Introduction
Ulcerative colitis (UC) is a chronic, non-specific
inflammatory disease that involves the rectum and colon of the
body; however, the etiology and pathogenesis of the disease have
yet to be fully elucidated. Studies have suggested that UC is
associated with a variety of factors, such as genetics, infection,
the immune system, and environmental and intestinal dysbiosis, as
well as damage to the intestinal tissues caused by immune cells and
inflammatory cytokines in the inflammatory signaling cascade
(1,2). In colonic mucosal lesions,
inflammation, mucosal congestion, edema and ulcers may be visible,
as well as the infiltration of immune cells (lymphocytes and
neutrophils) in the acute exacerbation stage (3). Following the administration of 5%
dextran sulfate sodium
(C6H7Na3O14S3,
DSS), mice gradually exhibit the symptoms of colitis, such as loose
stools, bloody diarrhea and weight loss, thus forming an
experimental colitis model similar to human UC (4).
Epigallocatechin-3-gallate (EGCG) is a type of
natural compound that can be extracted from green tea. EGCG has no
toxic effects but has a high biological activity and is known to
exert antibacterial, anti-inflammatory, anti-tumor, antioxidation
and anti-aging effects (5–8). Furthermore, it has been demonstrated
that EGCG can modulate the immune system to treat autoimmune
disease (9–11). EGCG is known to effectively treat
experimental colitis in mice, but its therapeutic mechanism
underlying this treatment is unclear (9,12). In a
previous study it was found that an imbalance in the number and
function of regulatory T cells (Tregs)/T helper 17 cells (Th17s)
and the secretion of certain cytokines, such as interleukin (IL)-6,
IL-10, IL-17 and transforming growth factor (TGF)-β1, had a crucial
role in the development of UC (13).
The aim of the present study was therefore to investigate the
mechanism of EGCG in the treatment of UC by measuring the levels of
proinflammatory [interleukin (IL)-6 and IL-17] and
anti-inflammatory [IL-10 and transforming growth factor (TGF)-β1]
cytokines in the plasma and the colonic protein expression of
hypoxia-inducible factor (HIF)-1α and signal transducer and
activator of transcription (STAT) 3 in a mouse model of colitis.
The Treg/Th17 balance was also examined.
Materials and methods
Experimental animals
Forty male BALB/c mice (age, 6–7 weeks; weight,
22–26 g) of specific pathogen-free status were obtained from the
China Experimental Animal Center of Southern Medical University
(Guangzhou, China; certificate of conformity no. SCXK Guangdong
2011-0015) for use in the present study. The mice were housed in
clean animal houses at a controlled temperature (22–25°C) and a
relative humidity of 55%, and were subjected to a 12-h light/dark
cycle. Food and drinking water were available ad libitum.
This study was carried out in strict accordance with the
recommendations in the Guide for the Care and Use of Laboratory
Animals of the National Institutes of Health. The animal use
protocol was reviewed and approved by the Institutional Animal Care
and Use Committee of the Second Clinical Medical College of Jinan
University (Shenzhen, China).
Treatment program
The 40 mice were fed with adaptability for one week,
and then randomly divided into four groups (n=10/group): Normal
control (NC), model (MD), EGCG (Qiyun Biotechnology Co., Ltd.,
Guangzhou, China) 50 mg/kg/day treatment (ELD) and EGCG 100
mg/kg/day treatment (EHD). The NC group was given free access to
sterile distilled water for 14 days, while the MD and EGCG
treatment groups received 5% DSS (Qiyun Biotechnology Co., Ltd.)
continuously for seven days; the DSS was replaced by distilled
water for seven days following the successful establishment of the
model. On day 8 of the experiment, the mice in the NC and MD groups
were given a daily gavage of 0.2 ml 0.5% ethanol for seven days,
while the ELD and EHD groups were administered EGCG at doses of 50
and 100 mg/kg/day for each mouse, respectively. A total of 0.2 ml
0.5% ethanol was then used to dissolve the EGCG for gavage
treatment for seven days. On the day 14 the mice were administered
100 mg/kg pentobarbital anesthesia, and eyeball blood was obtained
following the sacrifice of the mice. Blood plasma and spleen and
colon tissues were collected under sterile conditions.
Macroscopic and microscopic
assessment
The disease activity index (DAI) and spleen index
(SI) of the mice were measured and recorded daily, with reference
to the Murthy scoring system (14).
Details of the scoring system are shown in Table I. The DAI was calculated with the
following formula: DAI = (weight loss rate score + stool
consistency score + presence of blood in stools score)/3. The
spleens of the mice were washed with saline, and then dried and
weighed for the calculation of the SI using the following formula:
SI = spleen weight (mg)/body weight (g).
 | Table I.Murthy scoring system (10). |
Table I.
Murthy scoring system (10).
| Scores | Weight loss rate
(%) | Stool
stickiness | Presence of blood
in stool |
|---|
| 0 | (−) | Normal (granular,
with form) | Normal |
| 1 | 1–5 |
|
|
| 2 |
6–10 | Soft (mushy, not
adhering to the anus) | Occult blood
(+) |
| 3 | 11–15 |
|
|
| 4 | >15 | Diarrhea (watery,
adhering to the anus) | Bloody stools
(+) |
Specimen collection and pathology
assessment
The mice were sacrificed following pentobarbital
anesthetization and the abdominal cavity was opened. Measuring from
the anus, a 10-cm section of colon was cut along the longitudinal
axis of the mesentery and rinsed repeatedly with iced saline. The
section was then cut vertically into two parts, taking the lesion
as the center in the longitudinal axis. One colon sample was
immediately placed in 10% formalin solution at room temperature and
fixed for 48 h, prior to undergoing tissue dehydration, clearing,
embedding and staining with hematoxylin and eosin. The colon tissue
morphology was subsequently observed by microscopy. For the second
section of the colon tissue, the lesion was excised, dried by
filter paper, weighed and frozen at −70°C for tissue specimen
tests.
Lymphocyte extraction from spleen
tissue
Under sterile conditions, the spleen of each mouse
was obtained for lymphocyte separation. A 5-ml volume of lymphocyte
separation medium (Shenzhen Lvshiyuan Biotechnology, Co., Ltd.,
Shenzhen, China) was added to 35-mm petri dishes with homogenized
spleen tissue. Following the separation of the suspension, spleen
cell fluid was immediately transferred to a 15-ml centrifuge tube
with 200–500 µl RPMI-1640 medium (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). Samples were centrifuged at 800 × g for 30 min
at room temperature. The lymph cell layer was removed by suction
and 10 ml RPMI-1640 medium was added for reverse washing. The
samples were centrifuged at 250 × g for 10 min at room temperature,
and the lymphocytes were subsequently collected. The number of
lymphocytes was then adjusted to 2×106/reaction
tube.
