Introduction
Mycoplasma pneumoniae is responsible for
15–20% of all cases of community-acquired pneumonia (CAP) (1–3), which
is the main cause of hospitalization and mortality in Chinese
children (4). M. pneumoniae
is a common cause of pneumonia in older children and adolescents
(3,5–8);
however, contrary to previous thought, the incidence of M.
pneumoniae pneumonia (MPP) is also high among patients <1
year of age and among patients between 1 and 4 years of age
(6–9).
The classical radiological presentations of MPP
include lobar/segmental air-space consolidation and diffuse tiny
centrilobular nodules and bronchovascular thickening (10–13).
Previous studies have proposed that lobar alveolar consolidation is
rare or infrequent in pediatric MPP patients (14,15).
However, other reports have suggested that lobar consolidation may
occur more frequently than was generally recognized (16,17). The
lobar/segmental pattern is considered to account for 17–76.5% of
pediatric MPP cases (6,16–19).
Severe MPP often shows segmental or lobar infiltrates in one or
more lobes (20), which, taken in
the context of the estimated prevalence of MPP in pediatric
patients, suggests that the lobar/segmental pattern is an important
pathological category in pediatric MPP. However, large-scale,
population-based epidemiological and clinical research studies
analyzing segmental/lobar MPP remain rare.
In the present study, the epidemiological and
clinical features of segmental/lobar pattern MPP were
retrospectively analyzed in hospitalized children from 2000 to 2009
to improve the understanding of the prevalence and exploitable
therapeutic characteristics of pediatric MPP.
Patients and methods
Patients
Weifang Maternal and Child Health Hospital is
situated on the Shandong Peninsula in an eastern coastal area of
China. This hospital serves as a primary source of healthcare for
women, gravidae and children in Weifang. Weifang has a population
of nine million, moderate economic development and stable
infrastructure. In this study, a retrospective analysis of the
medical records of children with pneumonia (as defined by the
specifications in the International Classification of Diseases,
10th edition, ICD-10 code) who were admitted to Weifang Maternal
& Child Health Hospital between January 2000 and December 2009
was conducted.
Patients who presented with clinical signs and
symptoms of pneumonia underwent a chest radiograph, and the
pneumonia pattern was characterized based on the World Health
Organization Standardization of Interpretation of Chest Radiographs
for the diagnosis of CAP in children (21). Patients diagnosed with pneumonia were
included in this study if the chest radiographs were performed
during hospitalization and serological M. pneumoniae-IgM and
M. pneumoniae particles were detected by polymerase chain
reaction (PCR) in nasopharyngeal secretions ≥7 days following the
onset of disease. Patients >14 years of age or suffering from
known coexisting chronic, progressive or oncological illnesses were
excluded from the analysis. Furthermore, based on chest
radiographs, serological IgM tests and M. pneumoniae PCR
tests, the patients were categorized into the following groups: i)
lobar pneumonia (LP) if evidence of distinctive subsegmental,
segmental or lobar consolidation was observed on the chest
radiograph (20); ii) MPP if
positive serological M. pneumoniae IgM and PCR tests from
nasopharyngeal secretions were observed; iii) segmental/lobar
pattern M. pneumoniae pneumonia (S/L-MPP) (20) if tests satisfied the criteria for LP
and MPP, which is also characterized as air-space disease (10); and iv) non-segmental/lobar pattern
M. pneumoniae pneumonia (non-S/L-MPP) if patients presented
with MPP but did not fit the criteria for S/L-MPP. The non-S/L-MPP
category included patients with pulmonary perihilar linear
opacities or infiltrates or reticulonodular infiltrates by chest
radiography, as well as patients with an interstitial and bronchial
pattern of MPP.
A total of 18,739 patients with pneumonia were
admitted during the period of study, of which 7,319 were enrolled
in this study. Data were collected regarding age, gender, clinical
signs and symptoms, laboratory and radiological findings,
treatment, complications and duration of hospitalization.
Microflora were also detected using nasopharyngeal swab or sputum
specimens by culturing and processing in accordance with standard
microbiological procedures. A qualitatively indirect serum
agglutination assay based on the Gold-labeled Immunologic
Filtration Assay (GIFA; Kanghua Biotech Co., Ltd., Weifang, China)
was used to detect serological M. pneumoniae IgM (22,23).
The clinical features of the S/L-MPP patients were
evaluated according to age in the following groups: ≤3 years of age
(infants and young children), 4–6 years of age (pre-school-aged
children), and ≥7 years of age (school-aged children). The clinical
and laboratory findings were compared according to the pneumonia
pattern. This study was approved by the Institutional Review Board
of Weifang Maternal and Child Health Hospital, and written informed
consent was obtained from the guardians of the patients.
Statistical analysis
Statistical analyses were performed using the
Statistical Package for the Social Sciences for Windows version
11.5 (SPSS, Inc., Chicago, IL, USA). Continuous variables are
reported as the mean ± standard deviation. Since patient age may
have an association with the levels of certain laboratory indices,
including white blood cell count (WBC), erythrocyte sedimentation
rate (ESR) and C-reactive protein (CRP), these quantitative data
were transformed into categorical data (normal or abnormal).
Statistical significance was assessed using the Chi-square test or
Fisher's exact test for categorical variables, and the
t-test and one-way analysis of variation (ANOVA) for
continuous variables. The trends in the annual incidences of LP,
MPP and S/L-MPP were assessed using a trend test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Epidemiological characteristics
Of 18,739 children hospitalized with pneumonia from
January 2000 to December 2009, 7,319 were enrolled in this study,
of whom 4,282 were boys and 3,037 were girls, corresponding to a
male to female ratio of 1.4:1. Of the 7,319 patients enrolled, 823
children (11.2%; 489 boys and 334 girls) were diagnosed with LP,
1,933 children (26.4%; 1,110 boys and 823 girls) were diagnosed
with MPP, and 684 children (9.3%; 385 boys and 299 girls) were
diagnosed with S/L-MPP.
