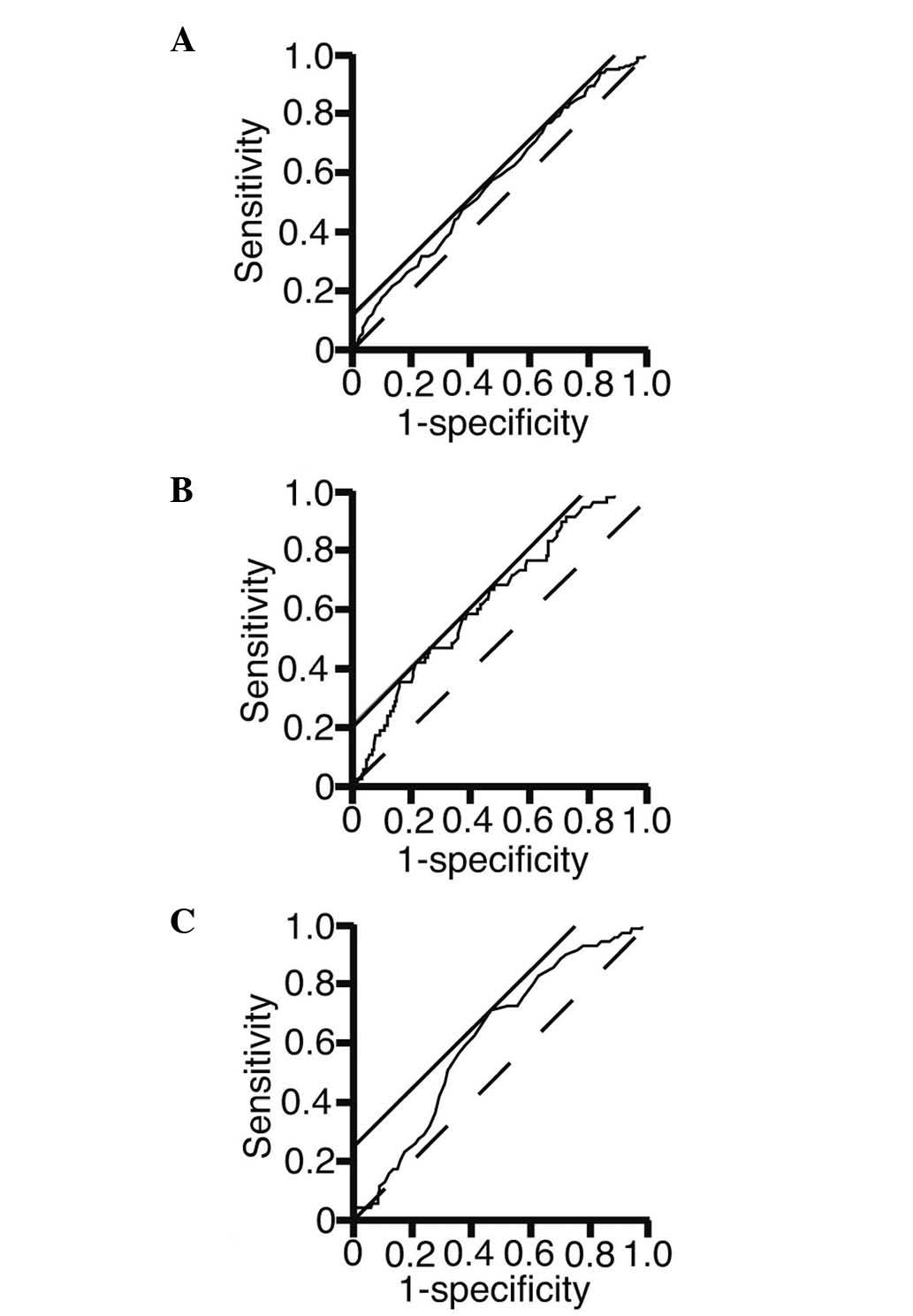
To address the possibility that the presence of
diverticula could be predicted using laboratory test variables, an
ROC analysis was performed (Fig. 2).