Flow cytometry
The lymphocyte samples (2×106/reaction
tube) were analyzed using flow cytometry with a Th17/Treg kit (BD
Biosciences, Franklin Lakes, NJ, USA) according to the
manufacturer's instructions. A total of 50 ng/ml phorbol
12-myristate 13-acetate (for the lymphocytes), 1 µg/ml ionomycin
and monensin were used to stimulate the cells for 5 h. After 5 h,
the cells were collected, stained, fixed and lysed in strict
accordance with the kit instructions, and 20 µl streaming
antibodies were added to each tube in turn, mixed and incubated for
30 min. The main antibodies used in the flow cytometry included
mouse cluster of differentiation 4 (CD4)-PerCP-Cyanine5.5,
IL-17A-phycoerythrin (PE) and Alexa Fluor 647-forkhead box protein
P3 (Foxp3). A parallel negative control group was set, and 0.2 mg/l
PE-rat immunoglobulin (Ig)G1 and 0.2 mg/l Alexa Fluor 647-Rat IgG2b
were added. The samples were loaded and the data were analyzed.
ELISA
Blood was obtained from the mice and treated with
heparin to prevent coagulation, prior to the supernatant being
centrifuged for 5 min at 1,000 × g (4°C). A total of 50 mg mouse
colon tissue was weighed using an electronic analytical balance and
cut into pieces as soon as possible with a small pair of
ophthalmological scissors. Following cutting, 1,200 µl
physiological saline at 0°C was added and the tissue was fully
homogenized on ice using a glass homogenizer and centrifuged for 10
min using a refrigerated high-speed centrifuge (Sigma, St. Louis,
MO, USA) at 4°C (1,000 × g). The supernatant was obtained, and
protein quantification was then performed using a NanoDrop™ 2000
spectrophotometer (Thermo Fisher Scientific, Inc.). According to
the specifications of the ELISA kit (Dakota Biotech Co. Ltd.,
Beijing, China), the IL-6, IL-10, IL-17 and TGF-β1 concentrations
in the plasma and colon tissues of the mice were detected.
Western blot analysis
A total of 100 mg mouse colon tissue was accurately
weighed using an electronic analytical balance, and plasma and
nuclear proteins were extracted from the cells using a nucleus and
cytoplasm tissue extraction kit in accordance with the
manufacturer's instructions (Thermo Fisher Scientific, Inc.). The
protein samples were quantified using a NanoDrop 2000
spectrophotometer and the protease inhibitor phenylmethylsulfonyl
fluoride was added. The proteins were then separated using SDS-PAGE
(10%) and transferred to a nitrocellulose membrane (Beijing Bayer
Di Biotechnology, Co., Ltd., Beijing, China). The membrane was
blocked with Western blocking buffer (P0023B) and incubated with
the primary and secondary antibodies, for 1 h each, and
subsequently developed using enhanced chemiluminescence for
analysis. Western washing liquid (P0023C) was used to wash the
membrane between incubations. Anti-mouse polyclonal STAT3 (1:500;
Bioworld Technology, Inc., St. Louis Park, MN, USA) and HIF-1α
(1:1,000; Cell Signaling Technology, Inc., Danvers, MA, USA)
primary antibodies were used to analyze the nuclear HIF-1α and
cytoplasmic STAT3 protein levels. Goat anti-rabbit horseradish
peroxidase secondary antibodies (1:2,000; Jackson ImmunoResearch
Laboratories, Inc., West Grove, PA, USA) were incubated with the
membranes with phosphate-buffered saline, containing 0.1% Tween-20,
5% w/v skimmed milk powder and 2% BSA diluent/blocking solution,
for 1 h at room temperature. In addition, levels where tested
against lamin or β-actin (Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA).
Statistical analysis
Experimental data are expressed as the mean ±
standard deviation. The statistical analysis of the experimental
data was performed using SPSS statistical software, version 20.0
(IBM SPSS, Armonk, NY, USA). The differences between the groups
were compared using single-factor analysis of variance, and
P<0.05 was considered to indicate a statistically significant
difference.
Results
Assessment of DAI and SI
No animals died during the experiment. During the
course of the experiment the activity, body weight, stool
consistency and presence of blood in the stools of the mice were
observed and recorded daily. The score of the NC group was 0.
Blood, weight loss and significant decreases in activity were
visible in the MD, ELD and EHD groups following the establishment
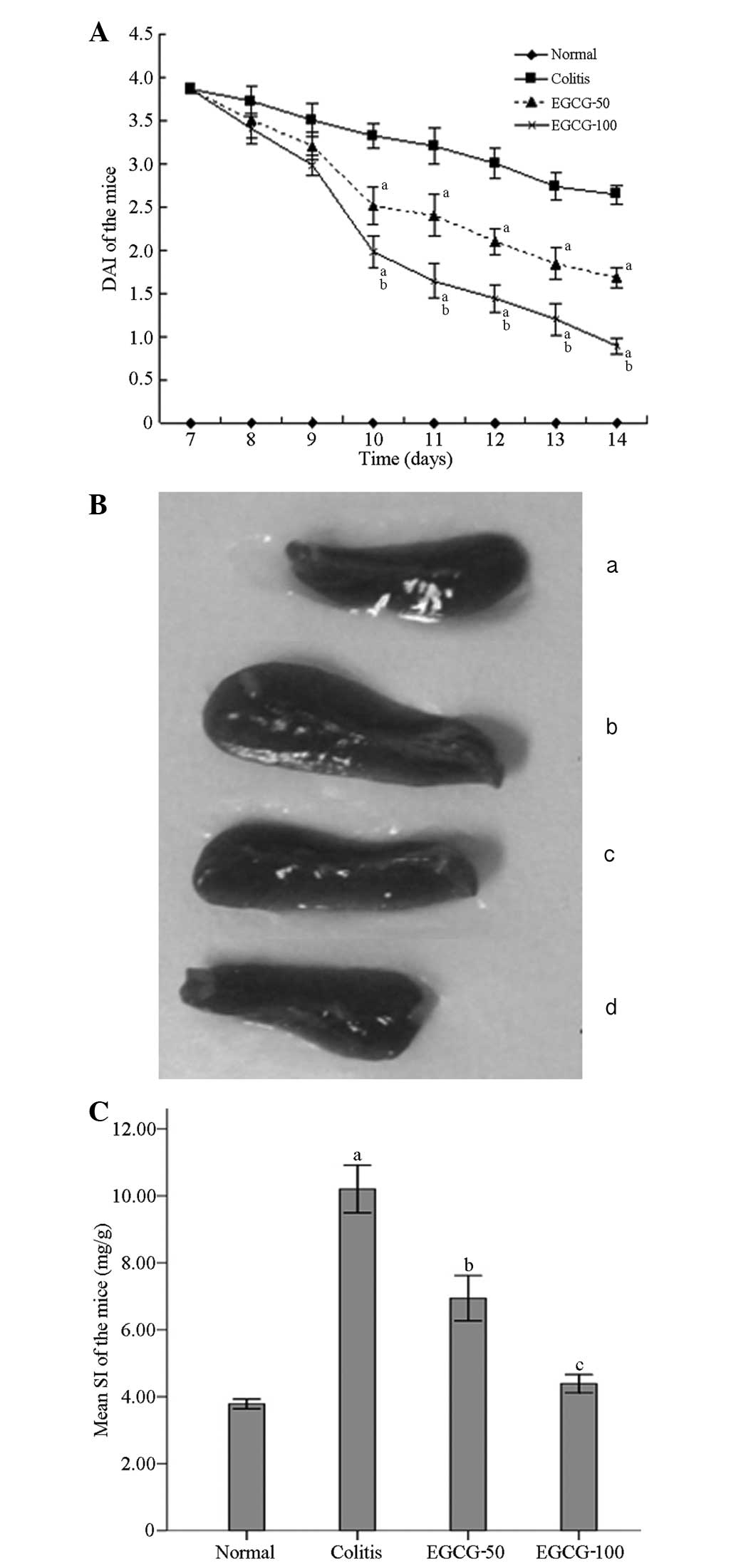
of the model using 5% DSS for seven days. As shown in Fig. 1A, the presence of blood in the
stools, the rate of weight loss and the DAI scores were
significantly reduced in the ELD and EHD groups between days 10 and
14 compared with those of the MD group. Compared with the score in
the ELD group, the DAI was decreased more significantly in the EHD
group. The spleen in the MD group was enlarged compared with that
in the NC group, while the spleen size and SI were significantly
reduced in the ELD and EHD groups as compared with the MD group
(Fig. 1B and C). The SI in the EHD
group was decreased to a greater extent than that in the EDL group
(Fig. 1C).