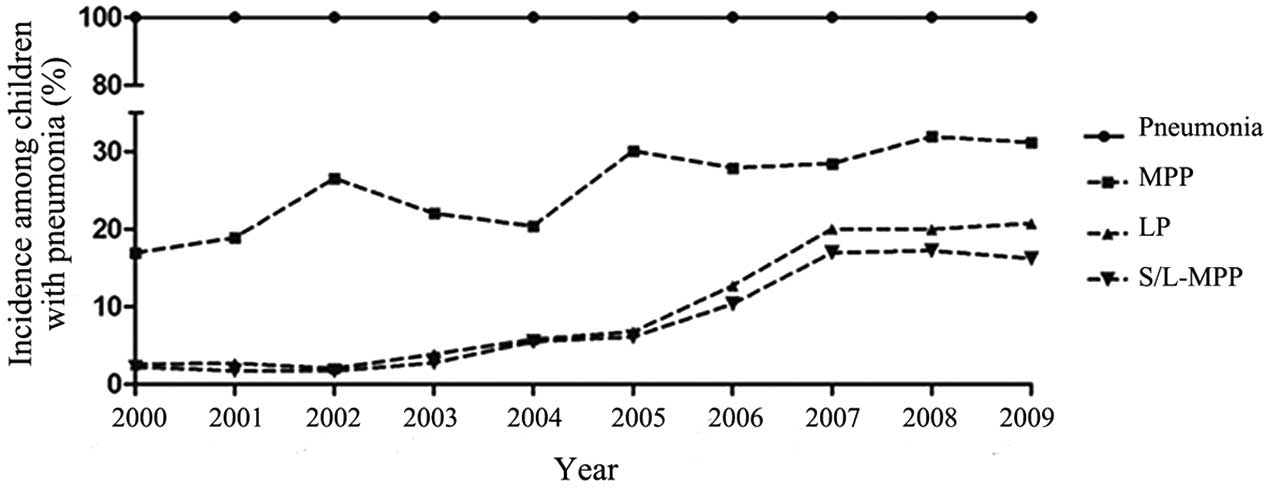
The annual incidence rates of MPP, LP and S/L-MPP
are displayed in Fig. 1. The annual
incidence of MPP showed an overall increasing trend over the
10-year course of the study (P<0.001); the highest incidence was
in 2008 (286/896 cases, 31.9%), and the lowest was in 2000 (85/502
cases, 16.9%). The annual incidence of LP and S/L-MPP showed an
initial similarly increasing trend (P<0.001), which accelerated
after 2005; the highest incidence for both diseases was in 2009
(22.9 and 16.2%, respectively), and the lowest incidence for both
diseases was in 2002 (2.0 and 1.7%, respectively).
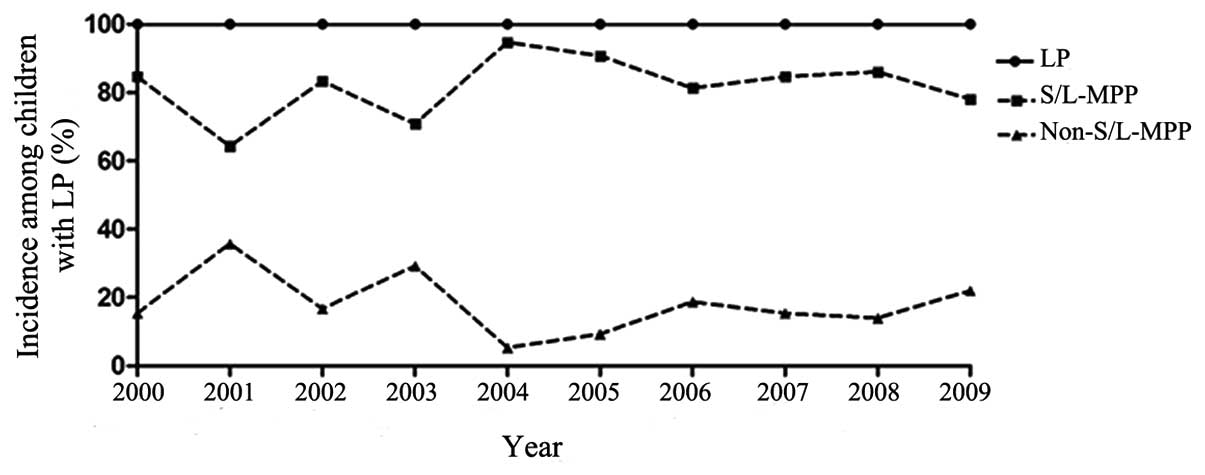
Among the 823 LP patients observed between 2000 and
2009, of which 83.1% were infected with M. pneumoniae; the
highest annual incidence was in 2004 (36/38 cases, 94.7%), and the
lowest incidence was in 2001 (9/14 cases, 64.3%). However, a
decreasing trend (P=0.028) was observed after 2004 (P<0.001;
Fig. 2).
