Introduction
Rhizoma Dioscoreae (RD) is a traditional
Chinese medicine described in the Pharmacopoeia of the People's
Republic of China, of which the RD polysaccharides (RDPS) are the
major active ingredient (1). Ko and
Hong (2) demonstrated the safety of
using Dioscoreae rhizome in the practice of
pharmacopuncture, and various studies have detected therapeutic
effects for RDPS. In particular, RDPS administered intragastrically
was shown to decrease the levels of malondialdehyde (MDA), nitric
oxide synthase and nitric oxide, alleviate liver inflammation, and
decrease the liver index and alanine transaminase activity in a
mouse model of chemokine (C-C motif) ligand-4-induced liver injury
(3). Furthermore, treatment of a
mouse model of D-galactose-induced mimetic aging with RDPS was
associated with increased superoxide dismutase (SOD), glutathione
peroxidase (GSH-Px) and Na+/K+-ATPase
activities, as well as decreased serum levels of MDA (4,5). In
rats, focal application of Dioscoreae rhizome extract at a
sciatic nerve crush injury site was associated with increased
levels of the axonal growth-associated protein and cyclin-dependent
kinase-1 in the distal portion of the injured nerve (6). Furthermore, previous in vitro
studies have suggested that RDPS is able to scavenge
1,1-diphenyl-2-picrylhydrazyl, OH and O2−
free radicals (7,8).
In acute or chronic ischemia/hypoxia, the necrosis
and apoptosis of neurons is mediated by the production of reactive
oxygen species (ROS) or activation of the mitochondrial apoptosis
pathway (9,10). The present study aimed to investigate
the neuroprotective effects of RDPS against in vitro
hypoxia-induced cerebral cortical neuron apoptosis. The results of
the present study suggested that RDPS was able to improve neuronal
cell viability, and inhibit hypoxia-induced apoptosis of neuronal
cells.
Materials and methods
Materials
Gibco neurobasal medium and B-27 supplement were
obtained from Thermo Fisher Scientific, Inc. (Waltham, MA, USA).
Equine serum, poly-D-lysine and trypsin were obtained from
Sigma-Aldrich (St. Louis, MO, USA). RDPS (extracted using a mixture
of distilled water, chloroform, n-butanol and ethanol, and diluted
with neurobasal medium; polysaccharide content, >95.0%) was
purchased from Nanjing Zelang Medical Technological Co., Ltd.
(Nanjing, China). Hoechst 33342, Annexin V-fluorescein
isothiocyanate (FITC) and Rhodamine 123 staining kits were obtained
from Nanjing KeyGen Biotech Co., Ltd. (Nanjing, China). MTT was
obtained from Beijing Probe Biotech Co., Ltd. (Beijing, China).
TransScript™ two-step reverse transcription-polymerase chain
reaction (RT-PCR) Supermix kit was obtained from Beijing TransGen
Biotech Co., Ltd. (Beijing, China).
Rats
A total of 52 pregnant Sprague-Dawley rats were bred
and housed at the Laboratory Animal Services Centre of the Jiangxi
College of Traditional Chinese Medicine (Nanchang, China). The rats
were house in a room that was free of noise and strong odors, with
a controlled temperature of 23±2°C and 60±5% relative humidity, and
were maintained in a 12 h light/12 h dark cycle. The rats had free
access to water and food. All experiments were performed in
accordance with the animal experimental guidelines established by
the Ministry of Science and Technology of the People's Republic of
China, and were approved by the ethics committee of Jiangxi
Province People's Hospital (Nanchang, China).
Cytotoxicity of RDPS
Cerebral cortical neurons in primary serum-free
culture
A neuronal suspension was prepared from the pregnant
rats, as outlined previously (11).
Briefly, the pregnant rats were anesthetized with 1.5 ml 10%
chloral hydrate (Sangon Biotech Co., Ltd., Shanghai, China) and
then fixed on the animal operating table. Their abdominal skin was
disinfected with a 75% alcohol gauze and laparotomy was performed.
The fetal rats were carefully removed from the uterus and their
brain was removed following removal of the scalp and skull. The
brain tissues were placed in cold D-Hank's solution (Gibco; Thermo
Fisher Scientific, Inc.) containing 4.5% glucose in a petri dish.
Subsequently, the fetal rats meninges and blood vessels were
removed under a Leica S6 E stereomicroscope (Leica Microsystems,
Wetzlar, Germany), and the brain cortex tissue was isolated and cut
into 1×1×1 mm tissue sections. The sections were then placed into a
0.25% pancreatic enzyme EDTA solution at 37°C for 20 min, followed
by termination of digestion by addition of 5 ml Dulbecco's Modified
Eagle medium (DMEM; Gibco, Thermo Fisher Scientific, Inc.)
supplemented with 10% horse serum and 10% fetal bovine serum for 5
min. The cells were isolated from the sections by a mechanical
method using a Pasteur pipette, and passed through a 200 mesh
stainless steel sieve. Next, the cells were counted and adjusted to
a concentration of 5×106/ml using DMEM.
Subsequently, 0.1 ml cells were seeded at a density
of 5×105 cells/ml into 96-well culture plates coated
with polylysine, and were subsequently stored in a 5%
CO2 incubator (Thermo Fisher Scientific, Inc.) at 37°C
with saturated humidity. After 4 h, Gibco Dulbecco's modified
Eagle's medium (Thermo Fisher Scientific, Inc.), supplemented with
10% equine serum, was removed from the plates, and the cell
cultures were incubated with 2.0% B-27 neurobasal medium for 4
days. Subsequently, the cells were incubated with RDPS (0.025,
0.05, 0.10, 0.25, 0.50, 1.0, 2.0, 4.0 or 8.0 g/l) for 48 h, after
which the culture medium (0.1 ml) was removed and added to 96-well
plates containing 0.5% MTT for 4 h. Following removal of the
culture media, 0.15 ml dimethyl sulfoxide (DMSO; Sigma-Aldrich) was
added to the wells, and the plates were agitated for 10 min.
Absorbance [optical density (OD)] values were measured at 490 nm
using a microplate reader (EL×800™; BioTek Instruments, Inc.,
Winooski, VT, USA).
Cerebral cortical neurons in primary serum-free
hypoxia/reoxygenation culture
Cytotoxicity and MTT analyses were conducted as
outlined previously (11). Briefly,
the neurons were treated with RDPS (0.025, 0.05, 0.10, 0.25, 0.50,
1.0 or 2.0 g/l) for 4 h, after which the 96-well plates were stored
for 12 h in a hypoxia incubator (YQX-II Anaerobic Incubator;
Shanghai Hengyue Medical Instruments Co., Ltd, Shanghai, China),
containing 85% nitrogen, 10% hydrogen and 5% CO2, at
37°C. Subsequently, the plates were transferred to a 5%
CO2 reoxygenation incubator for 24 h, after which the
culture media (0.1 ml) was removed and added to 96-well plates
containing 0.5% MTT for 4 h. Subsequently, the culture media was
removed, 0.15 ml DMSO was added to the wells, the plates were
agitated for 10 min, and the OD values were measured at 490 nm
using a microplate reader.