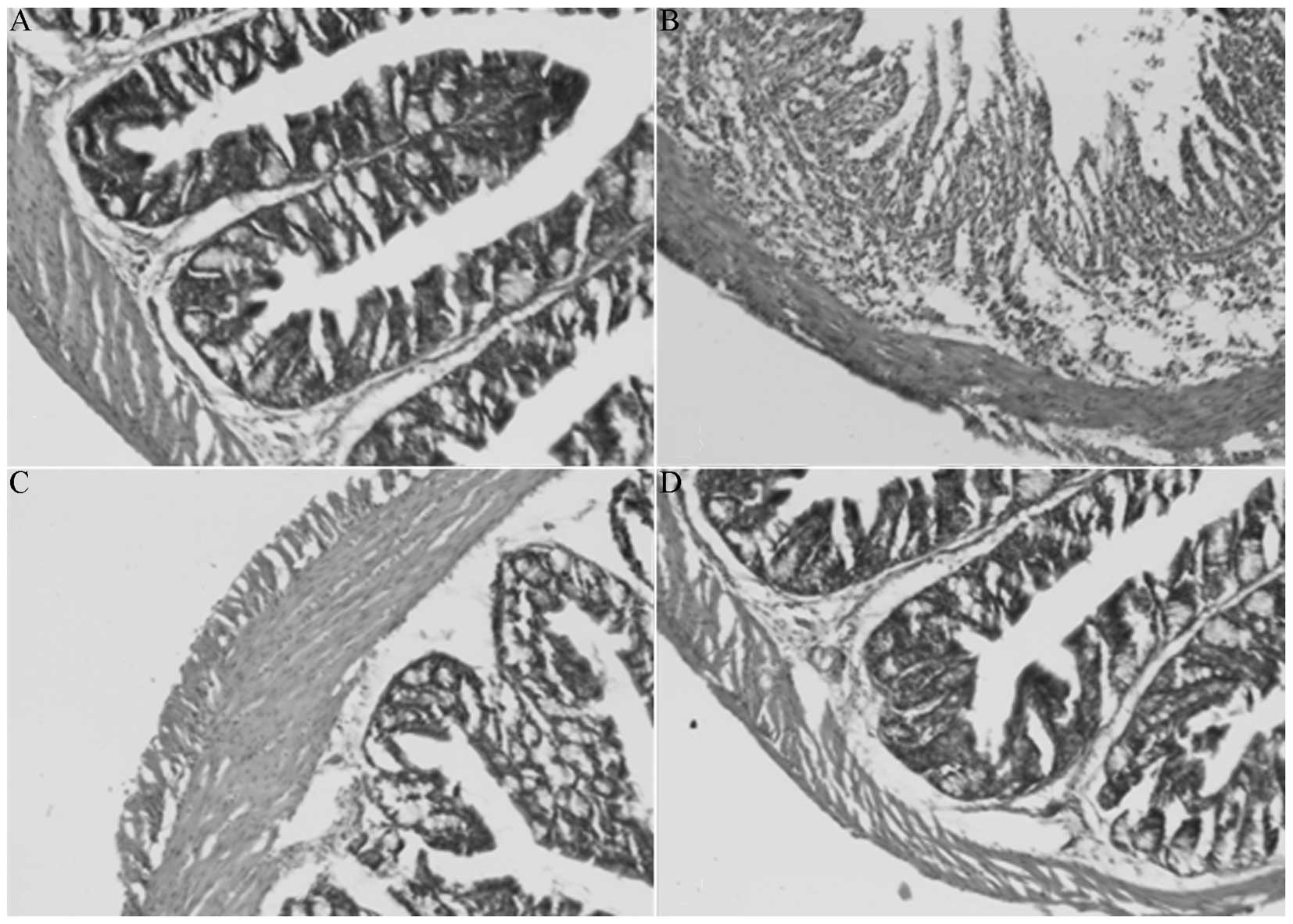
Pathological assessment
Compared with the NC group (Fig. 2A), the MD group (Fig. 2B) exhibited visible pathological
manifestations, including acute inflammation accompanied by mucosal
erosion, as well as edema, crypt reduction and the infiltration of
inflammatory cells, such as neutrophils, in the muscularis propria
and mucosa. Compared with the MD group, the colonic mucosal
inflammatory cell infiltration, erosion and edema in the ELD
(Fig. 2C) and EHD (Fig. 2D) groups were significantly
improved.
