Introduction
Fungal infections have emerged worldwide due to an
increasing number of immunocompromised patients, including patients
with cancer, acquired immune deficiency syndrome, solid-organ and
hematopoietic stem cell transplant recipients, premature neonates
and patients recovering from major surgery. Nosocomial bloodstream
fungal infection remains serious among hospitalized patients
(1–3). Invasive Candida are responsible
for the highest mortality rates (1,4,5). Candida albicans is the major
fungus causing mucosal and invasive infections in humans (3,6);
however, an increasing number of infections caused by
non-albicans Candida has also been reported (7–9). Azole
drugs are commonly used to treat infections caused by
Candidas; however, a number of reports have indicated that
Candidas are exhibiting reduced susceptibility to azole
drugs (1,3,10);
certain Candida species are even intrinsically resistant to
them (3,11). A search for novel antifungal agents
has therefore been undertaken for candidiasis.
Antimicrobial peptides (AMPs) are important
components of innate immunity for humans and several other forms of
life. The most significant characteristic of AMPs is their
broad-spectrum antimicrobial activity against bacteria, viruses and
fungi, including multi-drug-resistant microbes (12–14).
This antimicrobial activity is not associated with the rapid
emergence of resistance (13,15,16),
which has become a serious problem for conventional antibiotics.
Furthermore, unlike traditional antibiotics that inhibit specific
biosynthetic pathways, AMPs are multi-target agents. Studies into
AMPs may provide an insight into the innate immunity of
invertebrates and produce templates for designing novel,
broad-spectrum antibiotics that would function in humans (13); therefore, AMPs have emerged as the
most promising group of candidates for the development of a new
class of antibiotics (13,15).
AMPs are currently the focus of extensive research
in order to assess their possible use as a new class of
antibiotics. Possible problems in the stability, delivery and
pathogen-targeting may arise in the use of AMPs as antimicrobial
agents. To improve the application of such peptides, combinations
with antibiotics or other compounds are generally used to increase
the in vivo activity of AMPs (17).
Chromogranin A (CGA) is a protein expressed in all
types of neurons (18). A series of
studies on the antimicrobial function of the CGA-derived peptides
has been conducted (19–22). Catestatin (derived from the
C-terminus of bovine CGA) is expressed in murine skin in response
to injury and infection, and it has a potent antimicrobial activity
against bacteria, fungi and yeast. Similar activity has also been
reported for vasostatin-I (CGA1–76; corresponding to CGA residues
1–76), which can trigger the selective migration of human monocytes
and eosinophils. CGA1–76 and catestatin have been described to be
innate immunity components of mammals (19,21). A
study by Lugardon et al (19)
showed that the C-terminal moiety of bovine CGA1–76 exhibited a
potent antifungal activity and that the disulfide-bridge loop
Cys17-Cys38 was crucial for its antibacterial
activity, but not for the antifungal activity. In order to
investigate antifungal peptides derived from human CGA, we have
studied several recombinant peptides derived from the N-terminus of
human CGA: CGA18–76, CGA18–66 and CGA31–76 (corresponding to human
CGA residues 18–76, 18–66 and 31–76, respectively). This ongoing
research has shown that CGA-N46, a derived peptide containing amino
acids 31–76 of the N-terminus of human CGA, has antagonistic
activity against Candida albicans (23).
AMPs have a broad spectrum of activity and can act
as antibacterial, antifungal, antiviral and sometimes even as
anticancer peptides. Extensive research has been performed in the
field of antibacterial peptides, describing their identification,
characterization and mechanism of action (13,14,24,25). In
order to further our ongoing search for novel antimicrobial agents
that could be used in drug therapy, recombinant CGA-N46 was
expressed and purified in the present study, and the activities of
CGA-N46 against selected fungi, cancer cells, human erythrocytes
and normal fibroblast cells, as well as the stability of CGA-N46
and its effect in combination with conventional antibiotics, were
investigated.
Materials and methods
Reagents
MTT, Triton X-100, dimethylsulfoxide (DMSO),
fluconazole and terbinafine were purchased from Sigma-Aldrich
(Shanghai, China). All of the solvents used were of
high-performance liquid chromatography grade. The water used for
all experiments was supplied by a Milli-Q® water purification
system from Millipore (Beijing, China).
Culture and suspensions of fungal
strains
The fungal strains Candida glabrata, Candida
parapsilosis, Candida krusei, Candida tropicalis, Candida albicans,
Cryptococcus neoformans, Aspergillus fumigatus, Aspergillus flavus,
Aspergillus niger, Fusarium moniliforme, Microsporum canis,
Microsporum gypseum, Trichophyton rubrum and Trichophyton
mentagrophytes were supplied by the China Academy of Chinese
Medical Sciences (Beijing, China). For each experiment, strains
were sub-cultured onto Sabouraud dextrose (SD) agar (Oxoid Ltd.,
Basingstoke, UK) at 35°C for between 48 h and 7 days, depending on
the species. For yeasts, the inoculum suspension was prepared by
selecting 5 colonies measuring ≥1 mm in diameter and suspending the
material in 5 ml sterile 0.85% NaCl. The working suspension
(1–5×103 CFU/ml) was established by a 1:10 dilution with
SD broth (Oxoid Ltd.). For filamentous fungi, colonies were covered
with ~1 ml sterile 0.85% saline, and the suspensions were
established by gently probing the colonies with the tip of a
Pasteur pipette. The resulting mixture of conidia or
sporangiospores and hyphal fragments was withdrawn and transferred
to a sterile tube. Heavy particles were allowed to settle for 3–5
min, and the upper homogeneous suspension was then collected and
mixed. The density of the suspension was measured using a
microscope. The suspension was adjusted to 0.8–10×104
CFU/ml by diluting with SD broth.