The area under the curve, threshold value, sensitivity and
specificity are presented in Table
IV. The threshold values of the Hb, TG and UA levels were
12,400, 146 and 5.1 mg/dl, respectively. The sensitivity of the Hb
and UA levels at the threshold values was 76.5 and 71.0%,
respectively.
 | Table IV.Threshold values to predict the
presence of colon diverticula. |
Table IV.
Threshold values to predict the
presence of colon diverticula.
| Blood variable | AUC | Threshold value,
mg/dl | Sensitivity, % | Specificity, % |
|---|
| Hb | 0.56926 | 12400 | 76.5 | 34.1 |
| TG | 0.63512 | 146 | 47.5 | 75.3 |
| UA | 0.62105 | 5.1 | 71.0 | 53.5 |
Discussion
The correlation between laboratory data, with the
exception of age, and the presence of diverticula has not been
previously reported. In the present study, the presence of
diverticula was significantly associated with low Hb levels, and
high TG and UA levels. A non-significant trend with increasing age
was also revealed. The prevalence of diverticula may increase with
advancing age due to structural changes in the colon wall (10,13,14). To
the best of our knowledge, the present study is the first to report
an association between diverticula and low Hb, high TG and high UA
levels.
The current study revealed that the Hb levels were
lower in patients with diverticula than in those individuals
without; lower Hb levels were likely associated with the presence
of diverticula as this is a major cause of lower gastrointestinal
bleeding (15).
Higher TG levels are associated with metabolic
syndrome (16,17) and TG level decreases as metabolic
syndrome improves following lifestyle changes (18). In the present study, high TG levels
were associated with diverticula. These previous data, alongside
the data from the current study, indicate that diverticula may be
associated with metabolic syndrome. Foster et al (19) compared the prevalence of diverticula
between patients with and without ischemic heart disease and
demonstrated that diverticula occurred in 57 and 25% of patients,
respectively. This indicated an association with ischemic heart
disease, which itself has a known association with high TG levels
(20). Previous studies, together
with the present data, thus indicate that high TG levels may be
associated with diverticula.
The data in the current study clearly suggested that
the prevalence of diverticula was associated with higher UA levels;
to the best of our knowledge, the current study presents the first
report of this association. The molecular details, however, are not
known. The data on TG and UA presented in the current study may
suggest that a reduction in TG and UA levels decreases the risk of
diverticula.
Fernández et al (21) reported that predicting colonoscopic
outcomes is challenging when based on blood examination results
alone. The sensitivity of the Hb level at 12,400 mg/dl was 76.5% in
the current study, suggesting that low Hb levels may be due to
diverticulum bleeding. A notable finding was that the threshold
value of UA was 5.1 mg/dl, the same value as the median.
To conclude, low Hb levels and high TG and UA levels
are associated with the presence of diverticula. Therefore,
colonoscopy may be recommended for patients with high TG and UA
levels, in order to detect colon diverticula.
References
|
1
|
Meyers MA, Volberg F, Katzen B, Alonso D
and Abbott G: The angioarchitecture of colonic diverticula.
Significance in bleeding diverticulosis. Radiology. 108:249–261.
1973. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wilkins T, Baird C, Pearson AN and Schade
RR: Diverticular bleeding. Am Fam Physician. 80:977–983.
2009.PubMed/NCBI
|
|
3
|
Humes DJ and Spiller RC: Review article:
The pathogenesis and management of acute colonic diverticulitis.