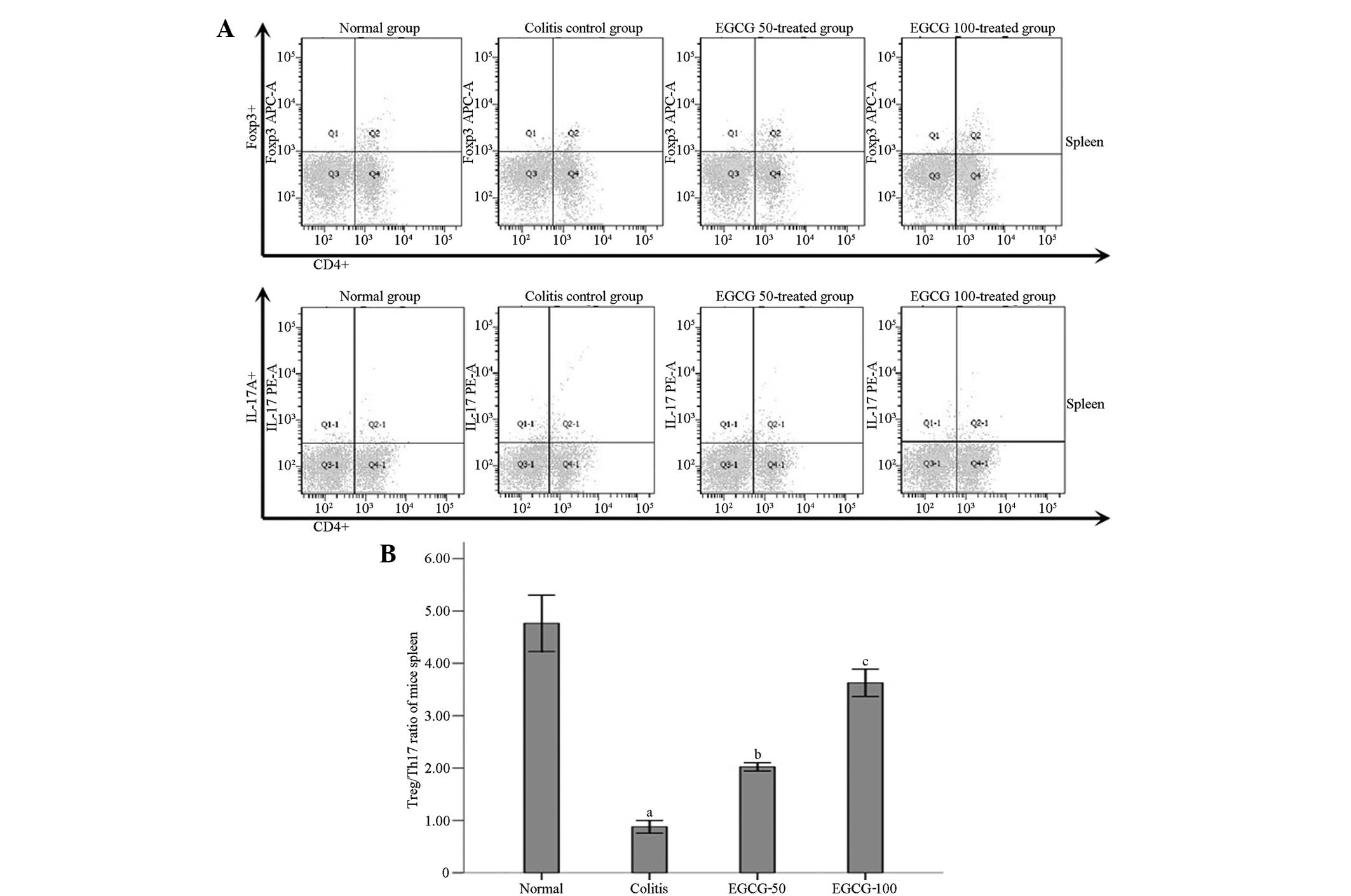
Treg/Th17 ratio
Compared with the NC group, the
CD4+IL-17+ (Th17)/CD4+ lymphocyte
ratio was significantly elevated in the MD group, while the
CD4+Foxp3+ (Treg)/CD4+ lymphocyte
ratio and the Treg/Th17 ratio were decreased significantly
(P<0.001) (Fig. 3). Compared with
the MD group, the levels of Th17s in the ELD and EHD groups were
decreased and the Treg/Th17 ratio was significantly increased
(P<0.001). The difference in the Treg/Th17 ratio between the EHD
and NC groups was not statistically significant (P=0.674), and a
statistically significant difference was also found in the ratio
between the ELD and NC groups (P=0.006). Compared with the ELD
group, the Treg/Th17 ratio in the EHD group showed a significant
increase (P<0.001).
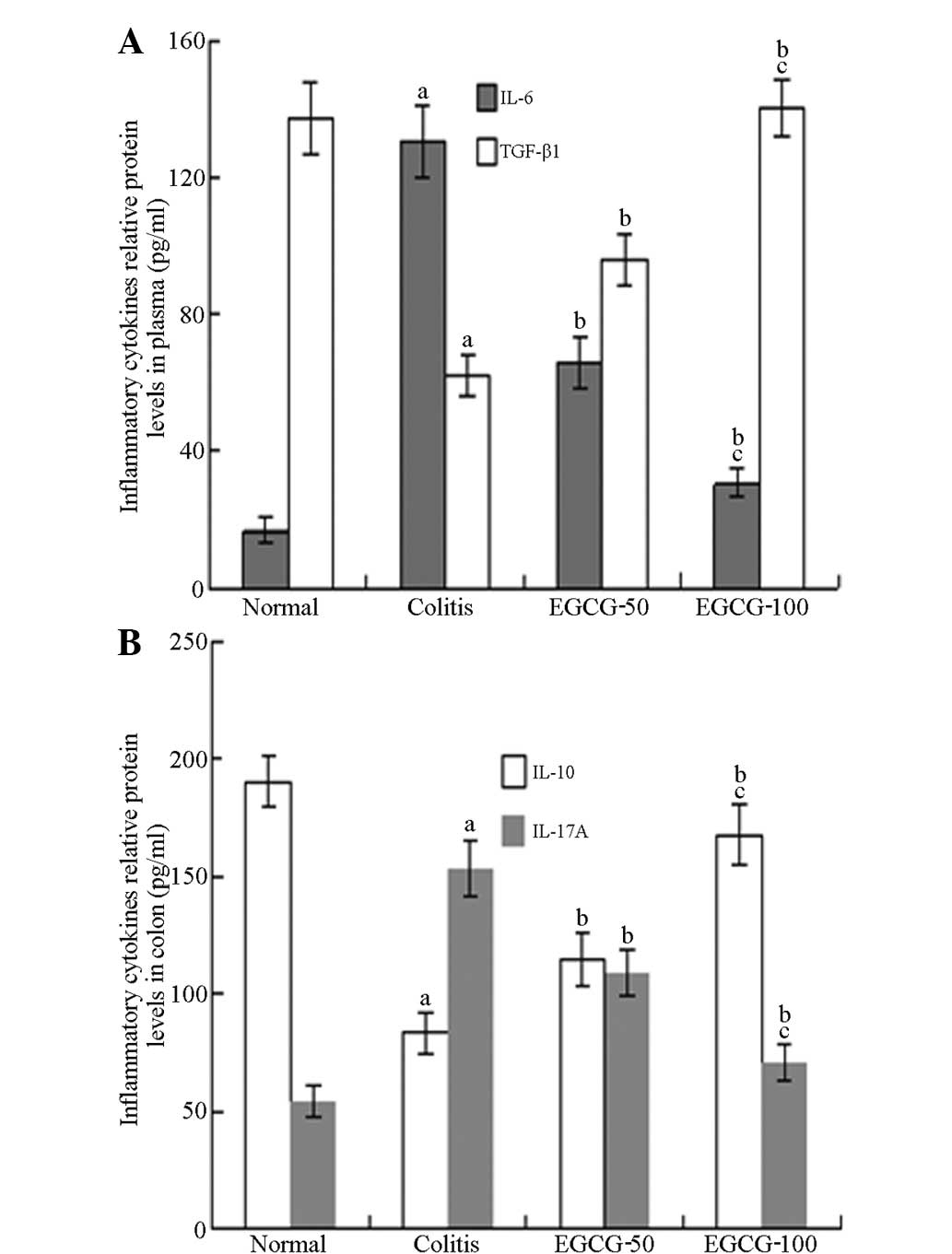
Cytokines
Compared with the NC group, the IL-6 and IL-17
levels of the MD group were significantly increased, while the
IL-10 and TGF-β1 levels were significantly decreased. Compared with
the MD group, the IL-6 and IL-17 levels in the ELD and EHD groups
were decreased while the IL-10 and TGF-β1 levels were significantly
increased. Compared with the ELD group, the IL-6 and IL-17 levels
in the EHD group were decreased to a greater extent, while the
IL-10 and TGF-β1 levels were increased to a greater extent
(Fig. 4).