Cell line culture
A549 lung cancer cells (American Type Culture
Collection, Manassas, VA, USA) were maintained at the Henan
University of Technology (Zhengzhou, China) in RPMI-1640 medium
(Gibco-BRL, Grand Island, NY, USA) supplemented with 10% fetal
bovine serum (Hyclone, Rockford, IL, USA), with 100 U/ml penicillin
and 100 µg/ml streptomycin at 37°C in a humidified atmosphere
containing 5% CO2. The normal primary chicken embryo
fibroblasts (CEFs) from specific pathogen-free chickens
(Experimental Animal Center, Jenan University of Technology,
Zhengzhou, China) were isolated using a standard protocol described
elsewhere (26). Fibroblasts were
grown as a monolayer in Dulbecco's modified Eagle's medium
(Gibco-BRL) containing 10% fetal bovine serum (Hyclone).
Antifungal assay
Minimum inhibitory concentrations (MICs) of CGA-N46
against the fungi were measured according to a modified version of
the broth microdilution method of the Clinical and Laboratory
Standards Institute (CLSI) (27).
Briefly, CGA-N46 was serially diluted to concentrations of between
0.62 µM and 3.2 mM in 20 mM phosphate-buffered saline (PBS) (pH
6.0), and 100-µl samples were dispensed into the wells of a
96-well, U-shaped plate. Each sample was mixed with 100 µl 2X
inoculum suspension (yeast, 1–5×103 CFU/ml; conidia or
sporangiospores, 0.8–10×104 CFU/ml) of a log-phase
fungal culture in SD broth. The cultures were incubated at 30°C
without agitation: C. glabrata, C. parapsilosis, C. krusei, C.
tropicalis and C. albicans were incubated for 48 h;
C. neoformans, A. fumigatus, A. flavus, A. niger and F.
moniliforme were incubated for 72 h; M. canis, M. gypseum,
T. rubrum and T. mentagrophytes were incubated for 96 h.
The MIC was defined as the lowest peptide concentration that
completely inhibited fungal growth. Cultures without CGA-N46 or SD
broth were employed as positive and negative controls,
respectively. Three replicates were set for each experiment.
Hemolysis assay
The hemolytic activity of CGA-N46 was determined by
measuring the amount of hemoglobin released by the lysis of human
erythrocytes using a modified version of the method described by
Huang et al (28). Briefly,
CGA-N46 was serially diluted using PBS in 96-well plates to give
100 µl sample solution in each well. Human erythrocytes that had
undergone anticoagulation using EDTAK2 (Sigma-Aldrich) were
collected by centrifugation at 1,000 × g for 5 min at 4°C, washed
twice with PBS and then diluted to a concentration of 2% in PBS.
The erythrocytes (100 µl of 2% solution) were added to each well to
achieve a final concentration of 1% human erythrocytes per well,
and the reactions were incubated at 37°C for 30 min. Following
incubation, the plates were centrifuged for 10 min and 100 µl
supernatant was transferred to a new 96-well, U-shaped plate.
Hemoglobin release was determined by measuring the absorbance of
the supernatant at a wavelength of 570 nm. Peptide samples were
diluted in a 2-fold series to determine the minimum concentration
at which hemolysis occurred. Erythrocytes in PBS and 1% (v/v)
Triton-X100 were used as the negative and positive controls,
respectively. The hemolysis of erythrocytes was calculated using
the following formula: Erythrocyte hemolysis = (Asample
- Anegative)/(Apositive -
Anegative) × 100%.
MTT assay
Cell viability was assessed using the MTT assay,
based on the reduction of MTT into formazan dye by active
mitochondria (24). Briefly, 50 µl
cell suspension (1×105 CFU/ml) was seeded in 96-well
microtiter plates. Following the attachment of the cells, various
concentrations of CGA-N46 (5 µl) were added to each well to give
final concentrations of between 0.1 and 1.6 mM for incubation at
37°C in 5% CO2 for 24, 48 and 72 h. A total of 10 µl MTT
solution (5 mg/ml MTT in PBS) was then added to each well and
incubated for an additional 4 h. After rinsing, 100 µl DMSO was
added to dissolve the MTT formazan crystals. The absorbance was
read using an ELISA reader (BioTek Instruments, Inc., Winooski, VT,
USA) at 490 nm. Cultures without CGA-N46 or SD broth were appointed
as the control and blank groups, respectively. The relative
cellular activity was calculated according to the following
equation: Cell survival (% of control) = (Atest -
Ablank)/(Acontrol - Ablank) × 100.