Grouping
The harvested rat neuronal cells were divided into
five groups, as follows: i) Normal control group (C), in which the
neurons (5×105 cells/ml) were cultured in an incubator
at 37°C, containing 5% CO2 and saturated humidity for 6
days; ii) apoptosis model group (A), in which neurons
(5×105 cells/ml) were cultured in an incubator at 37°C,
containing 5% CO2 and saturated humidity for 4 days,
after which the neurons were placed in a hypoxia incubator for 12
h, followed by transfer to a 5% CO2 reoxygenation
incubator for 24 h; iii) 0.025 g/l RDPS-treated group (RDPS1); iv)
0.05 g/l RDPS-treated group (RDPS2); v) 0.1 g/l RDPS-treated group
(RDPS3); and vi) 0.25 g/l RDPS-treated group (RDPS4). Generation of
the RDPS-treated groups involved culturing the rat neurons
(5×105 cells/ml) in an incubator containing 5%
CO2 at 37°C, with saturated humidity for 4 days, after
which the cells were incubated with the appropriate concentration
of RDPS for 4 h. Subsequently, the neurons were cultured under
hypoxic conditions for 12 h, and then incubated for 24 h in a 5%
CO2 reoxygenation incubator.
Hoechst 33342 fluorescence staining
Hoechst 33342 fluorescence staining was conducted as
outlined previously (11). The
apoptotic neurons were observed using fluorescence microscopy.
Briefly, the neurons (>200) were counted randomly under a high
power microscope (DMI 3000; Leica Microsystems GmbH, Wetzlar,
Germany) and the apoptotic rate was calculated as follows:
Apoptotic rate (%) = (number of apoptotic neurons/total number of
neurons) × 100%.
Annexin V FITC/propidium iodide (PI) double
staining and flow cytometric analysis
The neurons were digested using 0.02%
ethylenediaminetetraacetic acid (EDTA) and 0.125% pancreatin
solution (Sigma-Aldrich), and the resulting neuronal cell
suspension was centrifuged for 5 min at 300 × g, after which the
supernatant was removed. The neurons were washed twice with
phosphate-buffered saline (PBS), followed by centrifugation for an
additional 5 min (300 × g). The cells (1–5×105 cells/ml)
were suspended in 500 µl binding buffer, after which 5 µl
Annexin-FITC and 5 µl PI was added, with agitation. The cells were
incubated at room temperature in the dark for 10 min, followed by
centrifugation for 5 min at 300 × g. Subsequently, the labeling
liquid was removed and the cells were washed once with incubation
buffer. The cells were analyzed in a flow cytometer (Coulter Epics
xL; Beckman Coulter Inc., Brea, CA, USA) with argon ion
laser-excited fluorescence at 488 nm. Flowjo 7.6 software was used
to analyze the results of the flow cytometric analysis (Tree Star
Inc., Ashland, OR, USA).
Rhodamine 123 staining and flow cytometric
analysis
Rhodamine 123 staining was performed according to
the manufacturer's protocol. Briefly, hypoxia/reoxygenation
cultured neurons were digested using 0.02% EDTA and 0.125%
pancreatin solution, and the resulting neuronal cell suspension was
centrifuged for 5 min at 300 × g, after which the supernatant was
removed. The neurons were washed with PBS three times and Rhodamine
123 dye (final concentration, 0.005 g/l) was added. Following
incubation for 20 min, the neurons were washed three times with PBS
and incubated for 60 min. Subsequently, the neurons were collected
and analyzed in a flow cytometer with argon ion laser-excited
fluorescence at 488 nm. Flowjo 7.6 software was used to analyze the
fluorescence intensity.
Semi-quantitative PCR assay
The PCR assay (MyCycler™ Thermal Cycler; Bio-Rad
Laboratories, Inc., Hercules, CA, USA) was performed according to
the manufacturer's protocol. Briefly, total mRNA extraction was
performed as follows: TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) extraction for 5 min, followed by chloroform
treatment for 2 min, centrifugation at 12,000 × g for 15 min,
isopropyl alcohol treatment for 20 min, followed by further
centrifugation at 12,000 × g for 10 min. The supernatant was
removed and 75% ethanol precipitation was performed, followed by
centrifugation at 7,500 × g for 5 min, supernatant removal and air
drying. Diethylpyrocarbonate-treated water was then added, in order
to dissolve the mRNA, at 65°C for 10–15 min. The OD value of the
RNA was measured at 260 nm using a SmartSpec Plus ultraviolet
spectrophotometer (Bio-Rad Laboratories, Inc.).
The RNA OD value was used to calculate the
concentration of RNA, as follows: RNA concentration (mg/ml) = 40 ×
OD260 value × dilution ratio/1,000. Reverse
transcription of mRNA into cDNA was performed as follows: Total
mRNA (3 µl), 1 µl random primer (0.1 µg/ml), 10 µl 2X TS Reaction
mix, 1 µl TransScript™ RT/RI Enzyme mix and 5 µl ribonuclease-free
water, was mixed and incubated at 25°C for 10 min, 42°C for 30 min
and 85°C for 5 min.