Western blot analysis
As presented in Fig.
5, the nuclear HIF-1α and cytoplasmic STAT3 protein expression
in the MD group was significantly increased compared with that in
the NC group. Compared with the MD group, the expression of the
proteins in the ELD and EHD treatment groups was decreased
significantly, with a greater decrease observed in the EHD
group.
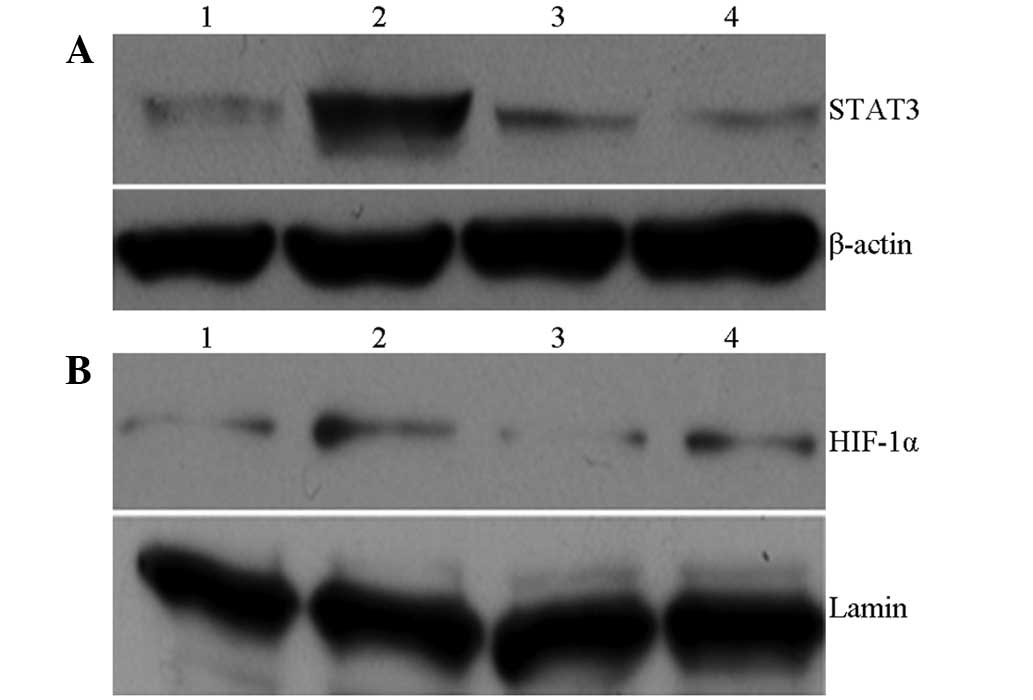 | Figure 5.Effect of EGCG on cell signaling
pathway proteins: (A) STAT3 and (B) HIF-1α. (A) Lane 1, normal
group; lane 2, colitis (model) group; lane 3, EGCG-50 group; lane
4, EGCG-100 group. (B) Lane 1, normal group; lane 2, colitis
(model) group; lane 3, EGCG-100 group; lane 4, EGCG-50 group. EGCG,
epigallocatechin-3-gallate; EGCG-50, EGCG at 50 mg/kg/day;
EGCG-100, EGCG at 100 mg/kg/day; STAT3, signal transducer and
activator of transcription 3; HIF-1α, hypoxia-inducible
factor-1α. |
Discussion
UC is a chronic inflammatory disease of the
intestine. The exact pathogenesis of the disease is not fully
understood, but it is believed to involve the abnormal activation
of the innate and adaptive immune systems. The immune inflammatory
response exhibits excessive hyperactivity, which negatively affects
the self-limiting nature of the reaction (15,16).
Immune cells and cell factors involved in intestinal tissue are
imbalanced and lead to immune injury (15,16). It
has previously been suggested that UC is mediated by Th2 cells
(17–21), while other studies have also
indicated that the Thl/Th2 axis is fundamental to the pathogenesis
of UC (22–25); however, the identification of Th17s
confirmed the immune bypass in UC and explained the anomalies of
the traditional Th1/Th2 imbalance (26–28). The
rebalancing of the Treg/Th17 ratio is considered to be an important
treatment strategy for UC (29,30). It
is known that Treg/Th17 imbalance is common in a variety of
autoimmune disorders, and that human inflammatory bowel disease
(IBD) is associated with Tregs, Th17s and the secreted cytokines.
The Treg/Th17 imbalance may therefore be a possible target for the
treatment of IBD (2,31,32). IBD
includes UC and Crohn's disease. The findings of the present study
further confirmed the existence of a Treg/Th17 ratio imbalance in
the mouse model of experimental colitis, a model reflecting human
UC, and were consistent with the results of previous reports
(33–35). Previous studies have found that EGCG
can treat UC, but the exact mechanism has yet to be fully
elucidated. We hypothesized that EGCG could treat UC by mediating
the rebalancing of the Treg/Th17 ratio, and attempted to explore
the specific mechanisms underlying the EGCG-induced adjustments in
the Treg/Th17 ratio.