Each experiment was repeated in triplicate.
Physicochemical stability
analysis
The physicochemical stability of CGA-N46 at
different temperatures and pHs was assessed. CGA-N46 was dissolved
in 20 mM PBS (pH 6.0) to form a solution (6.4 mM). The heat
stability of CGA-N46 was assessed by incubating the CGA-N46
solution at different temperatures (40–100°C) for 30 min and then
comparing the MICs of the heat-treated CGA-N46 solutions with the
MIC of the control. To determine the pH-stability of CGA-N46, the
pH of the CGA-N46 solutions was adjusted to 1.0–12.0 with 0.1 M HCl
or 1 M NaOH, and the solutions were maintained at room temperature.
After 60 min, the pH was adjusted back to 6.0 in order to assess
the antifungal activity against C. krusei. The MIC of
CGA-N46 was analyzed by the broth microdilution method of the CLSI
(27). A freshly prepared CGA-N46
solution in 20 mM PBS (pH 6.0) was used as control.
Checkerboard analysis
The combination effect of CGA-N46 with conventional
antibiotics was analyzed using the checkerboard method described by
Su et al (29), with slight
modification. Briefly, an inoculum of logarithmic-phase C.
krusei cells (1×103 CFU/ml) was cultured at 30°C for
20 h in each well of a 96-well, U-shaped culture plate containing
100 µl SD broth with CGA-N46 (3.2 mM to 0.62 µM) or an antibiotic
(10 µM to 0.1 nM) added, alone or in combination, at a specified
concentration. The lowest concentration of each drug combination
causing growth inhibition was plotted on an arithmetic scale and
any synergy or additive effect was identified from the shape of the
curve and the fractional inhibitory concentration (FIC) index. The
FIC index was calculated as follows: FIC index =
(A/MICA) + (B/MICB), where MICA
and MICB are the MICs of drugs A and B alone,
respectively, and A and B are the MICs of drugs A and B when used
in combination, respectively (30).
Drug interactions are usually classified as synergistic, additive
or antagonistic on the basis of the FIC index and are defined as
follows: Synergy, ≤0.5; additive effect, 0.5–1.0; indifference (or
no effect), 1.0–2.0; antagonism, >2.0 (29).
Statistical analysis
Experiments were performed three times, and
experimental data were analyzed using one-way analysis of variance
followed by Least Significant Difference and Duncan's tests using
PASW statistical software, version 18 (SPSS, Inc., Chicago, IL,
USA). The results are presented as the mean ± standard error of the
mean. P<0.01 was considered to indicate a statistically
significant difference.
Results
Antifungal activity
The antifungal activity spectrum of CGA-N46 was
indicated using the MICs of CGA-N46 against the tested fungi and
yeasts. As shown in Table I, CGA-N46
was active against yeasts (MICs of 0.1–0.8 mM), but had no effect
on the growth of filamentous fungi, even with peptide
concentrations up to 3.2 mM. The highest activity was found against
C. krusei, with an MIC of 0.1 mM.
 | Table I.Antifungal spectrum and MICs of
CGA-N46. |
Table I.
Antifungal spectrum and MICs of
CGA-N46.
| Strains | MICs (mM) |
|---|
| Candida
glabrata | 0.8 |
| Candida
parapsilosis | 0.8 |
| Candida
krusei | 0.1 |
| Candida
tropicalis | 0.2 |
| Candida
albicans | 0.2 |
| Cryptococcus
neoformans | ND |
| Aspergillus
fumigatus | ND |
| Aspergillus
flavus | ND |
| Aspergillus
niger | ND |
| Fusarium
moniliforme | ND |
| Microsporum
canis | ND |
| Microsporum
gypseum | ND |
| Trichophyton
rubrum | ND |
| Trichophyton
mentagrophytes | ND |
Hemolytic activity of CGA-N46
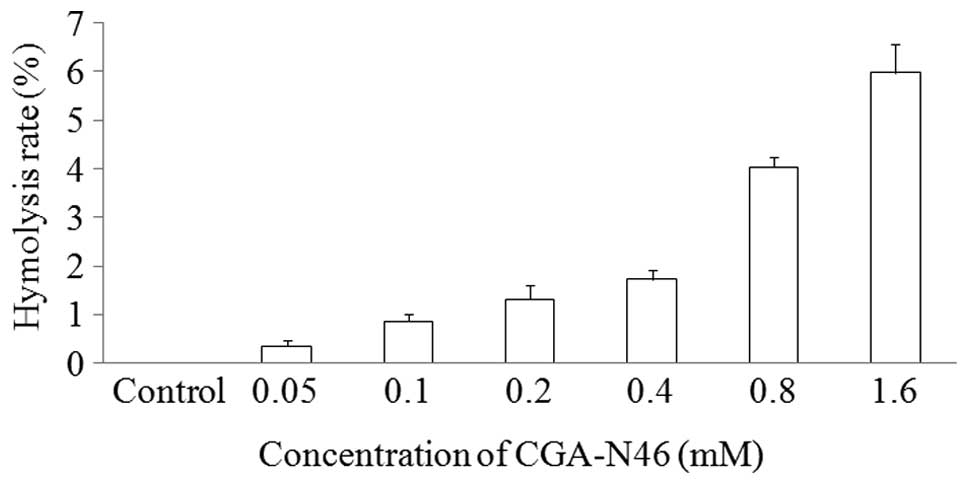
The hemolytic activity of CGA-N46 against the highly
sensitive human erythrocytes was determined as a measure of its
toxicity to mammalian cells. The release of hemoglobin was
monitored by measuring the absorbance at 570 nm. As negative and
positive controls, erythrocytes in PBS without CGA-N46 and 0.1%
(v/v) Triton X-100 in PBS were employed, respectively. CGA-N46 only
showed weak hemolytic activity at the concentration of 1.6 mM
(Fig. 1).