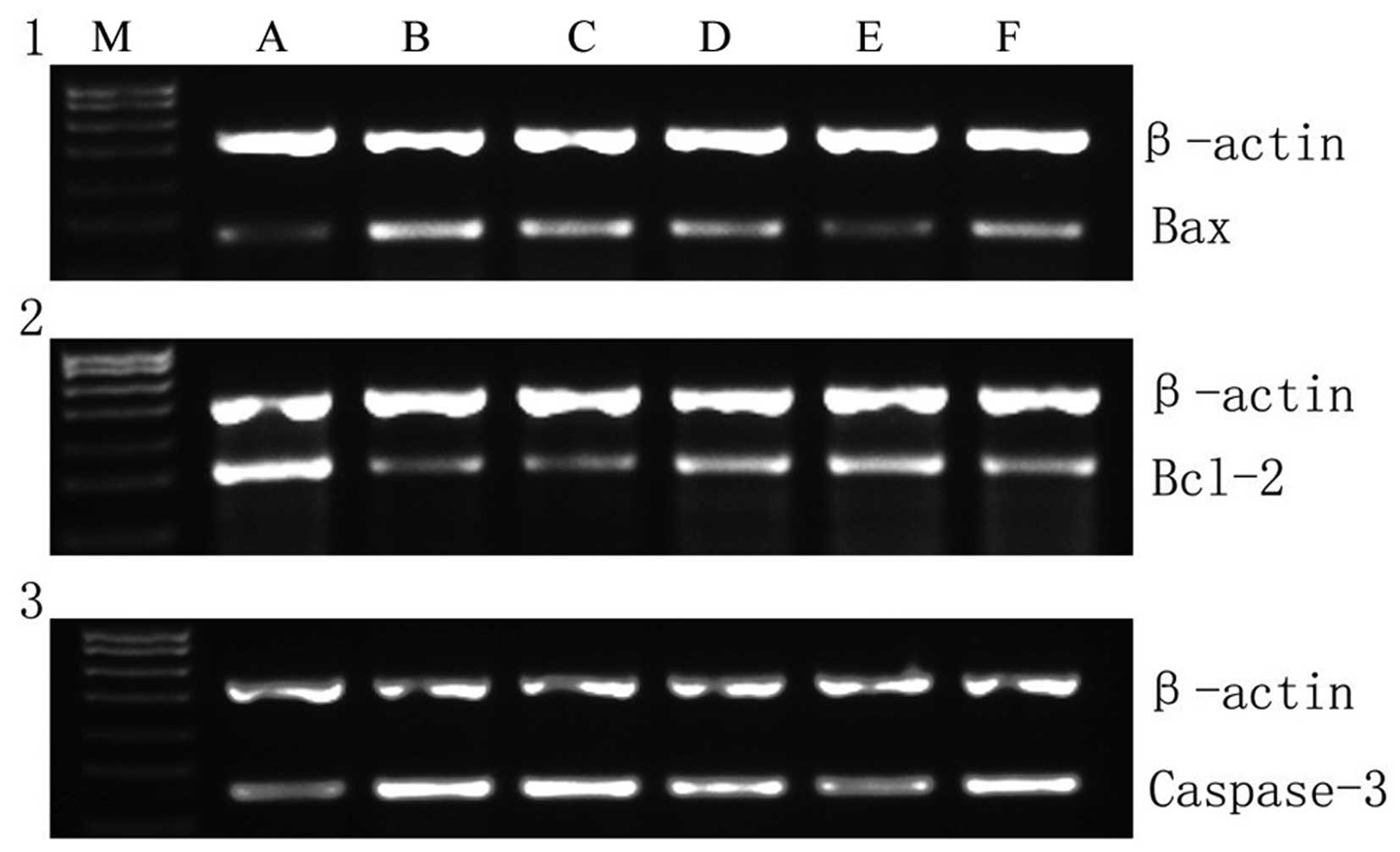
The β-actin, caspase-3, B-cell lymphoma 2 (Bcl-2)
and Bcl-2-associated X protein (Bax) genes were amplified according
to the following protocol: The cDNA (3 µl), 1 µl forward primer (10
µM), 1 µl reverse primer (10 µM), 25 µl 2X TransTap™ HiFi PCR
SuperMix II and 20 µl double distilled H2O, were mixed
and subjected to 32 PCR cycles (94°C for 5 min, 94°C for 30 sec,
55°C for 30 sec, 72°C for 1 min and 72°C for 10 min) for β-actin
and caspase-3, and 32 PCR cycles (94°C for 5 min, 94°C 30 sec, and
58°C for 30 sec, 72°C 1 min and 72°C for 10 min) for Bax and Bcl-2.
Agarose gel electrophoresis was performed using 5 µl of the PCR
products at 120 V for 45 min. The gray-scale value of each DNA band
was measured using Quantity One software (Bio-Rad Laboratories,
Inc.). The levels of gene expression were quantified by calculating
the ratio of the OD values of the respective gene:OD value of the
internal control.
The gene primer sequences were as follows: β-actin
(432bp) forward, 5′-TCA GGT CAT CAC TAT CGG CAA T-3′ and reverse,
5′-AAA GAA AGG GTG TAA AAC GCA −3′; caspase-3 (159bp) forward,
5′-GCA TGC CAT ATC ATC GTC AG-3′ and reverse, 5′-GGA CCT GTG GAC
CTG AAA AA-3′; Bax (173 bp) forward, 5′-GAT CAG CTC GGG CAC TTT
AG-3′ and reverse, 5′-TGC AGA GGA TGA TTG CTG AC-3′; and Bcl-2
(223bp) forward, 5′-ATG CCG GTT CAG GTA CTC AG-3′ and reverse,
5′-CGA CTT TGC AGA GAT GTC CA-3′.
Immunocytochemical staining
Immunocytochemical staining was performed, as
outlined previously (11). Briefly,
the cells were incubated with rabbit anti-rat Bcl-2 (dilution,
1:200; cat. no. D2010), rabbit anti-rat Bax (dilution, 1:200; cat.
no. 12910), or rabbit anti-caspase-3 (dilution, 1:400; cat. no.
E1410) polyclonal primary antibodies, followed by incubation with a
goat anti-rabbit immunoglobulin G secondary antibody (cat. no.
202012; all antibodies were purchased from Zhongshan Golden Bridge
Biotechnology, Beijing, China). The cells that were brown in
appearance under a light microscope (BX43; Olympus Corp., Tokyo,
Japan) were designated positive, whereas unstained or ‘buffy’ cells
were considered negative. A total of 200 cells were randomly
counted in order to calculate the positive rate, as follows:
Positive rate(%) = (the number of positive cells/total number of
cells) × 100%.
Statistical analysis
Data are presented as the mean ± standard deviation.
Experimental data that conformed to a normal distribution and
homogeneity of variance were analyzed using a one-way analysis of
variance, and post hoc tests were used for comparison between two
groups. Experimental data that did not fit a normal distribution or
homogeneity of variance were analyzed using a non-parametric test.
P<0.05 was considered to indicate a statistically significant
difference. For statistical analysis, the SPSS version 17.0
software (SPSS, Inc., Chicago, IL, USA) was used.
