Introduction
The extensively studied proinflammatory cytokine
interleukin (IL)-1β is, among other humoral factors, a crucial
mediator of the inflammatory response. IL-1β is secreted primarily
by blood monocytes and macrophages, and is known to induce fever in
the hypothalamic vascular network, facilitate neutrophil
extravasation and bone marrow production, and induce IL-6
production (1). The dysregulation of
IL-1β expression has been attributed to a number of systemic
conditions, including familial Mediterranean fever, Muckle-Wells
syndrome and diabetes mellitus type 2 (DMT2), while the inhibition
of IL-1β expression has resulted in the mitigation of rheumatoid
arthritis (RA), osteoarthritis and gout (2). IL-18 is an immunomodulatory cytokine
that has been associated with a Th1-mediated immune reaction via
the induction of interferon-γ. Furthermore, IL-18 has been
associated with a number of autoinflammatory conditions, including
systemic lupus erythematodes, Crohn's disease, psoriasis and
graft-versus-host disease (3–5). In a
previous mouse model of sepsis, animals with deletion or blockade
of IL-18 had increased survival rates (6). IL-18 is thought to be expressed
constitutively; however, the expression of IL-1β has to be induced
by extracellular stimuli, such as damage-associated molecular
pattern molecules or pathogen-associated molecular pattern
molecules via toll-like receptor (TLR)-activation (4,7).
Due to their highly relevant biological activities,
IL-1β and IL-18 undergo tightly regulated intracellular processing.
Prior to their secretion, IL-1β and IL-18 require the
caspase-1-dependent cleavage and subsequent activation from their
respective zymogen (pro-IL-1β and pro-IL-18) into bioactive forms
(4,5). The proteolytical activation of
caspase-1 is promoted by multiprotein complexes termed
inflammasomes (8). The majority of
known inflammasomes contain inactive caspase-1, in addition to the
nucleotide-binding oligomerization domain, leucin-rich repeats and
pyrin domain-containing protein (NLRP) and ultimately an
apoptosis-associated speck-like protein containing a caspase
recruitment domain (PYCARD) adaptor molecule (9). While the first described inflammasome
was associated with NLRP1, the most extensively characterized
inflammasome is NLRP3-containing (10). PYCARD is not essential to the NLRP1
inflammasome and is only necessary for the formation of NLRP3
inflammasomes; however, caspase-1 is obligatory and common to the
majority of inflammasomes (9).
Adrenaline and noradrenaline are known as the
prototypes of humoral stress signals, playing an important role as
pharmaceutical agents for the treatment of anaphylaxis, asthma and
for blood pressure control in intensive care medicine (11–15). The
catecholamines adrenaline and noradrenaline were first purified by
Takamine in 1901 and von Euler in 1946, respectively (12,14,16).
Additionally, there was increased interest in these catecholamines
following the identification of their immunomodulating properties
(17). In rheumatology, adrenergic
stimulation has been associated with proinflammatory effects in
acute diseases and anti-inflammatory effects in chronic diseases
(18). Sympathetic activation, as
induced by psychological stress, has been linked to chronic
low-grade inflammatory diseases, such as DMT2, cardiovascular
diseases, RA and the modulation of multiple sclerosis (19–21).
Previous studies that investigated the effect of
catecholamines on human monocytes revealed an increase in the
secretion of a number of proinflammatory cytokines, notably IL-1β
(22,23). Grisanti et al (22) demonstrated that the application of
phenylephrine (PE) increased the intracellular load of the 31-kD
progenitor pro-IL-1β and enhanced IL-1β protein expression in human
monocytes. Previous studies have indicated that adrenergic
stimulation induced p38 mitogen-activated protein kinase (MAPK)
phosphorylation. Although IL-1β generation does not seem to require
p38 MAPK activation, synergistic effects with the nuclear factor-κB
(NF-κB) pathway appear to be mediated via this signal in a number
of contexts (22,24). In addition to the gene expression of
pro-IL-1β, NLRP3 gene expression is induced by NF-κB activation
(25,26).