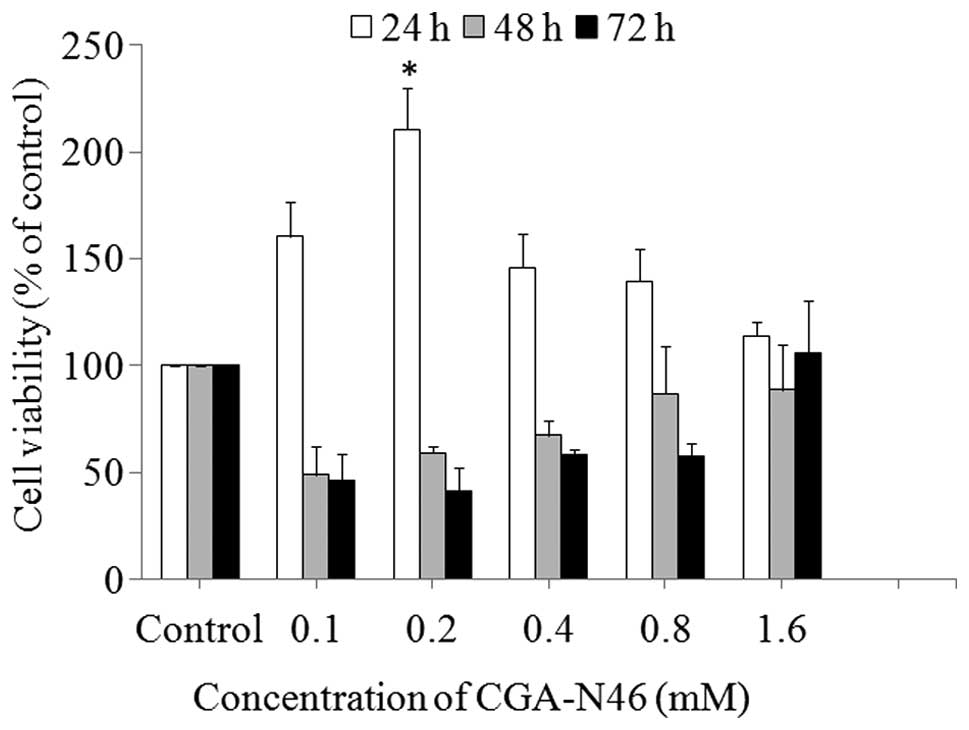
Effect of CGA-N46 on the growth of
primary CEF cells
The antiproliferative activity of CGA-N46 against
normal cells was assessed by investigating the effect on normal
primary CEF cells using the MTT colorimetric assay. As shown in
Fig. 2, CGA-N46 promoted the growth
of the cells up to 24 h of incubation, and the promoting effect
decreased as the concentration increased >0.2 mM. When the
incubation time was extended to 48 h and beyond, CGA-N46 showed an
inhibitory activity on cell growth, and the inhibitory activity
decreased with the increasing concentration. Compared with the
control, the effect of CGA-N46 on normal primary CEF cells was
reversible at concentrations <1.6 mM, and the growth promotion
and inhibition were not observed at the concentration of 1.6
mM.