Results
Effects of RDPS on cultured cerebral
cortical neuronal cell viability
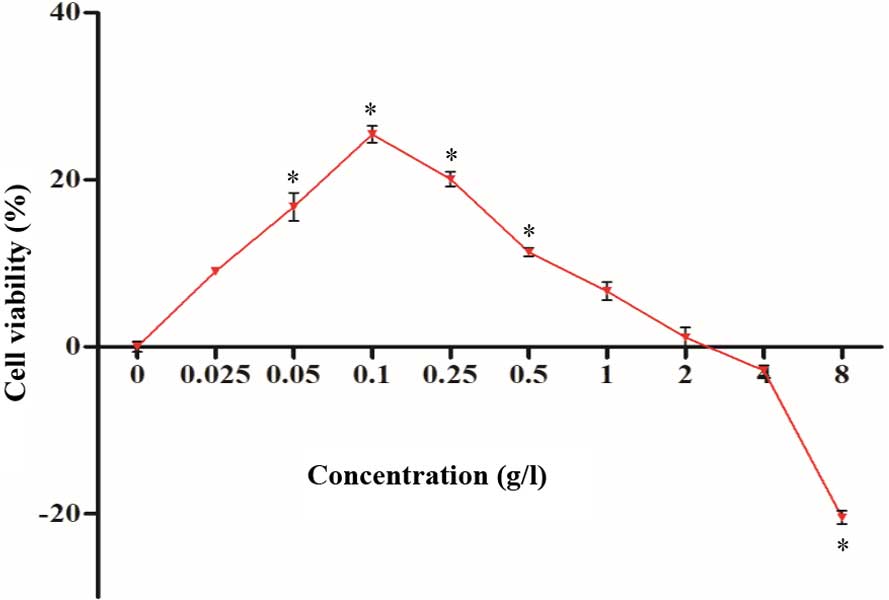
The cultured neurons were treated with RDPS for 48
h. MTT detection demonstrated that the neuronal cell viability was
markedly improved following treatment with 0.025–2.0 g/l RDPS, with
significant improvements being detected for cells treated with
0.05–0.5 g/l, as compared with the normal control (P<0.05;
Fig. 1). However, cytotoxicity was
detected following treatment of the neuronal cells with 8.0 g/l
RDPS (P<0.05; Fig. 1).
Effects of RDPS on
hypoxia/reoxygenation-cultured cerebral cortical neuronal cell
viability
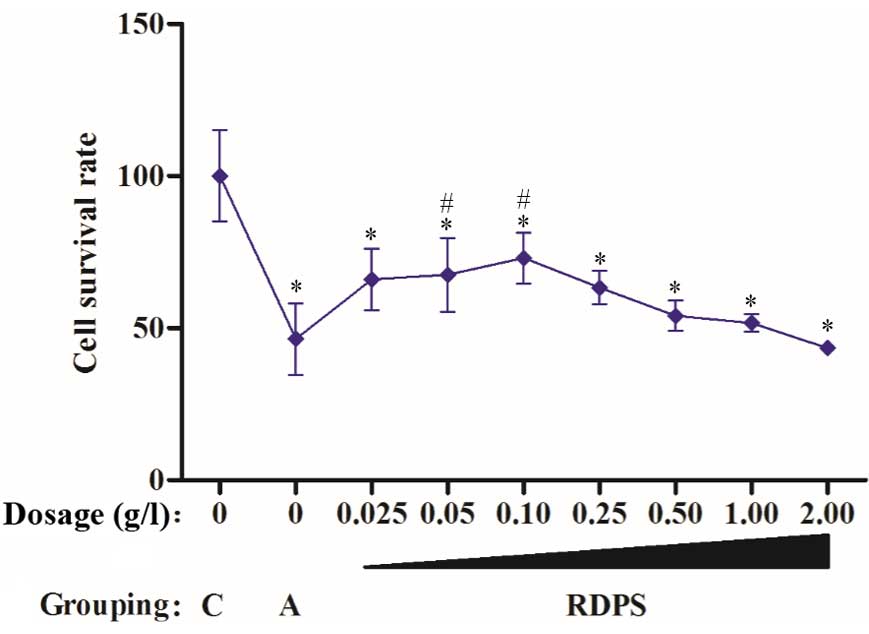
The cultured neurons were treated with RDPS for 48
h, after which the cells were cultured under hypoxic conditions for
12 h, followed by culturing under reoxygenating conditions for 24
h. The neuronal cell survival rate was markedly improved following
treatment with 0.025–1.0 g/l RDPS, and was significantly improved
following treatment with 0.05–0.10 g/l RDPS, as compared with the
apoptosis model (P<0.05; Fig. 2).
These results suggest that 0.05–0.10 g/l RDPS may protect neuronal
cells against hypoxia-induced apoptosis.
Effects of RDPS on
hypoxia/reoxygenation-induced cerebral cortical neuronal cell
apoptosis
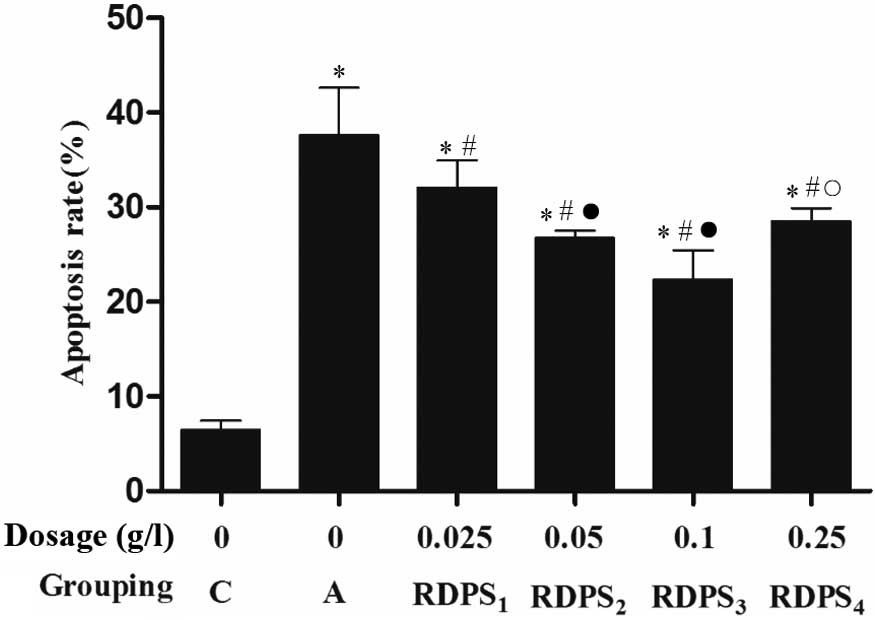
The neurons were treated with RDPS for 4 h, after
which the cells were cultured under hypoxic conditions for 12 h and
under reoxygenating conditions for 24 h. Hoechst 33342 fluorescence
staining demonstrated that treatment of the neuronal cells with
0.025–0.25 g/l RDPS, and particularly with 0.10 g/l RDPS,
significantly decreased the apoptotic rate, as compared with the
apoptosis model group (P<0.05; Fig.
3).