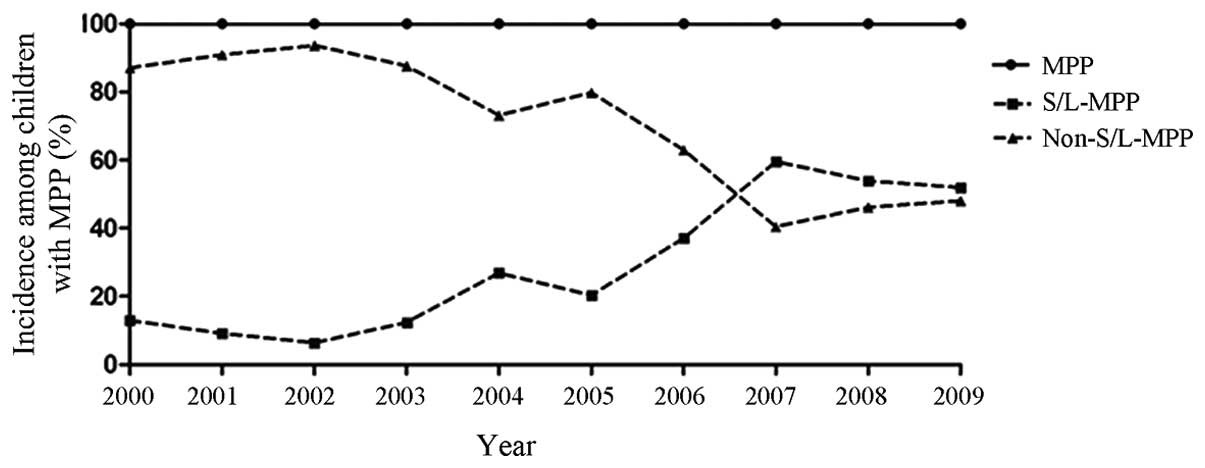
Between 2000 and 2009, 1,933 patients were diagnosed
with MPP, 35.4% of whom were further diagnosed with S/L-MPP. The
highest annual incidence occurred in 2007 (165/277 cases, 59.6%),
and the lowest incidence occurred in 2002 (10/157 cases, 6.4%). A
significant upward trend was observed in the annual incidence of
S/L-MPP in children with MPP (P<0.001), which is similar to that
observed in children suffering from general pneumonia (Fig. 3).
The age of children with MPP ranged from 8 months to
14 years (mean, 6.74±4.12 years), and the age distribution is
summarized in Table I. The age
distribution in non-S/L-MPP patients was significantly different
from the distribution observed in S/L-MPP patients (P<0.001).
The incidence of S/L-MPP was higher in children aged 4–6 years
(336/684 cases, 49.1%), whereas the incidence of non-S/L-MPP was
higher in patients ≥7 years of age (819/1,249 cases, 65.6%).
 | Table I.Clinical and treatment data of
children with MPP (n=1,933). |
Table I.
Clinical and treatment data of
children with MPP (n=1,933).
| Variables | No. | S/L-MPP
(n=684)a | Non-S/L-MPP
(n=1,249)b | P-value |
|---|
| Gender |
|
|
|
0.454 |
|
Male | 1,110 | 385 |
725 |
|
|
Female | 823 | 299 |
524 |
|
| Age (years) |
|
|
| <0.001 |
| ≤3 | 341 | 169 |
172 |
|
| 4 to
6 | 594 | 336 |
258 |
|
| ≥7 | 998 | 179 |
819 |
|
| Fever |
|
|
| <0.001 |
|
Yes | 1,658 | 617 | 1,041 |
|
| No | 275 | 67 |
208 |
|
| Duration of fever
(days) | 1,933 | 4.13±4.28 | 3.02±2.22 |
0.028 |
| Duration of cough
(days) | 1,993 | 9.43±7.62 | 7.86±5.92 |
0.084 |
| Gasping |
|
|
|
0.555 |
|
Yes | 499 | 182 |
317 |
|
| No | 1,434 | 502 |
932 |
|
| Pulmonary crackles
at onset |
|
|
|
0.436 |
|
Yes | 746 | 256 |
490 |
|
| No | 1,187 | 428 |
759 |
|
| Pleural
effusion |
|
|
| <0.001 |
|
Yes | 43 | 27 |
16 |
|
| No | 1,890 | 657 | 1,233 |
|
| Extrapulmonary
manifestations |
|
|
|
0.013 |
|
Yes | 444 | 179 |
265 |
|
| No | 1,489 | 505 |
984 |
|
| WBC |
|
|
| <0.001 |
|
Abnormal | 1,137 | 448 |
689 |
|
|
Normal | 796 | 236 |
560 |
|
| ESR |
|
|
|
0.697 |
|
Abnormal | 236 | 87 |
149 |
|
|
Normal | 1,324 | 471 |
854 |
|
| CRP |
|
|
|
0.002 |
|
Abnormal | 381 | 176 |
205 |
|
|
Normal | 1,054 | 393 |
661 |
|
| Co-infection with
bacteria |
|
|
|
0.008 |
|
Yes | 326 | 152 |
174 |
|
| No | 848 | 323 |
525 |
|
| Sensitivity to
macrolide antibiotics |
|
|
|
0.197 |
|
Yes | 1,860 | 653 | 1,207 |
|
| No | 73 | 31 |
42 |
|
|
Antibiotic-combination treatment |
|
|
|
0.004 |
|
Yes | 1,622 | 596 | 1,026 |
|
| No | 311 | 88 |
223 |
|
| Duration of
hospitalization (days) | 1,933 | 12.70±4.54 | 9.22±5.12 |
0.032 |
Clinical and laboratory assessment
according to pneumoniae pattern
Clinical data and the initial clinical assessment of
the children with MPP are presented in Table I. Relative to the children with
non-S/L-MPP (1,041/1,249 cases, 83.3%), those with S/L-MPP (617/684
cases, 90.2%) had a higher rate of fever (P<0.001). Moreover,
the duration of the fever for the S/L-MPP group (mean, 4.13±4.28
days) was significantly longer than that observed in the
non-S/L-MPP group (mean, 3.02±2.22 days, P=0.028). Additionally,
the duration of coughing was longer in the S/L-MPP group; however,
this difference in duration was not found to be statistically
significant (mean, 9.43±7.62 vs. 7.86±5.92 days, P=0.084).