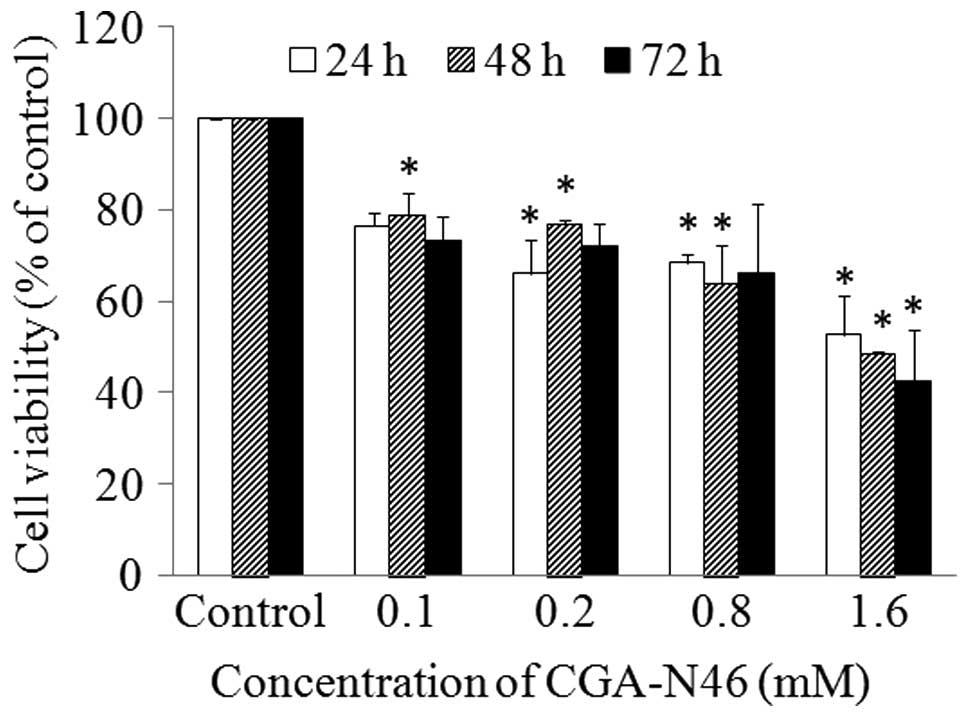
Effect of CGA-N46 on cancer cell
proliferation
The antiproliferative activity of CGA-N46 against
cancer cells was investigated using an A549 human lung cancer cell
line. A549 cells were treated with different concentrations of
CGA-N46 (0.62 µM to 3.2 mM) for 72 h, and 20 mM PBS (pH 6.0) was
used as a negative control. MTT was used to assess the effect of
CGA-N46 on A549 cell viability. As seen in Fig. 3, the number of metabolically active
cells decreased significantly as the concentration of CGA-N46
increased. The proliferation of A549 cells was inhibited by CGA-N46
in a dose-dependent manner.
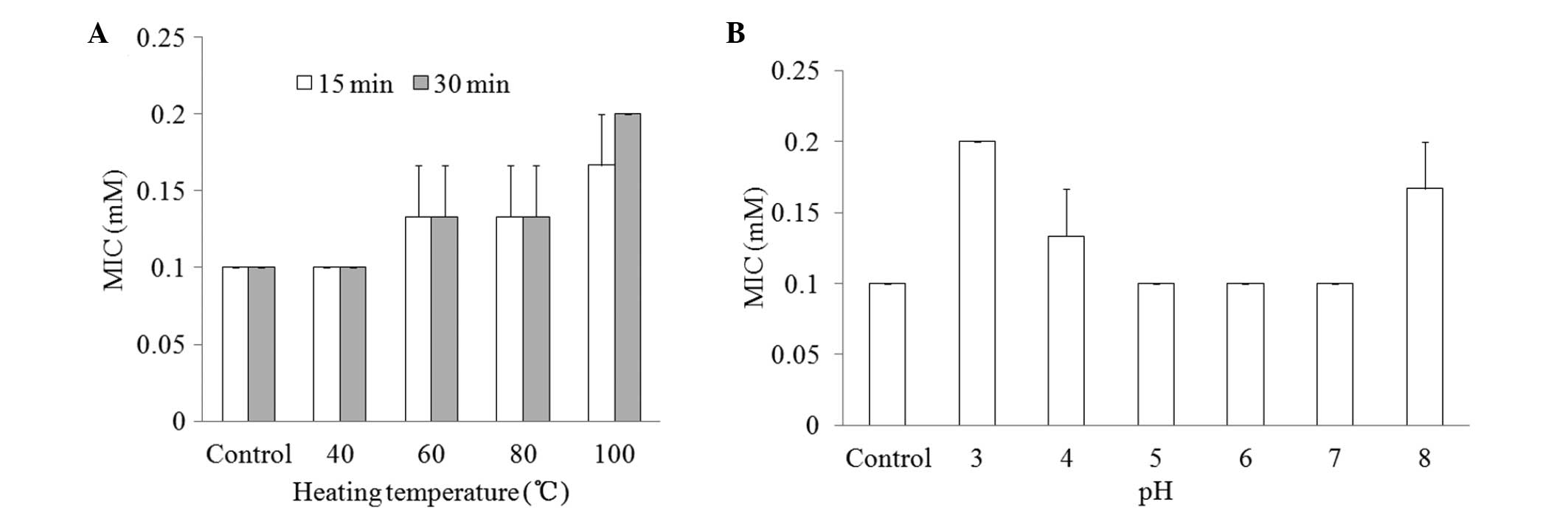
Physicochemical properties
To determine the effect of the physicochemical
factors heat and pH on the antifungal activity of CGA-N46, the MIC
values of CGA-N46 against C. krusei were examined and
compared following the treatment of the CGA-N46 with different
temperatures and pHs. As shown in Fig.
4, the MICs remained at 0.1 mM at a temperature <40°C or
within a broad pH range (5.0–7.0).