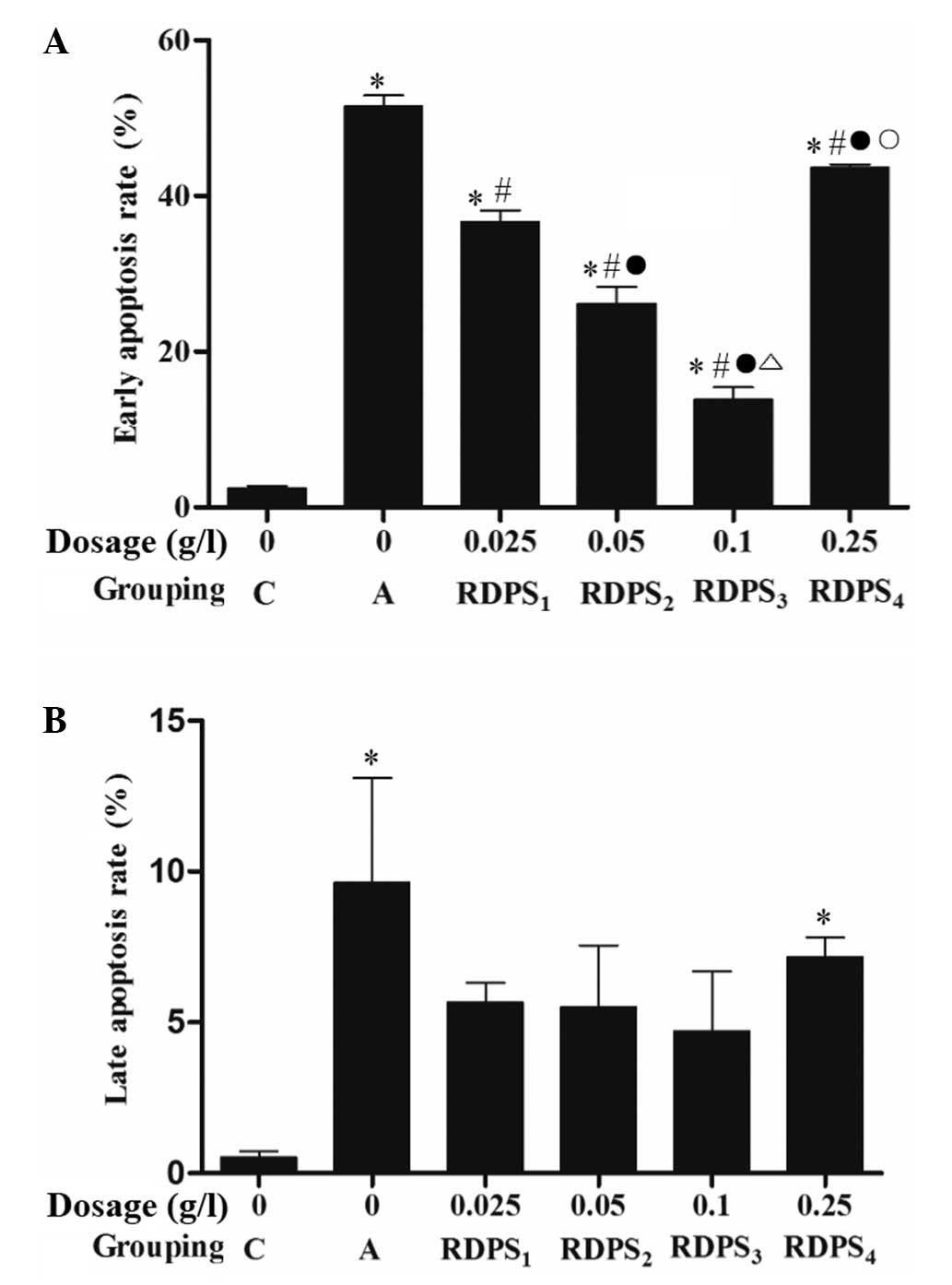
Annexin V/PI double staining demonstrated that
treatment with 0.025–0.25 g/l RDPS, in particular with 0.10 g/l,
significantly decreased the early apoptotic rate, as compared with
the apoptosis model group (P<0.05; Fig. 4A); however, there were no marked
differences in the rates of apoptosis at the late apoptotic stage
between the various RDPS-treated groups, as compared with that in
the apoptosis model group (P>0.05; Fig. 4B).
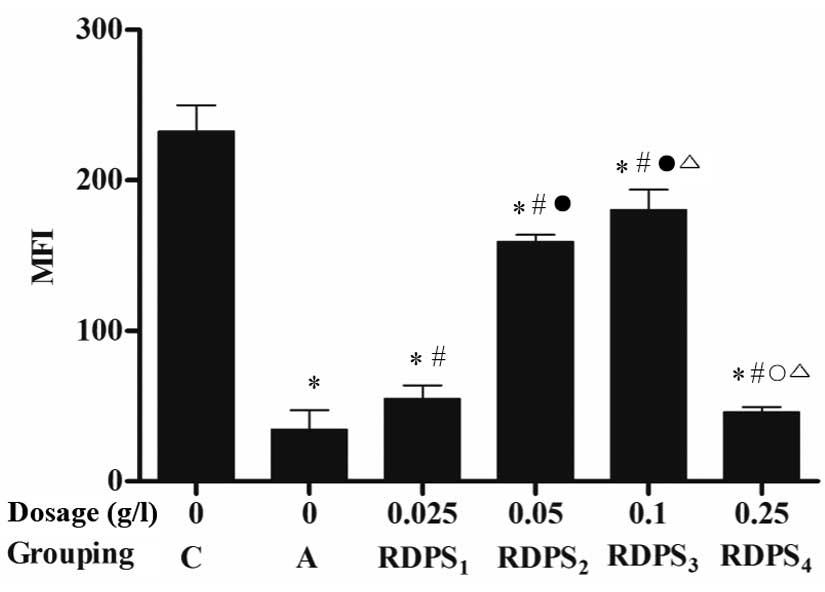
Treatment of the hypoxic neurons with 0.025–0.25 g/l
RDPS, and particularly 0.10 g/l RDPS, significantly increased the
mean fluorescence intensity (MFI) of Rhodamine 123 staining, as
compared with the apoptosis model group (P<0.05; Fig. 5). These results suggest that
treatment with RDPS attenuates hypoxia-induced mitochondrial injury
in neuronal cells.