Extrapulmonary manifestations, including erythematous maculopapular
rash, liver and kidney function lesions, and neurological
complications, occurred in 444 MPP patients (22.3%). The results
show that the incidence of extrapulmonary manifestations was
significantly higher in children with S/L-MPP (179/684 cases,
26.2%) than in children with non-S/L-MPP (265/1,249 cases, 21.2%;
P=0.013). The S/L-MPP group showed a higher rate of abnormal WBC
counts (P<0.001) and CRP levels (P=0.002) relative to the
non-S/L-MPP group. Nasopharyngeal swab or sputum specimen cultures
detected pathogens in 1,174 MPP patients, and 326 (27.8%) of these
patients were co-infected with bacteria, including Klebsiella
pneumoniae (112 cases, 34.4%), Streptococcus pneumoniae
(92 cases, 28.2%), Escherichia coli (67 cases, 20.5%),
Staphylococcus aureus (30 cases, 9.3%) and other pathogenic
bacteria (25 cases, 7.7%). The incidence of co-infection with
bacteria was significantly higher in children with S/L-MPP (152/475
cases, 32.0%) than in those with non-S/L-MPP (174/699 cases, 24.9%;
P=0.008; Table I).
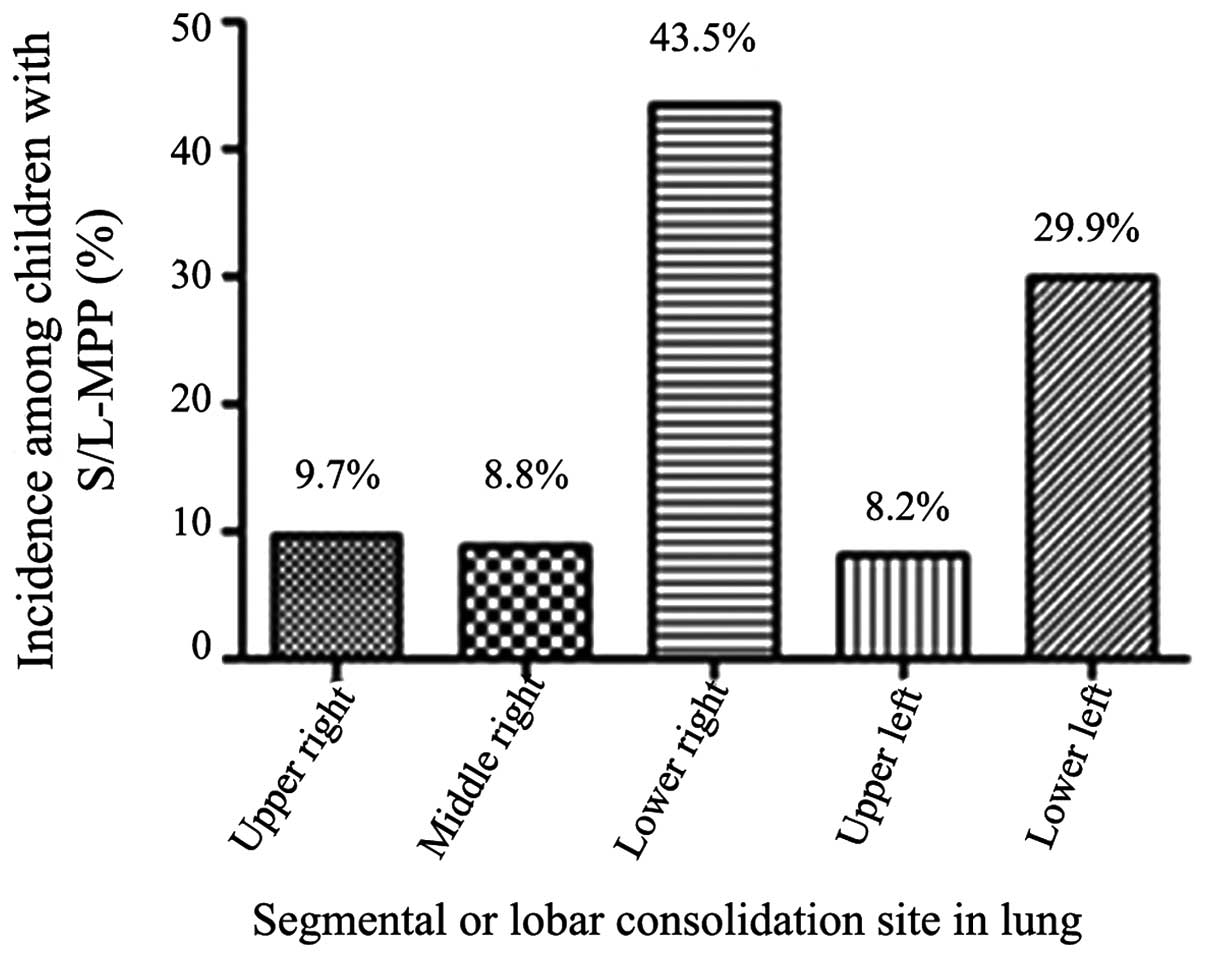
Radiological imaging demonstrated that
segmental/lobar consolidation was mainly located in the lower lung
lobe (501 cases, 73.3%), of which 297 cases were located in the
right lobe and 204 cases were in the left lobe (Fig. 4). The incidence of pleural effusion
in children with S/L-MPP (27/684 cases, 3.9%) was significantly
higher than that in children with non-S/L-MPP (16/1,249 cases,
1.3%; P<0.001).