Th17s are involved in the immune pathological
process of UC, possibly by releasing IL-17, IL-21, IL-22 and other
inflammatory cytokines (36). Tregs
are a CD4+ T-cell subset with immunosuppressive
activity. These cells inhibit the intestinal mucosal inflammation
cascade and inflammatory reaction amplification effect by
regulating the secretion of IL-10, TGF-β1 and other
anti-inflammatory cytokines and maintain intestinal immune balance
(37,38). Tregs act antagonistically with Thl7s
to maintain the relative stability of the body's immune status, so
that the body is held in a delicate and complex balance. Tregs
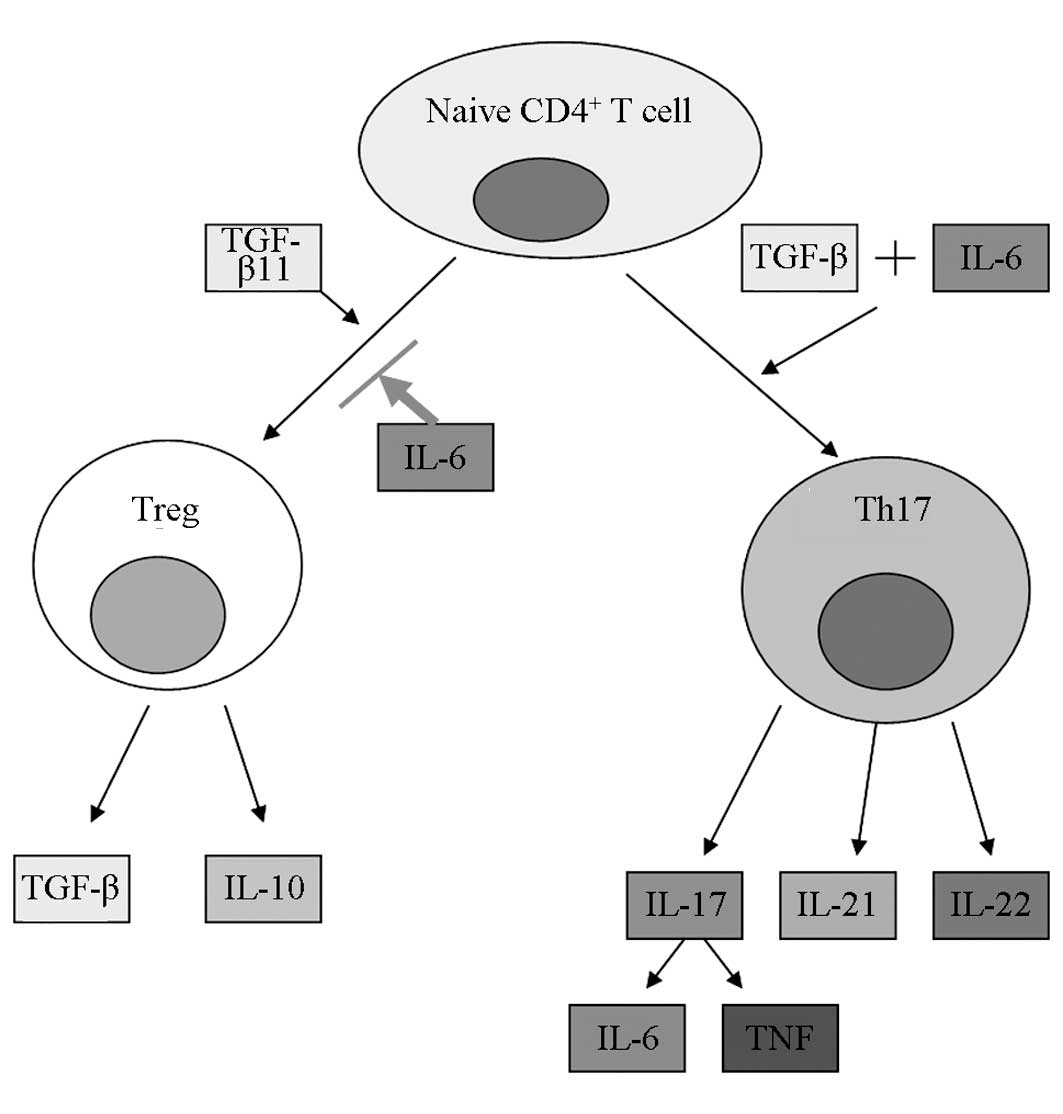
undergo a similar differentiation process to Thl7s. It has been
demonstrated that naive CD4+ T cells can differentiate
into Tregs following stimulation from TGF-β1 alone, but, in the
presence of IL-6, TGF-β1 and IL-6 act together to induce the
expression of the transcription factor retinoic acid-related orphan
receptor γt (RORγt), thus suppressing the production of Tregs and
promoting the naive CD4+ T cells to differentiate into
Th17s (39–41) (Fig.
6). At low concentrations, TGF-β1 acts synergistically with
IL-6 to induce RORγt generation; at high concentrations, TGF-β1 can
regulate the expression of Foxp3. The balance between Foxp3 and
RORγt could determine whether the naive T cells differentiate into
Th17s or Tregs following stimulation by antigens (42). In the absence of IL-6, TGF-β1 does
not induce the differentiation of the naive T cells into Th17s, but
instead promotes the differentiation to Tregs (43). In the absence of TGF-β, Th17s can
also be generated following stimulation by IL-6 or the combination
of IL-23 and IL-1β (44). IL-6 can
additionally inhibit the TGF-β-induced generation of Tregs
(45).
It has previously been shown that two pathways,
hypoxia-mammalian target of rapamycin-HIF-1α-Th17 and
IL-6-STAT3-HIF-1α-Th17, have a crucial role in Treg/Th17 imbalance
(26). In the first pathway, UC
leads to a sustained hypoxic state in the intestine, and the local
tissue hypoxia induces HIF-1α synthesis and promotes its entry into
the nucleus to exert its biological functions, resulting in the
upregulation of Th17 activity, the promotion of Foxp3 binding with
ubiquitin for degradation, and then the downregulation and
inhibition of Tregs (31,32,46). In
the second pathway, UC causes increases in the levels of
inflammatory cytokines, such as IL-6, leading to the upregulation
and activation of HIF-1α by the IL-6/STAT3 signaling pathway. STAT3
can also inhibit the ubiquitination and degradation of HIF-1α and
prolong its half-life, in addition to increasing the protein
expression of HIF-1α; as a consequence, increased HIF-1α is
available to activate Th17s (47–49).
IL-6 promotes the differentiation of Th17s (39,50–52), and
the activated Thl7s secrete proinflammatory cytokines such as IL-17
(53–55). IL-17 can further promote the release
of cytokines, such as IL-6 and TNF, and the activation of other
inflammatory cells (56). The
waterfall-like inflammatory cell and cytokine cascade may be
central to the disease progression of patients with UC (13). Reducing the concentration of IL-6
could reduce the induced expression of RORγt and inhibit the
IL-6-STAT3-HIF-1α-Th17 pathway, thereby reducing the generation of
Th17 cells (39,57). The waterfall-like effects could thus
be interrupted and the inhibition of Treg generation could be
reduced, thereby regulating the Treg/Th17 balance. EGCG has been
found to reduce the generation of IL-6 in patients with IBD
(58,59). Since IL-6 plays such an important
role in the mechanism of Treg/Th17 imbalance, we speculated that
EGCG could regulate the Treg/Th17 balance by reducing IL-6 levels
in a mouse model of experimental colitis.
In the present study it was found that, compared
with the MD group, the IL-6 levels in the ELD and EHD groups were
significantly reduced, with a more significant decrease in the EHD
group. This demonstrated that EGCG could significantly reduce the
release of IL-6 in mice with experimental colitis. It could be
concluded that EGCG reduced the induced expression of RORγt by
downregulating the cytokine IL-6 and inhibiting STAT3 and HIF-1α
protein expression, thus reducing the generation of Th17s and
simultaneously reducing the inhibition of IL-6 on Tregs, and
ultimately leading to the rebalancing of the Treg/Th17 ratio and
treating mice with experimental colitis. This mechanism would
explain the findings in the present study. The experiments also
confirmed that the effect of EGCG treatment was dose-related, as
the high-dose therapy produced superior results to the low-dose
therapy. In conclusion, EGCG represents a natural medicine that
exhibits potential for clinical application in the treatment of
UC.