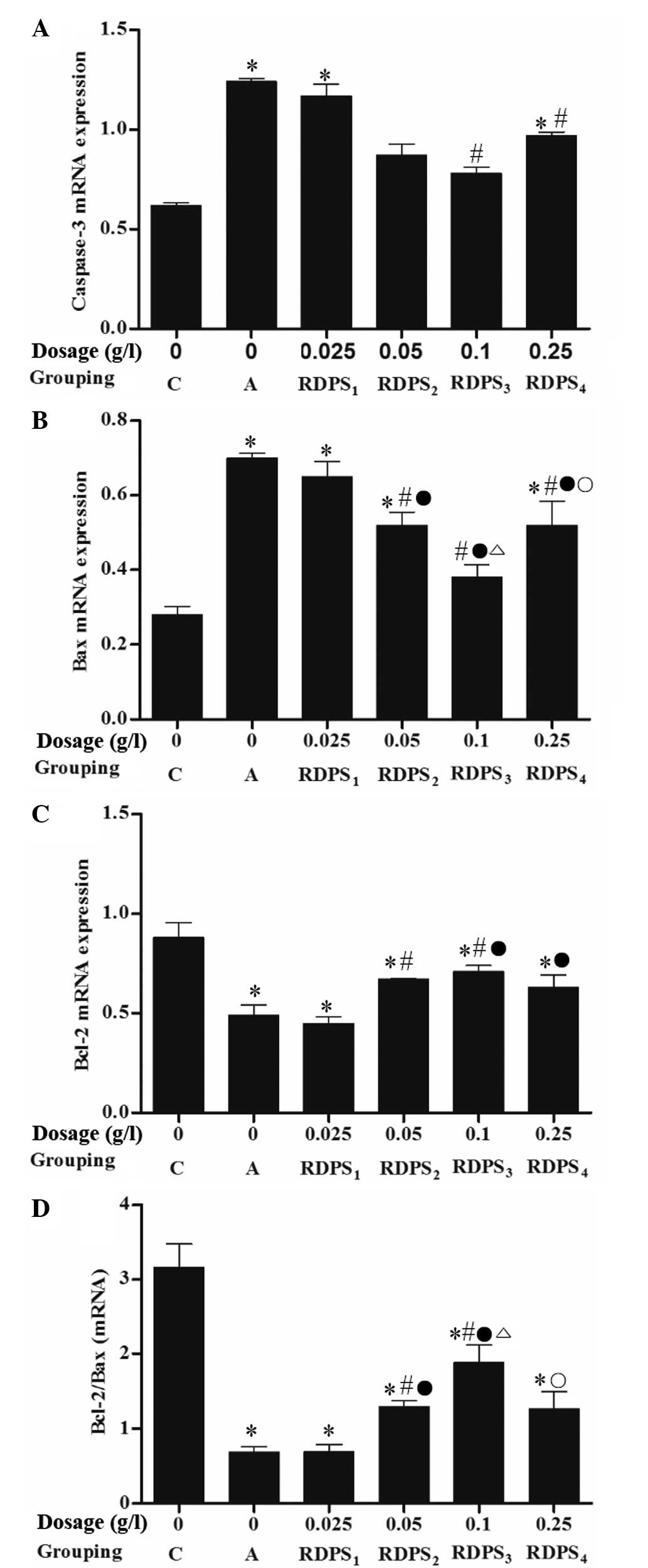
PCR demonstrated that the caspase-3 mRNA expression
levels in hypoxic neurons treated with 0.10–0.25 g/l RDPS, and the
Bax mRNA expression levels in hypoxic neurons treated with
0.05–0.25 g/l RDPS, were significantly decreased, as compared with
the apoptosis model group (P<0.05; Figs. 6 and 7). Conversely, the Bcl-2 mRNA expression
levels in hypoxic neurons treated with 0.05–0.10 g/l RDPS were
significantly increased, as compared with the apoptosis model group
(P<0.05; Figs. 6 and 7). Following treatment with 0.05–0.10 g/l
RDPS, the ratio of Bcl-2 mRNA:Bax mRNA was significantly increased
in the neurons cultured under hypoxia/reoxygenation conditions, as
compared with the apoptosis model group (P<0.05; Fig. 6). These results suggest that
decreased hypoxia-induced neuronal cell apoptosis following
treatment with RDPS may be due to a reduction in the expression
levels of apoptosis-regulating genes.
Immunocytochemical staining indicated that the
number of caspase-3-positive cells in the hypoxic neurons treated
with 0.025–0.25 g/l RDPS (in particular those treated with 0.10
g/l), and Bax-positive cells in the hypoxic neurons treated with
0.05–0.25 g/l RDPS (in particular 0.10 g/l) were significantly
decreased, as compared with the apoptosis model group (P<0.05;
Fig. 8A and B). Conversely, the
number of Bcl-2-positive cells in the hypoxic neurons treated with
0.05–0.25 g/l RDPS, particularly with 0.10 g/l, were significantly
increased, as compared with the apoptosis model group (P<0.05;
Fig. 8C). In addition, following
treatment with 0.025–0.25 g/l RDPS, the ratio of Bcl-2-positive
cells:Bax-positive cells was significantly increased in the neurons
cultured under hypoxia/reoxygenation conditions, as compared with
the apoptosis model group (P<0.05; Fig. 8D). These results suggest that
decreased hypoxia-induced neuronal cell apoptosis in the
RDPS-treated cells may be due to a decrease in the number of
caspase-3- and Bax-positive neurons, and an increase in the number
of Bcl-2-positive neurons, and the Bcl-2-positive:Bax-positive
neuronal ratio.