Although the IL-18 gene is speculated to be
expressed constitutively, there have been conflicting results
regarding the induction of IL-18 by TLR-dependent pathways. Certain
results suggested that high mobility group box-1 administration
stimulates pro-IL-18 synthesis via NF-κB and p38 MAPK in THP-1
macrophages, while LPS-stimulation does not induce the expression
of pro-IL-18 mRNA and protein in human primary peripheral blood
mononuclear cells (7,27).
The current pathophysiological understanding of the
aforementioned chronic inflammatory diseases demonstrates the
decisive role of inflammasomes. To the best of our knowledge, no
prior study has investigated the extent to which the expression and
activity of the inflammasomes is influenced by adrenergic stimuli.
Furthermore, the IL-1β release in parallel with the IL-18 response
has not previously been addressed within the context of adrenergic
stimulation. Therefore, the aim of the present study was to
determine whether the exposure of human primary monocytes to PE
co-stimulated with LPS was able to: i) Further increase IL-1β
secretion; ii) modulate the IL-18 response; and iii) alter the gene
expression profile of inflammasome components.
Materials and methods
Ethical approval
This study was performed in the University Hospital
Frankfurt, Goethe-University (Frankfurt, Germany) with the approval
of the institutional ethics committee (no. 312/10, in accordance
with the Declaration of Helsinki and following STROBE guidelines)
(28). All healthy volunteers
provided written informed consent in accordance with ethical
standards. A total of 21 healthy volunteers (age range, 21–62
years) were enrolled in this study, including 11 men and 10 women,
with a mean age of 31.9±11.7 years.
Blood sampling
Peripheral blood samples (9 ml) were obtained in
ethylenediaminetetraacetic acid (EDTA) tubes (Sarstedt AG & Co,
Nürmbrecht, Germany) and stored at room temperature.
Monocyte purification
Monocytes from healthy volunteers were isolated from
fresh blood samples using Ficoll density gradient centrifugation
(Ficoll solution, 1.077 g/ml; Biochrom GmbH, Berlin, Germany) at
600 × g for 20 min at room temperature. Following the removal of
the mononuclear cell layer, cells were washed twice in MACS buffer,
containing 2 mM EDTA (Sigma-Aldrich, St. Louis, MO, USA) and 0.5%
bovine serum albumin (Sigma-Aldrich) in Dulbecco's
phosphate-buffered saline without Mg2+ and
Ca2+ (Gibco Thermo Fisher Scientific, Inc., Karlsruhe,
Germany). Subsequently, monocytes were isolated by positive
selection using anti-CD14-coated magnetic beads from Miltenyi
Biotec (Bergisch Gladbach, Germany), according to the
manufacturer's instructions. The purity of the isolated
CD14+ cells (>96%) was confirmed by flow cytometry
(BD FACSCanto II; BD Biosciences, Heidelberg, Germany). Following
their isolation, CD14+ monocytes were immediately used
for experiments. A total of 1×105 cells in 200 µl
RPMI-1640 (Biochrom GmbH) were seeded in 48-well plates (BD
Biosciences, Franklin Lakes, NJ, USA) supplemented with 10%
heat-inactivated fetal calf serum, 100 IU/ml penicillin and 100
µg/ml streptomycin (Gibco; Thermo Fisher Scientific, Inc.) and 20
mM HEPES buffer (Sigma-Aldrich) and adhered for 1 h at 37°C in
humidified 5% CO2. Subsequently, nonadherent cells were
removed and adherent cells were washed and recultured in 200 µl
RPMI-1640 (with supplements) as described earlier.
Cell stimulation and ex vivo cytokine
expression assay
The cells were divided into four groups and
stimulated as follows: LPS group, 2 µg/ml LPS from Escherichia
coli 0127:B8 (Sigma-Aldrich); PE group, 10 µM PE (E4250;
Sigma-Aldrich); LPS + PE group, combination of LPS and PE treatment
at the aforementioned doses; and control (ctrl) group, untreated.
After incubation for 24 h, the supernatants were removed by manual
pipetting, stored at −80°C and subsequently assayed for IL-1β and
IL-18 expression using a Human IL-1β/IL-1F2 Quantikine® ELISA kit
(R&D Systems, Inc., Minneapolis, MN, USA) and a Human IL-18
ELISA kit (MBL International Corporation, Woburn, MA, USA),
according to the manufacturer's instructions.