Therapeutic characteristics
The medical and curative characteristics of the
1,933 hospitalized children with MPP were analyzed. Seventy-three
(3.8%) of the MPP cases were resistant to treatment with macrolide
antibiotics. S/L-MPP macrolide-resistant cases (31/684 cases, 4.5%)
were observed to be more prevalent than non-S/L-MPP
macrolide-resistant cases (42/1,249 cases, 3.4%); however, this
difference in prevalence was not found to be statistically
significant (P=0.197). The rate of antibiotic-combination treatment
for S/L-MPP patients (596/684 cases, 87.1%) was significantly
higher than that of non-S/L-MPP patients (1,026/1,249 cases, 82.1%;
P=0.004). Furthermore, the duration of hospitalization was observed
to be significantly longer for the patients in the S/L-MPP group
(mean, 12.70±4.54 days) than for the patients in the non-S/L-MPP
group (mean, 9.22±5.12 days, P=0.032; Table I).
Clinical and laboratory results
according to age in S/L-MPP
The 684 children with S/L-MPP included in this
analysis were divided into three groups according to age. The older
patients showed a higher rate of fever (P=0.048) and a longer
duration of fever (P=0.036) and cough (P=0.040). However, the older
patients presented with a lower rate of extrapulmonary
manifestations (P=0.017). Based on the laboratory findings, the
younger patients had a higher rate of bacterial co-infection than
the older patients (P=0.004; Table
II).
 | Table II.Clinical data of children with
S/L-MPP (n=684) according to age group. |
Table II.
Clinical data of children with
S/L-MPP (n=684) according to age group.
| Variables | No. | ≤3 years
(n=169)a | 4–6 years
(n=336)b | ≥7 years
(n=179)c | P-value |
|---|
| Gender |
|
|
|
| 0.077 |
|
Male | 385 | 92 | 179 | 114 |
|
|
Female | 299 | 77 | 157 | 65 |
|
| Fever |
|
|
|
| 0.048 |
|
Yes | 617 | 148 | 301 | 168 |
|
| No | 67 | 21 | 35 | 11 |
|
| Duration of fever
(days) | 684 | 3.49±3.22 | 3.68±4.64 | 5.25±4.77 |
0.036d |
| Duration of cough
(days) | 684 | 8.11±4.67 | 9.34±5.03 | 9.78±7.23 |
0.040d |
| Gasping |
|
|
|
| 0.075 |
|
Yes | 182 | 52 | 90 | 40 |
|
| No | 502 | 117 | 246 | 139 |
|
| Pulmonary crackles
at onset |
|
|
|
| 0.637 |
|
Yes | 256 | 65 | 118 | 73 |
|
| No | 428 | 104 | 218 | 106 |
|
| Pleural
effusion |
|
|
|
| 0.473 |
|
Yes | 27 | 6 | 12 | 9 |
|
| No | 657 | 163 | 324 | 170 |
|
| Extrapulmonary
manifestation |
|
|
|
| 0.017 |
|
Yes | 179 | 54 | 88 | 37 |
|
| No | 505 | 115 | 248 | 142 |
|
| WBC |
|
|
|
| 0.076 |
|
Abnormal | 448 | 122 | 217 | 109 |
|
|
Normal | 236 | 47 | 119 | 70 |
|
| ESR |
|
|
|
| 0.867 |
|
Abnormal | 87 | 19 | 50 | 18 |
|
|
Normal | 471 | 130 | 223 | 118 |
|
| CRP |
|
|
|
| 0.242 |
|
Abnormal | 176 | 46 | 78 | 52 |
|
|
Normal | 393 | 97 | 202 | 94 |
|
| Co-infection with
bacteria | 475 |
|
|
| 0.004 |
|
Yes | 152 | 48 | 70 | 34 |
|
| No | 323 | 59 | 167 | 97 |
|
| Duration of
hospitalization (days) | 684 | 12.83±5.63 | 12.52±7.72 | 11.32±8.65 |
>0.05e |
Discussion
MPP has been extensively researched; however,
systematic analysis of the epidemiological and clinical
differentiation of the segmental/lobar pattern from the other
radiographic patterns of pediatric MPP is rare. The present 10-year
study analyzed children who were hospitalized and presented with
the clinical features of S/L-MPP. The segmental/lobar pattern
accounted for 35.4% of MPP patients and 83.1% of LP patients.
Furthermore, pre-school children aged 4–6 years accounted for 56.6%
of the segmental/lobar pattern cases. The annual incidence of
S/L-MPP in children with MPP showed a rapidly increasing trend from
2000 to 2009. Compared with the non-segmental/lobar radiographic
MPP pattern, S/L-MPP is associated with more severe clinical
manifestations. The findings of the present study suggest that
S/L-MPP plays a more significant role in MPP cases than was
previously thought.
In this study, the mean prevalence of M.
pneumoniae infection among children with pneumonia (26.4%) was
similar to that in previous studies (3,5,24). Historically, endemic M.
pneumoniae disease transmission has been punctuated with cyclic
epidemics every 4–5 years (25,26). The
results of the present study show a similar, albeit less evident,
cycle from 2000 to 2009, which could be associated with differences
in the region, time and detection methods of the study. The annual
incidence of MPP showed a gradually increasing trend from 16.9 to
31.9% in this study, which is similar to that reported in other
studies (27,28). The age distribution of MPP in the
present study was also comparable to the age-related increase
reported in the literature (1,5,7).