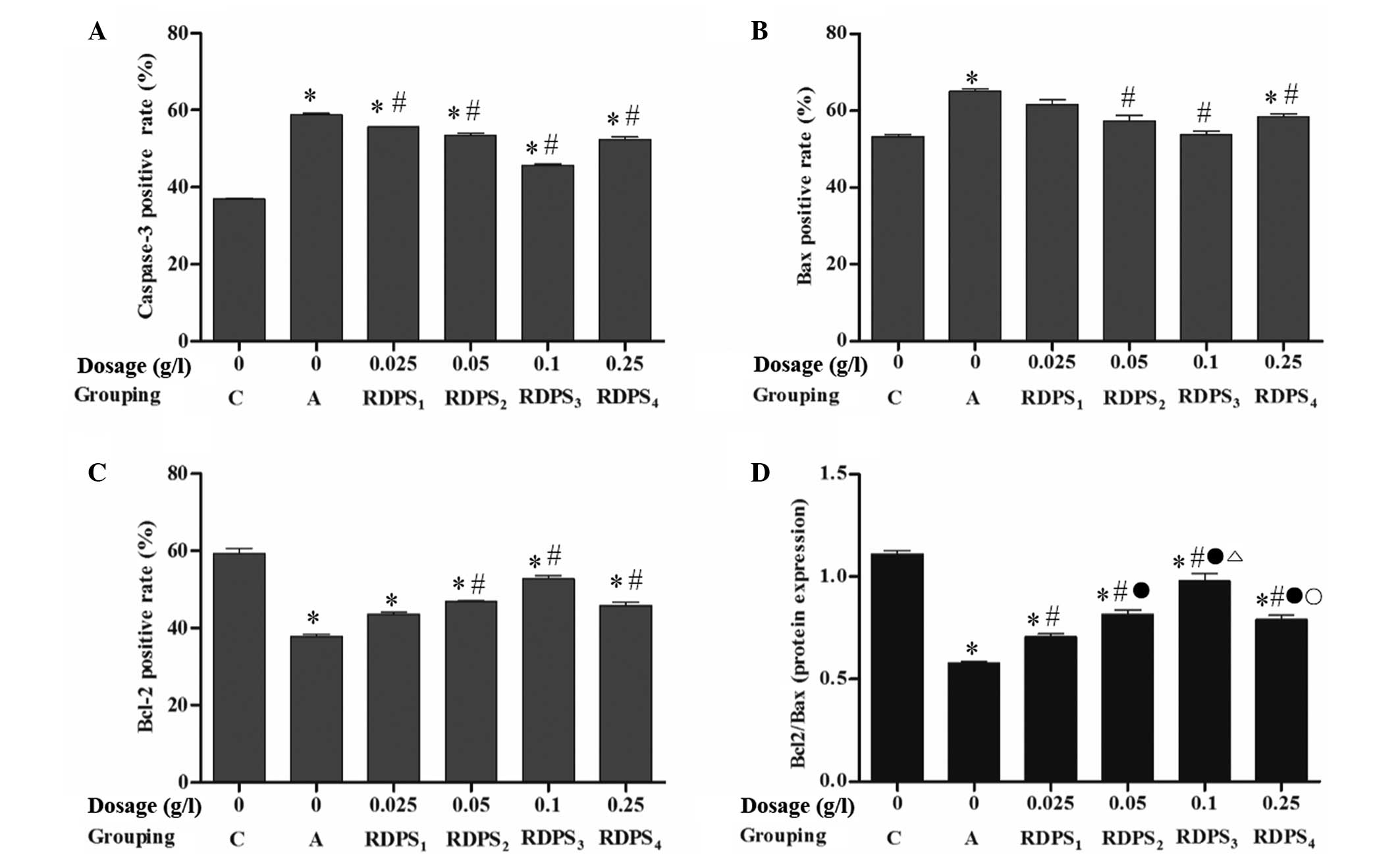 | Figure 8.Effects of RDPS on the number of (A)
caspase-3-, (B) Bax- and (C) Bcl-2-positive cells in hypoxic
neurons. Neuronal cells cultured under hypoxic conditions for 12 h,
under reoxygenating conditions for 24 h, were treated with 0,
0.025, 0.05, 0.10 or 0.25 g/l RDPS. The cells were analyzed using
immunocytochemical staining. Data are presented as the mean ±
standard deviation of triplicate experiments. *P<0.05 vs. the
normal control group; #P<0.05 vs. the apoptosis model
group; °P<0.05 vs. the RDPS1 group; ºP<0.05 vs. the RDPS3
group; ∆P<0.05 vs. the RDPS2 group. RDPS, Rhizoma
Dioscoreae polysaccharides; Bcl-2, B-cell lymphoma 2; Bax,
Bcl-2-associated X protein; C, the normal control group; A, the
apoptosis model group. |
Discussion
The pathological occurrence and development of a
stroke has previously been associated with the production of ROS,
which are produced at the mitochondrial membrane surface. Rhodamine
123 staining is widely used to measure alterations to mitochondrial
membrane potential, as it accumulates in the membrane in a manner
that is dependent on membrane polarization (12). In the present study, a reduction in
Rhodamine 123 MFI and cell viability, and an increase in the rate
of cell apoptosis, was detected in the neurons cultured under
hypoxic conditions. Treatment with RDPS significantly improved the
neuronal cell viability, attenuated mitochondrial injury, enhanced
the MFI of the mitochondria and decreased the rate of apoptosis in
the hypoxic neurons.
The result of the present study were consistent with
the hypothesis that RDPS exerts ROS scavenging activity: RDPS has
previously been shown to scavenge ROS and enhance the activities of
SOD, GSH-Px and Na+/K+-ATPase (4,5,7,8). In
addition, increased levels of oxidative stress, decreased levels of
GSH, catalase, GSH-Px and SOD, and induction of apoptosis, have
previously been detected in the brain tissue of a mouse model of
global cerebral ischemia/reperfusion (13). Intramitochondrial
Ca2+-dependent mitochondrial ROS production is a
molecular signal that culminates in the onset of mitochondrial
permeability transition (MPT), which may lead to apoptosis
(14,15). The opening of the MPT pore may
operate as a physiological Ca2+ release mechanism, and
may also contribute toward mitochondrial deenergization and the
release of pro-apoptotic proteins (15,16).
Appukuttan et al (17)
detected relocalization of soluble adenylyl cyclase to the
mitochondria, which was associated with the initiation of
mitochondrial depolarization, cytochrome c release, and
caspase-9/-3 cleavage and apoptosis, in cells cultured under
hypoxic/reoxygenating conditions.
Whether cell apoptosis occurs is dependent on the
balance between the expression of pro- and anti-apoptotic genes,
particularly the ratio of Bcl-2:Bax expression levels. In the
present study, the expression levels of caspase-3 and Bax were
significantly increased, and the expression levels of Bcl-2 and the
ratio of Bcl-2:Bax were significantly decreased, in neurons
cultured under hypoxic/reoxygenating conditions. However, RDPS was
demonstrated to significantly decrease the expression levels of
caspase-3 and Bax, and increase the expression levels of Bcl-2 and
the ratio of Bcl-2:Bax. These results suggested that RDPS may
attenuate hypoxia-induced neuronal cell apoptosis by decreasing the
mRNA and protein expression levels of apoptosis-initiating genes,
and increasing those of anti-apoptotic genes.
Members of the Bcl-2 family have previously been
demonstrated to function via conformation-induced insertion into
the outer mitochondrial membrane, in order to form channels or
pores that regulate the release of apoptogenic factors into the
cytosol. Furthermore, Bax heterodimerization with Bcl-2 was shown
to neutralize its pro-apoptotic activity. Bax monomers interact to
form an oligomeric channel that is permeable to cytochrome
c. The formation of this channel is blocked by Bcl-2 at
multiple sites; however, when Bax is present in excess, the
anti-apoptotic activity of Bcl-2 is antagonized, and apoptosis is
promoted (18).
Caspase-3 cleaves a protein with deoxyribonuclease
activity, and this cleavage activates a cascade of events that
culminate in the internucleosomal fragmentation of genomic DNA
(16). Previous studies have
detected increased expression levels of caspase-3 mRNA and Bax
protein, decreased Bcl-2 protein expression levels, and increased
cerebral infarct volumes in the brain tissue of rat models of
middle cerebral artery occlusion (19–21).