RNA isolation and semi-quantitative
reverse transcription polymerase chain reaction (RT-PCR)
Following the stimulation of CD14+
monocytes for 24 h, total RNA was isolated using an RNeasy kit
(Qiagen, Hilden, Germany) according to the manufacturer's
instructions. Residual DNA was removed using an RNase-free DNase
kit (Qiagen). The quality and quantity of RNA were determined
photometrically using a NanoDrop 1000 spectrophotometer (NanoDrop
Technologies, Wilmington, DE, USA). RNA was subsequently reverse
transcribed using an AffinityScript cDNA Synthesis kit (Agilent
Technologies, Waldbronn, Germany) and subjected to
semi-quantitative RT-PCR, as described previously (29). To determine the mRNA expression
levels of NLRP1, NLRP3, IL-1β, IL-18, caspase-1 and PYCARD, an
Mx3005P qPCR system (Agilent Technologies) was used with
gene-specific primers for human NLRP1 (GenBank no. NM_033004;
UniGene no. Hs.652273; cat. no. PPH06155E), NLRP3 (GenBank no.
NM_183395; UniGene no. Hs.159483; cat. no. PPH13170A), IL-1β
(GenBank no. NM_000576; UniGene no. Hs.126256; cat. no. PPH00171B),
IL-18 (GenBank no. NM_001562.2; UniGene no. Hs.83077; cat. no.
PPH00580C), CASP1 (GenBank no. NM_033292; UniGene no. Hs.2490; cat.
no. PPH00105B) and PYCARD (GenBank no. NM_013258; UniGene no.
Hs.499094; cat. no. PPH00907A) that were purchased from
SABiosciences (Frederick, MD, USA). Human glyceraldehyde
3-phosphate dehydrogenase (GAPDH; GenBank no. NM_002046; UniGene
no. Hs.592355; cat. no. PPH00150E; SABiosciences) was assayed as
the reference gene. PCR was conducted using a 1X RT2
SYBR Green/Rox qPCR Master Mix (SABiosciences) in a total volume of
25 µl, according to the manufacturer's instructions. A two-step
amplification protocol was conducted, as follows: Initial
denaturation at 95°C for 10 min, followed by 40 cycles of 15 sec
denaturation at 95°C and 60 sec annealing/extension at 60°C. The
specificity of amplification products was controlled using the
melting-curve analysis. Relative expression of target mRNA in each
sample was calculated using the comparative threshold-cycle (Ct)
method (ΔΔCt) method, as described previously (30). The quantity of target mRNA in each
sample was normalized against that of GAPDH mRNA in order to
determine ΔCt, and then against the quantity of a calibrator
consisting of ΔCt from unstimulated cells (ctrl group). Relative
gene expression is presented as a fold change compared with the
ctrl. Methods of monocyte purification, seeding and sampling were
similar in design to a previous study (31).
Statistical analysis
GraphPad Prism software, version 6.0 (GraphPad
Software Inc., La Jolla, CA, USA) was used to perform the
statistical analysis. Normality of all data was verified by the
Kolmogorov-Smirnov test. Data are presented as the mean ± standard
error of the mean. One-way analysis of variance with a Dunn
post-hoc test were used for comparison among the different groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
LPS and PE response in
CD14+ monocytes
To determine the monocyte function following
adrenergic (PE) and LPS stimulation, the ex vivo IL-1β and
IL-18 expression in the isolated CD14+ monocytes was
measured by ELISA. IL-1β expression was significantly increased
following LPS stimulation compared with that in the untreated ctrl
(475.1±73.0 vs. 3.8±1.7 pg/ml; P<0.05; Fig. 1A. The administration of PE alone had
no effect on IL-1β expression compared with that of the
unstimulated ctrl (1.2±1.1 vs. 3.8±1.7 pg/ml; Fig. 1A). However, treatment with LPS + PE
significantly increased IL-1β expression compared with all other
treatments [725.8±75.8 vs. 3.8±1.7 (ctrl), 475.1±73.0 (LPS) and
1.2±1.1 pg/ml (PE); P<0.05; Fig.