Acknowledgements
This study was supported by the Guangdong Provincial
Natural Science Foundation of China (no. 10151802001000002) and the
Shenzhen Key Science and Technology Projects (no. 200901003). The
authors would like to thank the Southern Medical Cell and
Microbiology Laboratory.
References
|
1
|
Sartor RB: Therapeutic manipulation of the
enteric microflora in inflammatory bowel diseases: antibiotics,
probiotics, and prebiotics. Gastroenterology. 126:1620–1633. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lei X, Liu M, Yang Z, et al: Thymoquinone
prevents and ameliorates dextran sulfate sodium-induced colitis in
mice. Dig Dis Sci. 57:2296–2303. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lichtenstein GR and Rutgeerts P:
Importance of mucosal healing in ulcerative colitis. Inflamm Bowel
Dis. 16:338–346. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yao J, Wang JY, Lai MG, et al: Treatment
of mice with dextran sulfate sodium-induced colitis with human
interleukin 10 secreted by transformed Bifidobacterium longum. Mol
Pharm. 8:488–497. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zaveri NT: Green tea and its polyphenolic
catechins: Medicinal uses in cancer and noncancer applications.
Life Sci. 78:2073–2080. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Higdon JV and Frei B: Tea catechins and
polyphenols: Health effects, metabolism, and antioxidant functions.
Crit Rev Food Sci Nutr. 43:89–143. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang CS, Maliakal P and Meng X: Inhibition
of carcinogenesis by tea. Annu Rev Pharmacol Toxicol. 42:25–54.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Caderni G, De Filippo C, Luceri C,
Salvadori M, Giannini A, Biggeri A, Remy S, Cheynier V and Dolara
P: Effects of black tea, green tea and wine extracts on intestinal
carcinogenesis induced by azoxymethane in F344 rats.
Carcinogenesis. 21:1965–1969. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brückner M, Westphal S, Domschke W,
Kucharzik T and Lügering A: Green tea polyphenol
epigallocatechin-3-gallate shows therapeutic antioxidative effects
in a murine model of colitis. J Crohns Colitis. 6:226–235. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Navarro-Perán E, Cabezas-Herrera J,
Sánchez-Del-Campo L, García-Cánovas F and Rodríguez-López JN: The
anti-inflammatory and anti-cancer properties of
epigallocatechin-3-gallate are mediated by folate cycle disruption,
adenosine release and NF-kappaB suppression. Inflamm Res.
57:472–478. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Miura Y, Chiba T, Tomita I, et al: Tea
catechins prevent the development of atherosclerosis in apoprotein
E-deficient mice. J Nutr. 131:27–32. 2001.PubMed/NCBI
|
|
12
|
Ran ZH, Chen C and Xiao SD:
Epigallocatechin-3-gallate ameliorates rats colitis induced by
acetic acid. Biomed Pharmacother. 62:189–196. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Eastaff-Leung N, Mabarrack N, Barbour A,
Cummins A and Barry S: Foxp3+ regulatory T Cells, Th17 effector
cells, and cytokine environment in inflammatory bowel disease. J
Clin Immunol. 30:80–89. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cooper HS, Murthy SN, Shah RS and
Sedergran DJ: Clinicopathologic study of dextran sulfate sodium
experimental murine colitis. Lab Invest. 69:238–249.
1993.PubMed/NCBI
|
|
15
|
Shen W and Durum SK: Synergy of IL-23 and
Th17 cytokines: New light on inflammatory bowel disease. Neurochem
Res. 35:940–946. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hardenberg G, Steiner TS and Levings MK:
Environmental influences on T regulatory cells in inflammatory
bowel disease. Semin Immunol. 23:130–138. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fuss IJ, Heller F, Boirivant M, Leon F,
Yoshida M, Fichtner-Feigl S, Yang Z, Exley M, Kitani A, Blumberg
RS, et al: Nonclassical CD1d-restricted NK T cells that produce
IL-13 characterize an atypical Th2 response in ulcerative colitis.
J Clin Invest. 113:1490–1497. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Heller F, Florian P, Bojarski C, Richter
J, Christ M, Hillenbrand B, Mankertz J, Gitter AH, Bürgel N, Fromm
M, et al: Interleukin-13 is the key effector Th2 cytokine in
ulcerative colitis that affects epithelial tight junctions,
apoptosis, and cell restitution. Gastroenterology. 129:550–564.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hunter MM, Wang A and McKay DM: Helminth
infection enhances disease in a murine TH2 model of colitis.
Gastroenterology. 132:1320–1330. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fuss IJ and Strober W: The role of IL-13
and NK T cells in experimental and human ulcerative colitis.
Mucosal Immunol. 1(Suppl 1): S31–S33. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Matsumura K, Nakase H, Yamamoto S, Yoshino
T, Takeda Y, Kasahara K, Ueno S, Uza N and Chiba T: Modulation of
the Th1/Th2 balance by infliximab improves hyperthyroidism
associated with a flare-up of ulcerative colitis. Inflamm Bowel
Dis. 15:967–968. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dohi T, Fujihashi K, Kiyono H, Elson CO
and McGhee JR: Mice deficient in Th1- and Th2-type cytokines
develop distinct forms of hapten-induced colitis. Gastroenterology.
119:724–733. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sawa Y, Oshitani N, Adachi K, Higuchi K,
Matsumoto T and Arakawa T: Comprehensive analysis of intestinal
cytokine messenger RNA profile by real-time quantitative polymerase
chain reaction in patients with inflammatory bowel disease. Int J
Mol Med. 11:175–179. 2003.PubMed/NCBI
|
|
24
|
Olsen T, Goll R, Cui G, Husebekk A, Vonen
B, Birketvedt GS and Florholmen J: Tissue levels of tumor necrosis
factor-alpha correlates with grade of inflammation in untreated
ulcerative colitis. Scand J Gastroenterol. 42:1312–1320. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Alex P, Zachos NC, Nguyen T, Gonzales L,
Chen TE, Conklin LS, Centola M and Li X: Distinct cytokine patterns
identified from multiplex profiles of murine DSS and TNBS-induced
colitis. Inflamm Bowel Dis. 15:341–352. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fuss IJ: Is the Th1/Th2 paradigm of immune
regulation applicable to IBD? Inflamm Bowel Dis. 14:S110–S112.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bogaert S, Laukens D, Peeters H, et al:
Differential mucosal expression of Th17-related genes between the
inflamed colon and ileum of patients with inflammatory bowel
disease. BMC Immunol. 11:612010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu ZJ, Yadav PK, Su JL, Wang JS and Fei
K: Potential role of Th17 cells in the pathogenesis of inflammatory
bowel disease. World J Gastroenterol. 15:5784–5788. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Heo YJ, Joo YB, Oh HJ, et al: IL-10
suppresses Th17 cells and promotes regulatory T cells in the CD4+ T
cell population of rheumatoid arthritis patients. Immunol Lett.