Generally, M. pneumoniae is considered an
atypical bacterium that causes a form of CAP with radiological
manifestations of interstitial lung disease and with a very slow
and benign course (29). However,
segmental/lobar consolidation has been suggested to occur more
frequently in MPP than is generally appreciated (16). Furthermore, segmental/lobar MPP has
been shown to account for 17–76.5% of pediatric cases of MPP and
has shown an increasing trend in incidence (6,16–19). In
a study conducted in 1978 of 59 patients with documented M.
pneumoniae, 20% were found to have a single homogeneous
infiltrate that appeared as a bacterial lobar pneumonia (16). In another large urban survey
conducted in 1980, lobar infiltrates were reported in 17% of MPP
patients (19). Esposito et
al reported that the lobar/segmental pattern occurred in 35.3%
of MPP patients (2–12 years old) in 2001 (18). In a study conducted in 2004, Phares
et al observed that 44% (38/85 cases) of MPP patients had
alveolar consolidations (6). In
2008, Defilippi et al (17)
reported that consolidations were the most frequent finding (76.5%,
62/76 cases) in chest X-rays of children with M. pneumoniae
infection. Collectively, these studies indicate that the rate of
lobar/segmental consolidation in MPP may be increasing each year.
The data in the present study show that the incidence of
segmental/lobar consolidation was 35.4% in children with MPP and
9.3% in those with pneumonia. The data also show that the annual
incidence of S/L-MPP in children with MPP showed a rapidly
increasing trend between 2000 and 2009, which was particularly
evident after 2003. The highest incidence of S/L-MPP occurred in
2007 accounting for 59.6% of MPP cases and with a slight reduction
observed after 2007. A gradual increase in the incidence of the
segmental/lobar pattern suggests that severe MPP has increased in
the most recent years of the present analysis. Moreover, the
majority of LP patients (83.1%) were infected with M.
pneumoniae; however, this trend showed a gradual reduction
after 2004. The reasons for these observed trends are complex.
Mixed infection (7,26,30) and
macrolide-resistant (31,32) MPP have displayed altered trends in
recent years. Liu et al reported that the rate of occurrence
of macrolide-resistant M. pneumoniae was 83% (44/53 cases)
between 2005 and 2008 (31) and
increased to 90% in 2010 (32) in
Shanghai, China. These time-related factors may be associated with
the change in S/L-MPP incidence.
The data in the present study show that S/L-MPP
predominantly occurs in pre-school-aged children (4–6 years old,
56.6%). M. pneumoniae is considered to be the primary
causative agent of pneumonia in children aged 5–10 years (7) and has also been recognized as the
primary causative agent of pneumonia in pre-school-aged children
(27). Typically, an age-related
increase in the incidence of pneumonia due to M. pneumoniae
infection is observed (33,34). Additionally, Wu et al reported
that in 1,009 children <18 years of age who were hospitalized
for LP or pneumococcal pneumonia, 64% were <5 years of age; the
age-specific incidence increased gradually and was highest in
patients between 4 and 5 years of age, after which the incidence
decreased yearly (35). In the
present study, the age distribution of S/L-MPP intersected with
that of MPP and LP, indicating that children <7 years of age
with MPP could be more likely to present with an accompanying
severe pulmonary lesion. This age distribution differs from that
reported in a study conducted in Korea by Youn et al
(20), in which the frequency of
S/L-MPP was significantly higher in patients ≥6 years of age
(69.1%) when compared with patients between 2 and 5 years of age
(40.7%) or patients <2 years of age (20.7%). This difference in
the age-specific incidence may be associated with differences in
age grouping, region, number of patients studied and the condition
of the patients (particularly in older children).
The typical chest X-ray manifestation of MPP is a
subsegmental patchy interstitial or alveolar (or both) infiltrate
in one or more areas that occasionally occurs bilaterally (19). In the present study, it was observed
that S/L-MPP is more often unilateral (86.5%) and confined to the
lower lobes (73.3%, especially in the right), which differs from an
earlier study on unilateral occurrence (35 and 50%) in the lower
lobes (50.0%) (34). Pleural
effusion is an unusual observation in MPP. In the present study,
the incidence of pleural effusion among MPP patients (2.2%) was
lower than that previously reported (5–20%) (34); however, the incidence in S/L-MPP
patients (3.9%) was significantly higher than that of non-S/L-MPP
patients (1.3%), suggesting that S/L-MPP is associated with more
severe pulmonary lesions.
MPP is referred to as walking or atypical pneumonia,
and the degree of consolidation may exceed what would be expected
based on the severity of the clinical manifestations. Consistent
with a previous study, the present study shows that relative to
non-S/L-MPP, S/L-MPP is associated with more severe manifestations,
including fevers of higher grade and longer duration, longer
durations of cough, increased incidence of pleural effusion and
additional extrapulmonary manifestations (20). Furthermore, it was found that younger
children with S/L-MPP had a greater rate of febrile and
extrapulmonary manifestations and a reduced duration of fever and
cough. Anerythematous maculopapular rash was the most common
extrapulmonary manifestation, whereas neurological complications
were rare. These results suggest that S/L-MPP is associated with
more severe clinical manifestations, particularly in younger
children.