1A].
IL-18 expression was significantly decreased by LPS
stimulation compared with that of the untreated ctrl (1.7±0.5 vs.
4.4±0.7 pg/ml; P<0.05; Fig. 1B).
Stimulating monocytes with PE did not significantly alter IL-18
expression compared with the unstimulated ctrl (4.8±0.9 vs. 4.4±0.7
pg/ml; Fig. 1B). However, the
combination treatment of monocytes with LPS + PE significantly
reduced IL-18 expression compared with that in the untreated ctrl
and PE-stimulated cells [2.7±0.6 vs. 4.4±0.7 (ctrl) and 4.8±0.9
pg/ml (PE); P<0.05; Fig. 1B] and
showed a tendency to reverse the LPS-induced IL-18 suppression;
however, this effect was not statistically significant (Fig. 1B).
Effect of LPS and PE on the gene
expression of inflammasome components
To elucidate the basal expression characteristics of
NLRP1 and NLRP3 inflammasome components following LPS and/or PE
stimulation in parallel to the inflammasome functionality, gene
expression analyses of all involved inflammasome components were
performed in CD14+ monocytes from healthy volunteers
following LPS and/or PE stimulation (Fig. 2).
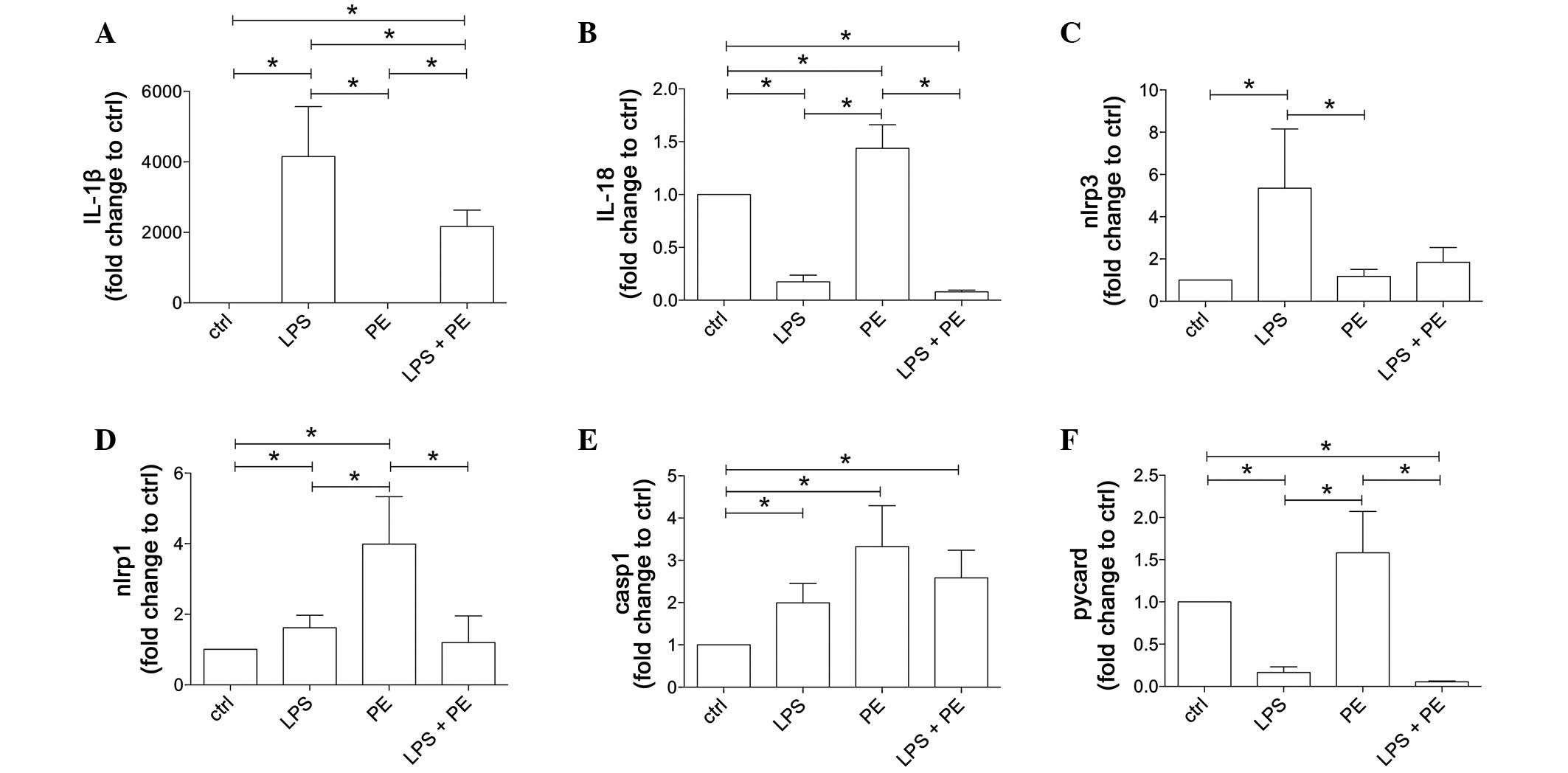 | Figure 2.LPS and/or PE stimulation results in
distinct gene expression pattern of inflammasome components in
CD14+ monocytes, which were isolated from peripheral
mononuclear blood cells from healthy volunteers (n=21) by positive
selection using CD14-magnetic beads. Cells were stimulated with LPS
and/or PE and the gene expression levels (A) IL-β, (B) IL-18, (C)
NLRP3, (D) NLRP1, (E) caspase-1 and (F) PYCARD were measured. Data
are shown as the mean ± standard error of the mean. *P<0.05. IL,
interleukin; LPS, lipopolysaccharide; PE, phenylephrine; NLRP,
NACHT, LRR and PYD domains-containing protein; casp1, caspase-1;