Aliment Pharmacol Ther. 39:359–370. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Marion Y, Lebreton G, Le Pennec V, Hourna
E, Viennot S and Alves A: The management of lower gastrointestinal
bleeding. J Visc Surg. 151:191–201. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jansen A, Harenberg S, Grenda U and Elsing
C: Risk factors for colonic diverticular bleeding: A Westernized
community based hospital study. World J Gastroenterol. 15:457–461.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tsuruoka N, Iwakiri R, Hara M, Shirahama
N, Sakata Y, Miyahara K, Eguchi Y, Shimoda R, Ogata S, Tsunada S,
et al: NSAIDs are a significant risk factor for colonic
diverticular hemorrhage in elder patients: Evaluation by a
case-control study. J Gastroenterol Hepatol. 26:1047–1052. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Aldoori WH, Giovannucci EL, Rimm EB, Wing
AL and Willett WC: Use of acetaminophen and nonsteroidal
anti-inflammatory drugs: A prospective study and the risk of
symptomatic diverticular disease in men. Arch Fam Med. 7:255–260.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Weizman AV and Nguyen GC: Diverticular
disease: Epidemiology and management. Can J Gastroenterol.
25:385–389. 2011.PubMed/NCBI
|
|
9
|
Afshar S and Kurer MA: Laparoscopic
peritoneal lavage for perforated sigmoid diverticulitis. Colorectal
Dis. 14:135–142. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Granlund J, Svensson T, Olén O, Hjern F,
Pedersen NL, Magnusson PK and Schmidt PT: The genetic influence on
diverticular disease - a twin study. Aliment Pharmacol Ther.
35:1103–1107. 2012.PubMed/NCBI
|
|
11
|
Crowe FL, Appleby PN, Allen NE and Key TJ:
Diet and risk of diverticular disease in Oxford cohort of European
Prospective Investigation into Cancer and Nutrition (EPIC):
Prospective study of British vegetarians and non-vegetarians. BMJ.
343:d41312011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Peery AF, Barrett PR, Park D, Rogers AJ,
Galanko JA, Martin CF and Sandler RS: A high-fiber diet does not
protect against asymptomatic diverticulosis. Gastroenterology.
142:266–272.e1. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Song JH, Kim YS, Lee JH, Ok KS, Ryu SH,
Lee JH and Moon JS: Clinical characteristics of colonic
diverticulosis in Korea: A prospective study. Korean J Intern Med.
25:140–146. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Heise CP: Epidemiology and pathogenesis of
diverticular disease. J Gastrointest Surg. 12:1309–1311. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wilkins T, Khan N, Nabh A and Schade RR:
Diagnosis and management of upper gastrointestinal bleeding. Am Fam
Physician. 85:469–476. 2012.PubMed/NCBI
|
|
16
|
Tomizawa M, Kawanabe Y, Shinozaki F, Sato
S, Motoyoshi Y, Sugiyama T, Yamamoto S and Sueishi M: Elevated
levels of alanine transaminase and triglycerides within normal
limits are associated with fatty liver. Exp Ther Med. 8:759–762.
2014.PubMed/NCBI
|
|
17
|
Tomizawa M, Kawanabe Y, Shinozaki F, Sato
S, Motoyoshi Y, Sugiyama T, Yamamoto S and Sueishi M: Triglyceride
is strongly associated with nonalcoholic fatty liver disease among
markers of hyperlipidemia and diabetes. Biomed Rep. 2:633–636.
2014.PubMed/NCBI
|
|
18
|
Yamaoka K and Tango T: Effects of
lifestyle modification on metabolic syndrome: A systematic review
and meta-analysis. BMC Med. 10:1382012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Foster KJ, Holdstock G, Whorwell PJ, Guyer
P and Wright R: Prevalence of diverticular disease of the colon in
patients with ischaemic heart disease. Gut. 19:1054–1056. 1978.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lisak M, Demarin V, Trkanjec Z and
Basić-Kes V: Hypertriglyceridemia as a possible independent risk
factor for stroke. Acta Clin Croat. 52:458–463. 2013.PubMed/NCBI
|
|
21
|
Fernández E, Linares A, Alonso JL,
Sotorrio NG, de la Vega J, Artimez ML, Giganto F, Rodríguez M and
Rodrigo L: Colonoscopic findings in patients with lower
gastrointestinal bleeding send to a hospital for their study. Value
of clinical data in predicting normal or pathological findings. Rev
Esp Enferm Dig. 88:16–25. 1996.(In Spanish). PubMed/NCBI
|