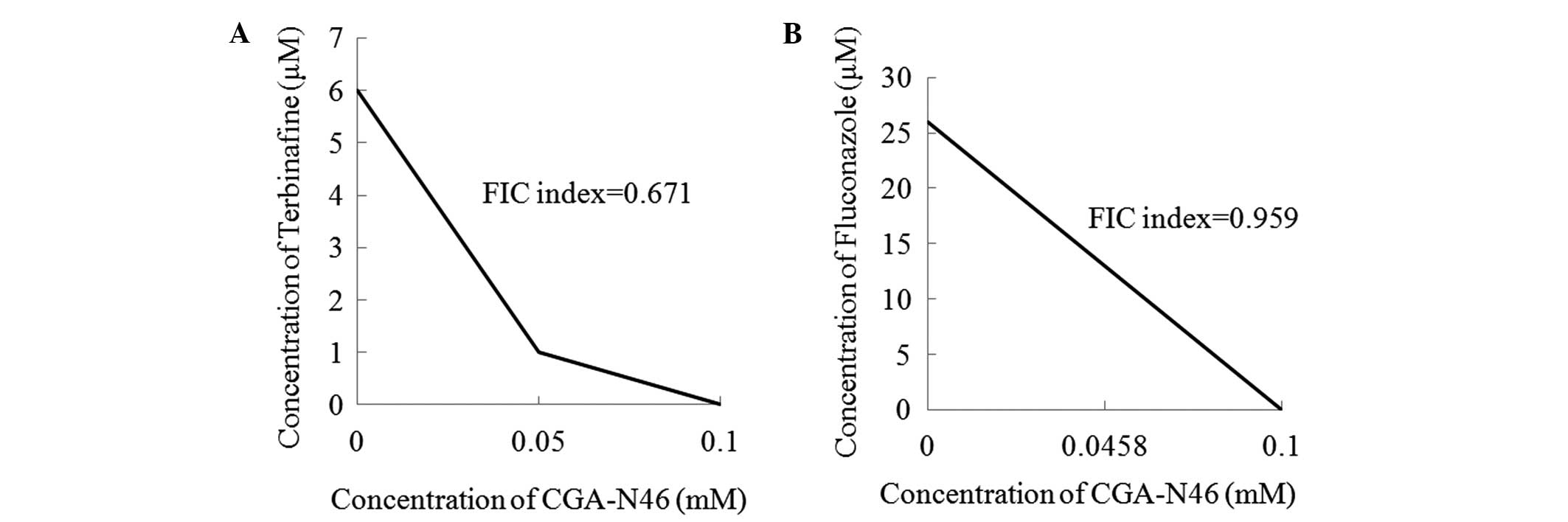
Combination effect of CGA-N46 and
antibiotics
The combination effects of CGA-N46 with terbinafine
and fluconazole against C. krusei are shown in Fig. 5. The MICs of terbinafine, fluconazole
and CGA-N46 alone were 6 µM, 26 µM and 0.1 mM, respectively. When
terbinafine was combined with CGA-N46, the MICs were 1 µM and 0.05
mM, respectively. When fluconazole and CGA-N46 were used in
combination, the MICs were 13 µM and 0.0458 mM, respectively. The
antifungal activities of the conventional antibiotics and CGA-N46
were increased when the agents were applied in combination. The FIC
indices of CGA-N46 in combination with terbinafine and fluconazole,
respectively, were 0.671 and 0.959, showing that the antifungal
activities of the combinations against C. krusei were
additive (FIC index 0.5–1.0).
Discussion
The majority of AMPs are alkaline cationic peptides
(25,31). It has been postulated that their
mechanism of action against microbes involves compromising the
membrane of the target organism (14,31). It
is generally accepted that the positive charge allows the
electrostatic attraction of AMPs with the phospholipid membranes of
microbes; therefore, environmental pH has an effect on the charges
of the alkaline cationic peptides. The acidic metabolites produced
by fungi in the local microenvironment of the human body may
neutralize the alkaline cationic peptides, and thus impede the
electrostatic attraction of the AMPs with fungal outer phospholipid
membranes (32). CGA-N46 has almost
zero charge at physiological pH due to its calculated pI of 7.38;
therefore, it has a broad spectrum of pH stability. CGA-N46 may be
a good model for researching the mechanisms of non-cationic
AMPs.
CGA-N46 exerted an antiproliferative effect on
Candida spp. and A549 cancer cells in a concentration- and
time-dependent manner. It had no hemolytic activity at the MIC of
CGA-N46 against yeasts. Furthermore, the results showed that
CGA-N46 had no significant effect on normal primary CEF cells at a
concentration of 1.6 mM and a reversible effect at concentrations
<1.6 mM. This observation was consistent with that for Magainin
II (33). CGA-N46 therefore
represents a member of a novel, non-cationic, α-helical peptide
family with antimicrobial and anticancer activities but without
toxicity to erythrocytes, indicating the possibility for use in
treatment for infection and cancer.
AMPs have different mechanisms of action from
conventional antibiotics (14,15).