PYCARD, apoptosis-associated speck-like protein containing a
caspase recruitment domain. |
The gene expression of the IL-1β precursor was
notably induced as a result of LPS stimulation
(4,151.0±1,417.0–fold vs. ctrl) compared with the unstimulated ctrl
and PE-stimulated monocytes (1.9±0.8-fold vs. ctrl; P<0.05;
Fig. 2A). PE treatment did not
change IL-1β gene expression compared with ctrl. Co-stimulation of
monocytes with LPS + PE significantly enhanced IL-1β gene
expression compared with the expression of the ctrl or PE-treated
cells; however, LPS + PE led to significantly reduced values
compared with those in the cells treated with LPS alone
[2,170.0±461.0-fold (LPS + PE) vs. 1.9±0.8-fold (PE) or
4,151.0±1,417.0–fold (LPS) change against the ctrl; P<0.05;
Fig. 2A].
IL-18 gene expression was significantly enhanced in
the PE-stimulated cells compared with that in the ctrl, LPS- or LPS
+ PE-stimulated samples [1.4±0.2-fold (PE) vs. 0.2±0.06-fold (LPS)
and 0.1±0.01-fold (LPS + PE) change against the ctrl; P<0.05;
Fig. 2B]. By contrast, IL-18
expression was markedly reduced in the LPS- and LPS + PE-stimulated
cells compared with the ctrl cells.
The expression of NLRP3 was significantly increased
in the LPS-stimulated cells compared with the ctrl and all other
groups [5.4±2.7-fold (LPS) vs. 1.2±0.3-fold (PE) and 1.8±0.7-fold
(LPS + PE) change against the ctrl; P<0.05; Fig. 2C.
Stimulation of CD14+ monocytes with LPS
or PE significantly increased NLRP1 gene expression compared with
the untreated ctrl (P<0.05, Fig.
2D). However, PE-induced NLRP1 expression was markedly enhanced
compared with all other groups (P<0.05), while LPS + PE
decreased NLRP1 gene expression compared with PE [3.9±1.3-fold (PE)
vs. 1.6±0.3-fold (LPS) and 1.2±0.7-fold (LPS + PE) change against
the ctrl; P<0.05; Fig. 2D.