Similarly, Huang et al (22)
detected significantly increased expression levels of caspase-3/9,
and an elevated neurocyte apoptosis rate, in the brain tissue of a
C57BL/6 mouse model of bilateral common carotid artery
occlusion.
In conclusion, the present study demonstrated that
RDPS was able to improve the viability of hypoxic neuronal cells,
which may be associated with its effects on mitochondrial function
and the expression levels of apoptosis-regulating proteins. In
particular, RDPS was able to decrease the mRNA and protein
expression levels of Bax and caspase-3, and increase the mRNA and
protein expression levels of Bcl-2, culminating in an increase in
the ratio of Bcl-2:Bax in hypoxic neurons. The results of the
present study suggest that RDPS may be considered for the
prevention and treatment of ischemic cerebral diseases and
ageing.
Acknowledgements
The present study was supported by the Social
Development Key Research Project of the Jiangxi Provincial
Department of Science and Technology (grant no. 2007BS22602).
References
|
1
|
Yuan SL: Research advances on chemical
compositions and bioactivities of Dioscorea opposita Thunb. Shi Pin
Yan Jiu Yu Kai Fa. 29:176–179. 2008.(In Chinese).
|
|
2
|
Ko MK and Hong KE: Evaluation of the
safety of Sanyak (Dioscoreae rhizoma) pharmacopuncture according to
the extraction method: A double-blind randomized controlled trial.
J Acupunct Meridian Stud. 6:41–51. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sun S, Zhao J, Guan ST, Zhang H and Liu X:
Effect of yam polysaccharide on the contents of free radicals and
tumor necrosis factor α in mice of CCl4-induced liver injury. Shan
Xi Yi Ke Da Xue Xue Bao. 42:452–454. 2011.(In Chinese).
|
|
4
|
Zan T, Tao J and Wang SR: The antiaging
effects of water-soluable polysaccharide from Rhizoma Dioscorea
opposita on mice. Yao Xue Jin Zhan. 23:356–360. 1999.(In
Chinese).
|
|
5
|
Tang W, Zhu ML and Song MH: Experimental
studies on the effect of Chinese yam polysaccharides on anti-aging
of mice. Huang Gang Zhi Ye Ji Shu Xue Yuan Yuan Bao. 4:23–25.
2002.(In Chinese).
|
|
6
|
Lee JM, Namgung U and Hong KE:
Growth-promoting activity of Sanyak (Dioscoreae rhizoma) extract on
injured sciatic nerve in rats. J Acupunct Meridian Stud. 2:228–235.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shang XY, Ren J, Cao G, Xu CL, Niu WN and
Qin CG: Preparation and antioxidant properties of polysaccharides
from Dioscorea opposita thumb roots. Hua Xue Yan Jiu. 21:72–76.
2010.(In Chinese).
|
|
8
|
Xu XQ, Liu ZF, Huo NR, Zhao Y, Tian X and
Lei TT: In vitro anti-oxidation capacity and immunomodulatory
efficacy in mice of yam polysaccharide. Zhong Guo Liang You Xue
Bao. 27:42–46. 2012.(In Chinese).
|
|
9
|
Kesaraju S, Nayak G, Prentice HM and
Milton SL: Upregulation of Hsp72 mediates anoxia/reoxygenation
neuroprotection in the freshwater turtle via modulation of ROS.
Brain Res. 1582:247–256. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee KY, Bae ON, Weinstock S, Kassab M and
Majid A: Neuroprotective effect of asiatic acid in rat model of
focal embolic stroke. Biol Pharm Bull. 37:1397–1401. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hu WX, Xiang Q, Wen Z, He D, Wu XM and Hu
GZ: Neuroprotective effect of Atractylodes macrocephalaon
polysaccharides in vitro on neuronal apoptosis induced by hypoxia.
Mol Med Rep. 9:2573–2581. 2014.PubMed/NCBI
|
|
12
|
Huang M, Camara AK, Stowe DF, Qi F and
Beard DA: Mitochondrial inner membrane electrophysiology assessed
by rhodamine-123 transport and fluorescence. Ann Biomed Eng.
35:1276–1285. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ciftci O, Oztanir MN and Cetin A:
Neuroprotective effects of β-myrcene following global cerebral
ischemia/reperfusion-mediated oxidative and neuronal damage in a
C57BL/J6 mouse. Neurochem Res. 39:1717–1723. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim JS, Wang JH and Lemasters JJ:
Mitochondrial permeability transition in rat hepatocytes after
anoxia/reoxygenation: Role of Ca2+-dependent
mitochondrial formation of reactive oxygen species. Am J Physiol
Gastrointest Liver Physiol. 302:G723–G731. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Christophe M and Nicolas S: Mitochondria:
A target for neuroprotective interventions in cerebral
ischemia-reperfusion. Curr Pharm Des. 12:739–757. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Martin LJ: Mitochondrial and cell death
mechanisms in neurodegenerative diseases. Pharmaceuticals (Basel).
3:839–915. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Appukuttan A, Kasseckert SA, Micoogullari
M, Flacke JP, Kumar S, Woste A, Abdallah Y, Pott L, Reusch HP and
Ladilov Y: Type 10 adenylyl cyclase mediates mitochondrial Bax
translocation and apoptosis of adult rat cardiomyocytes under
simulated ischaemia/reperfusion. Cardiovasc Res. 93:340–349. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gustafsson AB and Gottlieb RA: Bcl-2
family members and apoptosis, taken to heart. Am J Physiol Cell
Physiol. 292:C45–C51. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang J, Li J, Lu J, Zhang Y, Zhu Z and Wan
H: Synergistic protective effect of astragaloside
IV-tetramethylpyrazine against cerebral ischemic-reperfusion injury
induced by transient focal ischemia. J Ethnopharmacol. 140:64–72.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
He B, Chen P, Yang J, Yun Y, Zhang X, Yang
R and Shen Z: Neuroprotective effect of 20(R)-ginsenoside Rg(3)
against transient focal cerebral ischemia in rats. Neurosci Lett.
526:106–111. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang G, Liu A, Zhou Y, San X, Jin T and
Jin Y: Panax ginseng ginsenoside-Rg2 protects memory impairment via
anti-apoptosis in a rat model with vascular dementia. J
Ethnopharmacol. 115:441–448. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang XP, Tan H, Chen BY and Deng CQ:
Astragalus extract alleviates nerve injury after cerebral ischemia
by improving energy metabolism and inhibiting apoptosis. Biol Pharm
Bull. 35:449–454. 2012. View Article : Google Scholar : PubMed/NCBI
|