Previous studies have indicated that the limitations of AMPs, which
are long amino acid sequences, are poor bioavailability and
susceptibility to protease degradation and pathogen targeting
(14,17). To overcome these limitations,
combination with conventional antibiotics or other compounds is
generally used. Combined with minocycline or azole antifungal
agents, lactoferricin has synergistic effects against an
antibiotic-resistant strain of Staphylococcus aureus
(34). The bactericidal effect was
enhanced when human β-defensin was used in combination with the
antimicrobial agents (17).
Fluconazole and terbinafine are two antibiotics to which
Candidas are prone to be resistant (35). In the present study, CGA-N46 was
combined with these two drugs. In the checkerboard assay, the
combination of CGA-N46 with each of the drugs exhibited enhanced
antifungal activity. The combinations of CGA-N46 with terbinafine
and fluconazole demonstrated additive effects against C.
krusei. The results of this study may prove in the clinical
application of AMPs in combination with other compounds.
Acknowledgements
This study was supported by the National Science
Foundation of China (grant nos. 31071922, 31271948 and 21306040)
and Henan University of Technology (no. 11JCYJ10). The authors
would like to express their appreciation to Dr Jin Huang of Henan
University of Technology for performing the SPSS analysis.
References
|
1
|
Bassetti M, Taramasso L, Nicco E, Molinari
MP, Mussap M and Viscoli C: Epidemiology, species distribution,
antifungal susceptibility and outcome of nosocomial candidemia in a
tertiary care hospital in Italy. PLoS One. 6:e241982011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ortega M, Marco F, Soriano A, Almela M,
Martínez JA, López J, Pitart C and Mensa J: Candida species
bloodstream infection: Epidemiology and outcome in a single
institution from 1991 to 2008. J Hosp Infect. 77:157–161. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Scorzoni L, de Lucas MP, Mesa-Arango AC,
Fusco-Almeida AM, Lozano E, Cuenca-Estrella M, Mendes-Giannini MJ
and Zaragoza O: Antifungal efficacy during Candida krusei
infection in non-conventional models correlates with the yeast in
vitro susceptibility profile. PLoS One. 8:e600472013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gudlaugsson O, Gillespie S, Lee K, Van de
Berg J, Hu J, Messer S, Herwaldt L, Pfaller M and Diekema D:
Attributable mortality of nosocomial candidemia, revisited. Clin
Infect Dis. 37:1172–1177. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wisplinghoff H, Bischoff T, Tallent SM,
Seifert H, Wenzel RP and Edmond MB: Nosocomial bloodstream
infections in US hospitals: Analysis of 24,179 cases from a
prospective nationwide surveillance study. Clin Infect Dis.
39:309–317. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pushpanathan M, Rajendhran J, Jayashree S,
Sundarakrishnan B, Jayachandran S and Gunasekaran P: Direct cell
penetration of the antifungal peptide, MMGP1, in Candida
albicans. J Pept Sci. 18:657–660. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Trick WE, Fridkin WE, Edwards SK, Hajjeh
JR, Gaynes RA and National RP: Nosocomial Infections Surveillance
System Hospitals: Secular trend of hospital-acquired candidemia
among intensive care unit patients in the United States during
1989–1999. Clin Infect Dis. 35:627–630. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Arendrup MC: Epidemiology of invasive
candidiasis. Curr Opin Crit Care. 16:445–452. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pemán J, Cantón E, Quindós G, Eraso E,
Alcoba J, Guinea J, Merino P, Ruiz-Pérez-de-Pipaon MT,
Pérez-del-Molino L, Linares-Sicilia MJ, et al: FUNGEMYCA Study
Group; Epidemiology, species distribution and in vitro antifungal
susceptibility of fungaemia in a Spanish multicentre prospective
survey. J Antimicrob Chemother. 67:1181–1187. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Leroy O, Gangneux JP, Montravers P, Mira
JP, Gouin F, Sollet JP, Carlet J, Reynes J, Rosenheim M, Regnier B
and Lortholary O: AmarCand Study Group: Epidemiology, management
and risk factors for death of invasive Candida infections in
critical care: A multicenter, prospective, observational study in
France (2005–2006). Crit Care Med. 37:1612–1618. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Muñoz P, Sánchez-Somolinos M, Alcalá L,
Rodríguez-Créixems M, Peláez T and Bouza E: Candida krusei
fungaemia: Antifungal susceptibility and clinical presentation of
an uncommon entity during 15 years in a single general hospital. J
Antimicrob Chemother. 55:188–193. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lata S, Mishra N and Raghava G: AntiBP2:
Improved version of antibacterial peptide prediction. BMC
Bioinformatics. 11(Suppl 1): S192010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lu J and Chen ZW: Isolation,
characterization and anti-cancer activity of SK84, a novel
glycine-rich antimicrobial peptide from Drosophila virilis.
Peptides. 31:44–50. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Güell I, Micaló L, Cano L, Badosa E, Ferre
R, Montesinos E, Bardají E, Feliu L and Planas M: Peptidotriazoles
with antimicrobial activity against bacterial and fungal plant
pathogens. Peptides. 33:9–17. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hancock RE: Peptide antibiotics. Lancet.