The expression of caspase-1 precursor was
significantly increased in all stimulated groups compared with the
untreated ctrl (P<0.05); however, there were no statistically
significant differences between the stimulation groups (Fig. 2E).
The expression of PYCARD was decreased in the LPS-
and LPS + PE-stimulated cells compared with the ctrl cells;
however, stimulation with PE alone led to a nonsignificant increase
in PYCARD mRNA expression compared with the ctrl cells (Fig. 2F). Furthermore, the expression of
PYCARD was significantly increased in the PE-stimulated cells
compared with the LPS and LPS + PE groups [1.6±0.5-fold (PE) vs.
0.2±0.07-fold (LPS) and 0.1±0.01-fold (LPS + PE) change against the
ctrl; P<0.05; Fig. 2F.
Discussion
Inflammasomes are intracellular molecular
multiprotein platforms that are responsible for the
caspase-1-mediated cleavage of pro-IL-1β and pro-IL-18 into their
active IL-1β and IL-18 forms. The relevance of inflammasomes in a
number of autoimmune diseases has been described previously
(32); however, little is currently
known about the possible effects of ‘stress signals’, such as
endogenous or clinically administered catecholamines, on
inflammasome expression and activation.
The results of the present study indicated a potent
and distinct effect of catecholamines on inflammation-induced
inflammasome activation. Although LPS was proven to be an effective
inflammasome activator as demonstrated by increased IL-1β
expression, PE alone did not induce any effects on the IL-1β
expression. However, the co-stimulation of monocytes with LPS + PE
significantly potentiated the LPS-induced IL-1β expression
(Fig. 1A). These findings are
consistent with the results of Grisanti et al (22), which presented increased levels of
supernatant IL-1β in primary human monocytes co-stimulated with LPS
+ PE compared with LPS-stimulation alone, results which may be
associated with increased p38 MAPK activity. However, in contrast
to the observed increase in pro-IL-1β concentration within the
cytosol in Gristanti et al (22), the gene expression levels of IL-1β
were reduced in the present study. These data indicate that the
enhancing effects of PE co-stimulation on IL-1β secretion following
LPS application may occur on a post-genomic level, possibly as a
result of increased IL-1β mRNA stability, as has been described
previously for p38 MAPK activation (33,34).
However, the present study and the prior study by Grisanti et
al (22) indicate that
PE-stimulation alone does not stimulate IL-1β expression in human
primary monocytes.
Regarding IL-18, the results of the present study
were unexpected. LPS treatment reduced the gene expression and
secretion of the constitutively low-expressed IL-18, and while PE
alone did not seem to have any effect on IL-18 secretion, it
appeared to partly restore IL-18 secretion following LPS
application. However, this trend was not statistically significant
(Fig. 1B). There is consistency
between the IL-18 gene transcription and protein expression levels
observed in the present study and the results of Puren et al
(7), who identified no increase in
pro-IL-18 protein expression following LPS stimulation, and a
reduction in pro-IL-18 mRNA expression after 24 h of LPS exposure.
In order to account for the nonsignificant increase in
IL-18-secretion detected following co-stimulation with LPS + PE
compared with LPS alone, we hypothesize a compensatory effect,
mediated by PE, which may involve increased inflammasome activity
or pro-IL-18 mRNA stability.
The evaluation of IL-1β and IL-18 expression may be
used as an indicator of inflammasome activity. Previous studies
have suggested that LPS alone may not be a sufficient stimulus for
the secretion of IL-1β and inflammasome activation in primary human
monocytes, and have identified ATP binding to P2X7 receptor as a
necessary secondary signal (4,35).
However, recent studies observed stable IL-1β responses and
inflammasome activation in response to LPS stimulation alone
(36–38). The inflammasome activation and
subsequent expression of IL-1β and IL-18 is dependent on
intracellularly available components, which are required for
inflammasome assembly (39).