A clinical study has reported that children with
S/L-MPP have significantly lower WBC and lymphocyte counts but
higher CRP levels than children with bronchopneumonic patterns
(20). However, consistent with
another study, the present study shows that S/L-MPP is associated
with higher rates of abnormal WBC counts and CRP levels than
non-S/L-MPP (36). Furthermore, the
increased rate of abnormal WBC counts (72.2%) may be associated
with the increased febrile rate and co-infections in the younger
patients. Co-infection with S. pneumoniae is present in
>30% of M. pneumoniae CAP patients (27,30). In
the present study, 27.8% of MPP patients were co-infected with
another pathogen, and the most common co-infectious agent isolated
was K. pneumoniae; however, S. pneumoniae has not
been reported as a co-infectious agent (27). In the present study, S/L-MPP patients
showed a higher rate of bacterial co-infection (32.0%) than
non-S/L-MPP patients (24.9%), and this was particularly evident in
children <3 years of age (44.9%). Extensive microbiological
testing was not conducted, therefore the possibility that some
children could have been co-infected with Chlamydia or a
viral pathogen (27) cannot be
excluded. Children with S/L-MPP in the present study had a longer
duration of hospitalization and a higher rate of antibiotic
combination therapy than those with non-S/L-MPP. Generally,
lobar/segmental consolidation on chest radiographic findings is
considered to be an air-space disease (10), which is a severe pathological
manifestation in patients with MPP. Taking into account the
severity of the illness, the antibiotic resistance patterns and
comorbid conditions, empirical antibiotic combination therapy was
relatively frequent in patients with S/L-MPP.
The limitations of this study include the fact that
the study subjects only included those who were diagnosed with
pneumonia by radiographic analysis, which is typically performed on
patients with more severe clinical manifestations. The subjects
enrolled in this study could have had more severe clinical and
pathological manifestations than patients who do not typically
undergo a chest X-ray, which may have contributed to a
case-selection bias. Another limitation is the fact that M.
pneumoniae cultures and serological PCR were unavailable;
therefore, serological M. pneumoniae IgM and nasopharyngeal
PCR may have produced false negative and false positive
results.
In conclusion, it was found that a segmental/lobar
pattern existed in 35.4% of MPP patients, and the annual incidence
of S/L-MPP has increased in recent years. The incidence of S/L-MPP
was the highest in pre-school children aged 4–6 years, in whom
S/L-MPP presents with more severe clinical manifestations than
non-S/L-MPP. With knowledge of these new epidemiological
characteristics, clinicians are recommended to consider empirical
antibiotic-combination treatment for MPP, particularly in
pre-school-aged children.
Acknowledgements
The authors thank Yongliang Fen, PhD and Hong Xu,
PhD, MD for statistical support and methodological guidance and
Xiaolan Chen, MD, Jiahai Yu, MD, Ping Guo, MD, and Yahui Zhou, MD
for collection and classification of the data.
References
|
1
|
Foy HM: Infections caused by Mycoplasma
pneumoniae and possible carrier state in different populations of
patients. Clin Infect Dis. 17(Suppl 1): S37–S46. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Marston BJ, Plouffe JF, File TM Jr,
Hackman BA, Salstrom SJ, Lipman HB, Kolczak MS and Breiman RF: The
Community-based Pneumonia Incidence Study Group: Incidence of
community-acquired pneumonia requiring hospitalization: Results of
a population-based active surveillance study in Ohio. Arch Intern
Med. 157:1709–1718. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Waites KB and Talkington DF: Mycoplasma
pneumoniae and its role as a human pathogen. Clin Microbiol Rev.
17:697–728. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu G, Talkington DF, Fields BS, Levine
OS, Yang Y and Tondella ML: Chlamydia pneumoniae and Mycoplasma
pneumoniae in young children from China with community-acquired
pneumonia. Diagn Microbiol Infect Dis. 52:7–14. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Heiskanen-Kosma T, Korppi M, Jokinen C,
Kurki S, Heiskanen L, Juvonen H, Kallinen S, Stén M, Tarkiainen A,
Rönnberg PR, et al: Etiology of childhood pneumonia: Serologic
results of a prospective, population-based study. Pediatr Infect
Dis J. 17:986–991. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Phares CR, Wangroongsarb P, Chantra S,
Paveenkitiporn W, Tondella ML, Benson RF, Thacker WL, Fields BS,
Moore MR, Fischer J, et al: Epidemiology of severe pneumonia caused
by Legionella longbeachae, Mycoplasma pneumoniae and Chlamydia
pneumoniae: 1-year, population-based surveillance for severe
pneumonia in Thailand. Clin Infect Dis. 45:e147–e155. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Korppi M, Heiskanen-Kosma T and Kleemola
M: Incidence of community-acquired pneumonia in children caused by
Mycoplasma pneumoniae: Serological results of a prospective,
population-based study in primary health care. Respirology.
9:109–114. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Layani-Milon MP, Gras I, Valette M,
Luciani J, Stagnara J, Aymard M and Lina B: Incidence of upper
respiratory tract Mycoplasma pneumoniae infections among
outpatients in Rône-Alpes, France, during five successive winter
periods. J Clin Microbiol. 37:1721–1726. 1999.PubMed/NCBI
|
|
9
|
Principi N and Esposito S: Emerging role
of Mycoplasma pneumoniae and Chlamydia pneumoniae in paediatric
respiratory-tract infections. Lancet Infect Dis. 1:334–344. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
John SD, Ramanathan J and Swischuk LE:
Spectrum of clinical and radiographic findings in pediatric
mycoplasma pneumonia. Radiographics. 21:121–131. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nambu A, Saito A, Araki T, Ozawa K,
Hiejima Y, Akao M, Ohki Z and Yamaguchi H: Chlamydia pneumoniae:
Comparison with findings of Mycoplasma pneumoniae and Streptococcus
pneumoniae at thin-section CT. Radiology. 238:330–338. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Reittner P, Muller NL, Heyneman L, Johkoh
T, Park JS, Lee KS, Honda O and Tomiyama N: Mycoplasma pneumoniae
pneumonia: Radiographic and high-resolution CT features in 28
patients. AJR Am J Roentgenol. 174:37–41. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee I, Kim TS and Yoon HK: Mycoplasma
pneumoniae pneumonia: CT features in 16 patients. Eur Radiol.