127:150–156. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kleczynska W, Jakiela B, Plutecka H, et
al: Imbalance between Th17 and regulatory T-cells in systemic lupus
erythematosus. Folia Histochem Cytobiol. 49:646–653. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Horiuchi A, Hayashi T, Kikuchi N, et al:
Hypoxia upregulates ovarian cancer invasiveness via the binding of
HIF-1α to a hypoxia-induced, methylation-free hypoxia response
element of S100A4 gene. Int J Cancer. 131:1755–1767. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chu W, Wan L, Zhao D, et al: Mild
hypoxia-induced cardiomyocyte hypertrophy via up-regulation of
HIF-1α-mediated TRPC signalling. J Cell Mol Med. 16:2022–2034.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
OGarra A and Vieira P: Regulatory T cells
and mechanisms of immune system control. Nat Med. 10:801–805. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Veltkamp C, Ruhwald R, Giesem T,
Autschbach F, Kaden I, Veltkamp R, Sartor RB and Stremmel W:
CD4+CD25+ cell depletion from the normal CD4+ T cell pool prevents
tolerance toward the intestinal flora and leads to chronic colitis
in immunodeficient mice. Inflamm Bowel Dis. 12:437–446. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Oconnor W Jr, Kamanaka M, Booth CJ, Town
T, Nakae S, Iwakura Y, Kolls JK and Flavell RA: A protective
function for interleukin 17A in T cell-mediated intestinal
inflammation. Nat Immunol. 10:603–609. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fiocchi C: Inflammatory bowel disease:
evolutionary concepts in biology, epidemiology, mechanisms and
therapy. Curr Opin Gastroenterol. 29:347–349. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gupta S: Immune homeostasis: regulatory T
cells (Treg) and molecules. J Clin Immunol. 28:617–618. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Unutmaz D and Pulendran B: The gut feeling
of Treg cells: IL-10 is the silver lining during colitis. Nat
Immunol. 10:1141–1143. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bettelli E, Carrier Y, Cao W, et al:
Reciprocal developmental pathways for the generation of pathogenic
effector TH17 and regulatory T cells. Nature. 441:235–238. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lina C, Conghua W, Nan L and Ping Z:
Combined treatment of etanercept and MTX reverses Th1/Th2,
Th17/Treg imbalance in patients with rheumatoid arthritis. J Clin
Immunol. 31:596–605. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ben Aissa-Fennira F, Sassi A, Bouguerra A
and Benammar-Elgaaied A: Immunoregulatory role for a public IgM
idiotype in the induction of autoimmune diseases in Mycoplasma
pneumoniae infection. Immunol Lett. 136:130–137. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhou L, Lopes JE, Chong MM, et al:
TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by
antagonizing RORgammat function. Nature. 453:236–240. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li MO and Flavell RA: TGF-beta: A master
of all T cell trades. Cell. 134:392–404. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ghoreschi K, Laurence A, Yang XP, et al:
Generation of pathogenic T(H)17 cells in the absence of TGF-β
signalling. Nature. 467:967–971. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang J, Pae M, Meydani SN and Wu D: Green
tea epigallocatechin-3-gallate modulates differentiation of naïve
CD4+ T cells into specific lineage effector cells. J Mol Med
(Berl). 91:485–495. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Nutsch K and Hsieh C: When T cells run out
of breath: the HIF-1α story. Cell. 146:673–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Jung JE, Kim HS, Lee CS, et al: STAT 3
inhibits the degradation of HIF-1alpha by pVHL-mediated
ubiquitination. Exp Mol Med. 40:479–485. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sun H, Zhang Y, Gao P, et al: Adiponectin
reduces C-reactive protein expression and downregulates STAT3
phosphorylation induced by IL-6 in HepG2 cells. Mol Cell Biochem.
347:183–189. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Li Y, Yu C, Zhu WM, et al: Triptolide
ameliorates IL-10-deficient mice colitis by mechanisms involving
suppression of IL-6/STAT3 signaling pathway and down-regulation of
IL-17. Mol Immunol. 47:2467–2474. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Veldhoen M, Hocking RJ, Atkins CJ,
Locksley RM and Stockinger B: TGFbeta in the context of an
inflammatory cytokine milieu supports de novo differentiation of
IL-17-producing T cells. Immunity. 24:179–189. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Mangan PR, Harrington LE, OQuinn DB, Helms
WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR and Weaver
CT: Transforming growth factor-beta induces development of the
T(H)17 lineage. Nature. 441:231–234. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Volpe E, Servant N, Zollinger R, Bogiatzi
SI, Hupé P, Barillot E and Soumelis V: A critical function for
transforming growth factor-beta, interleukin 23 and proinflammatory
cytokines in driving and modulating human T(H)-17 responses. Nat
Immunol. 9:650–657. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Harrington LE, Hatton RD, Mangan PR,
Turner H, Murphy TL, Murphy KM and Weaver CT: Interleukin
17-producing CD4+ effector T cells develop via a lineage distinct
from the T helper type 1 and 2 lineages. Nat Immunol. 6:1123–1132.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
54
|
Annunziato F, Cosmi L, Santarlasci V,
Maggi L, Liotta F, Mazzinghi B, Parente E, Filì L, Ferri S, Frosali
F, et al: Phenotypic and functional features of human Th17 cells. J
Exp Med. 204:1849–1861. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lubberts E: Th17 cytokines and arthritis.
Semin Immunopathol. 32:43–53. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Komiyama Y, Nakae S, Matsuki T, Nambu A,
Ishigame H, Kakuta S, Sudo K and Iwakura Y: IL-17 plays an
important role in the development of experimental autoimmune
encephalomyelitis. J Immunol. 177:566–573. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Yang XO, Panopoulos AD, Nurieva R, Chang
SH, Wang D, Watowich SS and Dong C: STAT3 regulates
cytokine-mediated generation of inflammatory helper T cells. J Biol
Chem. 282:9358–9363. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Dryden GW, Fernandez-Botran GR and Qazzaz
HMH: EGCG reduces proinflammatory cytokine production and induces
apoptosis in activated CD14+ macrophages, CD4+CD45+RO T cells, and
mixed macrophage/T cell populations, but not CD4+CD45+RA T cells
from IBD patients and controls. Gastroenterology. 140:S–838.
2011.
|
|
59
|
Dryden GW, Lam A, Beatty K, Qazzaz HH and
McClain CJ: A pilot study to evaluate the safety and efficacy of an
oral dose of (−)-epigallocatechin-3-gallate-rich polyphenon E in
patients with mild to moderate ulcerative colitis. Inflamm Bowel
Dis. 19:1904–1912. 2013.PubMed/NCBI
|