349:418–422. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Foubister V: Superpeptide to treat
Candida albicans. Drug Discov Today. 8:380–381. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Maisetta G, Batoni G, Esin S, Luperini F,
Pardini M, Bottai D, Florio W, Giuca MR, Gabriele M and Campa M:
Activity of human beta-defensin 3 alone or combined with other
antimicrobial agents against oral bacteria. Antimicrob Agents
Chemother. 47:3349–3351. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Eiden LE: Is chromogranin a prohormone?
Nature. 325:3011987. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lugardon K, Raffner R, Goumon Y, Corti A,
Delmas A, Bulet P, Aunis D and Metz-Boutigue MH: Antibacterial and
antifungal activities of vasostatin-1, the N-terminal fragment of
chromogranin A. J Biol Chem. 275:10745–10753. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lugardon K, Chasserot-Golaz S, Kieffer AE,
Maget-Dana R, Nullans G, Kieffer B, Aunis D and Metz-Boutigue MH:
Structural and biological characterization of chromofungin, the
antifungal chromogranin A-(47–66)-derived peptide. J Biol Chem.
276:35875–35882. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Briolat J, Wu SD, Mahata SK, Gonthier B,
Bagnard D, Chasserot-Golaz S, Helle KB, Aunis D and Metz-Boutigue
MH: New antimicrobial activity for the catecholamine
release-inhibitory peptide from chromogranin A. Cell Mol Life Sci.
62:377–385. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Radek KA, Lopez-Garcia B, Hupe M, Niesman
IR, Elias PM, Taupenot L, Mahata SK, O'Connor DT and Gallo RL: The
neuroendocrine peptide catestatin is a cutaneous antimicrobial and
induced in the skin after injury. J Invest Dermatol. 128:1525–1534.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li R, Zhang T, Luo J, Wang F, Gu Q, Gan J
and Xiao F: Antifungal activity fragments of N domain of
chromogranin A. Zhong Shan Da Xue Xue Bao. 45:64–67. 2006.(In
Chinese).
|
|
24
|
Aleinein RA, Hamoud R, Schäfer H and Wink
M: Molecular cloning and expression of ranalexin, a bioactive
antimicrobial peptide from Rana catesbeiana in Escherichia coli and
assessments of its biological activities. Appl Microbiol
Biotechnol. 97:3535–3543. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mochon AB and Liu H: The antimicrobial
peptide histatin-5 causes a spatially restricted disruption on the
Candida albicans surface, allowing rapid entry of the peptide into
the cytoplasm. PLoS Pathog. 4:e10001902008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kong BW, Lee J, Bottje WG, Lassiter K, Lee
J, Gentles LE, Chandra YG and Foster DN: Microarray analysis of
early and late passage chicken embryo fibroblast cells. Poult Sci.
92:770–781. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
National Committee for Clinical Laboratory
Standards (NCCLS): Reference method for broth dilution antifungal
susceptibility testing of yeasts: Approved standard (2nd). Wayne,
USA: NCCLS. 1–13. 1997.
|
|
28
|
Huang J, Hao D and Chen Y, Xu Y, Tan J,
Huang Y, Li F and Chen Y: Inhibitory effects and mechanisms of
physiological conditions on the activity of enantiomeric forms of
an α-helical antibacterial peptide against bacteria. Peptides.
32:1488–1495. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Su Y, Ma L, Wen Y, Wang H and Zhang S:
Studies of the in vitro antibacterial activities of several
polyphenols against clinical isolates of methicillin-resistant
Staphylococcus aureus. Molecules. 19:12630–12639. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Eliopoulos GM and Moellering RC:
Antimicrobial combinations. Antibiotics in Laboratory Medicine.
Lorian V: (Baltimore, MD). Williams and Wilkins. 432–492. 1991.
|
|
31
|
Hancock RE and Diamond G: The role of
cationic antimicrobial peptides in innate host defences. Trends
Microbiol. 8:402–410. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tlaskalová-Hogenová H, Stepánková R,
Hudcovic T, Tucková L, Cukrowska B, Lodinová-Zádníková R, Kozáková
H, Rossmann P, Bártová J, Sokol D, et al: Commensal bacteria
(normal microflora), mucosal immunity and chronic inflammatory and
autoimmune diseases. Immunol Lett. 93:97–108. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lehmann J, Retz M, Sidhu SS, Suttmann H,
Sell M, Paulsen F, Harder J, Unteregger G and Stöckle M: Antitumor
activity of the antimicrobial peptide magainin II against bladder
cancer cell lines. Eur Urol. 50:141–147. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wakabayashi H, Teraguchi S and Tamura Y:
Increased Staphylococcus-killing activity of an
antimicrobial peptide, lactoferricin B, with minocycline and
monoacylglycerol. Biosci Biotechnol Biochem. 66:2161–2167. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Olafsson JH, Sigurgeirsson B and Baran R:
Combination therapy for onychomycosis. Br J Dermatol. 149:15–18.
2003. View Article : Google Scholar : PubMed/NCBI
|