16:719–725. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
George RB, Weill H, Rasch JR, Mogabgab WJ
and Ziskind MM: Roentgenographic appearance of viral and
mycoplasmal pneumonias. Am Rev Respir Dis. 96:1144–1150.
1967.PubMed/NCBI
|
|
15
|
Murray HW, Masur H, Senterfit LB and
Roberts RB: The protean manifestations of Mycoplasma pneumoniae
infection in adults. Am J Med. 58:229–242. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brolin I and Wernstedt L: Radiographic
appearance of mycoplasmal pneumonia. Scand J Respir Dis.
59:179–189. 1978.PubMed/NCBI
|
|
17
|
Defilippi A, Silvestri M, Tacchella A,
Giacchino R, Melioli G, Di Marco E, Cirillo C, Di Pietro P and
Rossi GA: Epidemiology and clinical features of Mycoplasma
pneumoniae infection in children. Respir Med. 102:1762–1768. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Esposito S, Blasi F, Bellini F, Allegra L
and Principi N: Mowgli Study Group: Mycoplasma pneumoniae and
Chlamydia pneumoniae infections in children with pneumonia. Eur
Respir J. 17:241–245. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Foy HM, Kenny GE, McMahan R, Mansy AM and
Grayston JT: Mycoplasma pneumoniae pneumonia in an urban area. Five
years of surveillance. JAMA. 214:1666–1672. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Youn YS, Lee KY, Hwang JY, Rhim JW, Kang
JH, Lee JS and Kim JC: Difference of clinical features in childhood
Mycoplasma pneumoniae pneumonia. BMC Pediatr. 10:482010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
World Health Organization Pneumonia
Vaccine Trial Investigators' Group. Standardization of
interpretation of chest radiographs for the diagnosis of pneumonia
in children. http://apps.who.int/iris/bitstream/10665/66956/1/WHO_V_and_B_01.35.pdfAccessed.
November 29–2011
|
|
22
|
Daxboeck F, Bauer CC, Assadian O and
Stanek G: An unrecognized epidemic of Mycoplasma pneumoniae
infection in Vienna. Wien Klin Wochenschr. 118:208–211. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Musatovova O, Kannan TR and Baseman JB:
Genomic analysis reveals Mycoplasma pneumoniae repetitive element
1-mediated recombination in a clinical isolate. Infect Immun.
76:1639–1648. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nolevaux G, Bessaci-Kabouya K, Villenet N,
Andrèoletti L, Laplanche D, Carquin J, Abély M, De Champs C and
Motte J: Epidemiological and clinical study of Mycoplasma
pneumoniae respiratory infections in children hospitalized in a
pediatric ward between 1999 and 2005 at the Reims University
Hospital. Arch Pediatr. 15:1630–1636. 2008.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hong JY, Nah SG, Nam SG, Choi EH, Park JY
and Lee HJ: Occurrence of Mycoplasma pneumoniae pneumonia in Seoul,
Korea, from 1986–1995. Korean J Pediatr. 40:607–613. 1997.
|
|
26
|
Kang KS and Woo HO: Pattern of occurrence
of Mycoplasma pneumoniae pneumonia in admitted children, southern
central Korea, from 1989 to 2002. Korean J Pediatr. 46:474–479.
2003.
|
|
27
|
Principi N, Esposito S, Blasi F and
Allegra L: Mowgli Study Group: Role of Mycoplasma pneumoniae and
Chlamydia pneumoniae in children with community-acquired lower
respiratory tract infections. Clin Infect Dis. 32:1281–1289. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu YC, Zhu LJ, Xu D, Tao XF, Li SX, Tang
LF and Chen ZM: Epidemiological characteristics and meteorological
factors of childhood Mycoplasma pneumoniae pneumonia in Hangzhou.
World J Pediatr. 7:240–244. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Marrie TJ: Pneumococcal pneumonia:
Epidemiology and clinical features. Semin Respir Infec. 14:227–236.
1999.
|
|
30
|
Toikka P, Juvén T, Virkki R, Leinonen M,
Mertsola J and Ruuskanen O: Streptococcus pneumoniae and Mycoplasma
pneumoniae coinfection in community acquired pneumonia. Arch Dis
Child. 83:413–414. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu Y, Ye X, Zhang H, Xu X, Li W, Zhu D
and Wang M: Antimicrobial susceptibility of Mycoplasma pneumoniae
isolates and molecular analysis of macrolide-resistant strains from
Shanghai, China. Antimicrob Agents Chemother. 53:2160–2162. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu Y, Ye X, Zhang H, Xu X, Li W, Zhu D
and Wang M: Characterization of macrolide resistance in Mycoplasma
pneumoniae isolated from children in Shanghai, China. Diagn
Microbiol Infect Dis. 67:355–358. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ito I, Ishida T, Osawa M, Arita M,
Hashimoto T, Hongo T and Mishima M: Culturally verified Mycoplasma
pneumoniae pneumonia in Japan: A long-term observation from
1979–99. Epidemiol Infect. 127:365–367. 2001.PubMed/NCBI
|
|
34
|
Othman N, Isaacs D and Kesson A:
Mycoplasma pneumoniae infections in Australian children. J Paediatr
Child Health. 41:671–676. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wu PS, Huang LM, Chang IS, Lu CY, Shao PL,
Tsai FY and Chang LY: The epidemiology of hospitalized children
with pneumococcal/lobar pneumonia and empyema from 1997 to 2004 in
Taiwan. Eur J Pediatr. 169:861–866. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cockcroft DW and Stilwell GA: Lobar
pneumonia caused by Mycoplasma pneumoniae. Can Med Assoc J.
124:1463–1468. 1981.PubMed/NCBI
|