According to the present results, the gene expression of NLRP1 may
be enhanced by PE alone in the absence of TLR or interleukin
receptor stimulation. This may elucidate the role of NLRs in
chronic, stress-induced, sterile inflammatory diseases, such as
DMT2, RA or multiple sclerosis. Notably, co-stimulation with PE
negated the positive effects of LPS on the gene expression levels
of NLRP3. In previous experiments by the present authors (31), a reduction in NLRP3 gene expression
was observed in patients suffering polytrauma (unpublished data).
It remains unclear whether a surge in endogenous or administered
circulating catecholamines following trauma may mediate this
phenomenon. The present results indicate that adrenergic
stimulation on human monocytes may lead to the suppression of NLRP3
and enhancement of NLRP1 inflammasome gene expression. Furthermore,
a qualitative comparison of the zymogen and NLR gene expression
patterns observed may support the hypothesis that IL-1β secretion
is mediated by the NLRP3 inflammasome, as IL-18 appears to be
associated with NLRP1 activity.
However, considering the secretion of inflammasome
dependent cytokines and the expression profile of associated genes
following LPS and/or PE application, the effects of adrenergic
stimulation on monocytes cannot be exclusively attributed to a
modification of gene expression, but appear to involve an unclear
post-genomic regulation. Notably, the present results do not
support the hypothesis of genomic mechanisms underlying the marked
increase of IL-1β secretion following co-stimulation with LPS + PE
compared with LPS alone, as none of the analyzed constituents of
the secretory mechanism presented further mRNA upregulation.
Further studies are required to determine whether there is an
interaction at a post-genomic level. Although there is an evident
potential therapeutic interest associated with the study of
neuroimmunologic interactions, the present and previous studies
demonstrate the complexity of these processes. As the network of
associations between catecholamines and cytokines is currently
without an adequate model, further studies are required.
Acknowledgements
The authors thank Kerstin Kontradowitz, Katrin
Jurida and Alexander Schaible for their technical assistance, and
Dr Dirk Henrich for his intellectual contribution to the study
(Department of Trauma, Hand and Reconstructive Surgery, University
Hospital Frankfurt, Goethe-University, Frankfurt, Germany).
References
|
1
|
Dinarello CA: Interleukin-1beta. Crit Care
Med. 33(Suppl 12): S460–S462. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dinarello CA: A clinical perspective of
IL-1β as the gatekeeper of inflammation. Eur J Immunol.
41:1203–1217. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Boraschi D and Dinarello CA: IL-18 in
autoimmunity: Review. Eur Cytokine Netw. 17:224–252.
2006.PubMed/NCBI
|
|
4
|
Dinarello CA: Immunological and
inflammatory functions of the interleukin-1 family. Annu Rev
Immunol. 27:519–550. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lebel-Binay S, Berger A, Zinzindohoué F,
Cugnenc PH, Thiounn N, Fridman WH and Pagès F: Interleukin-18:
Biological properties and clinical implications. Eur Cytokine Netw.
11:15–26. 2000.PubMed/NCBI
|
|
6
|
Dinarello CA and Fantuzzi G:
Interleukin-18 and host defense against infection. J Infect Dis.
187(Suppl 2): S370–S384. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Puren AJ, Fantuzzi G and Dinarello CA:
Gene expression, synthesis and secretion of interleukin 18 and
interleukin 1beta are differentially regulated in human blood
mononuclear cells and mouse spleen cells. Proc Natl Acad Sci USA.
96:2256–2261. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Martinon F, Burns K and Tschopp J: The
inflammasome: A molecular platform triggering activation of
inflammatory caspases and processing of proIL-beta. Mol Cell.
10:417–426. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schroder K and Tschopp J: The
inflammasomes. Cell. 140:821–832. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dagenais M, Skeldon A and Saleh M: The
inflammasome: In memory of Dr. Jurg Tschopp. Cell Death Differ.
19:5–12. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Arnold JJ and Williams PM: Anaphylaxis:
Recognition and management. Am Fam Physician. 84:1111–1118.
2011.PubMed/NCBI
|
|
12
|
Bennett MR: One hundred years of
adrenaline: The discovery of autoreceptors. Clin Auton Res.
9:145–159. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Donohue JF and Ohar JA: New combination
therapies for asthma. Curr Opin Pulm Med. 7:62–68. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
von Euler US: Visceral functions of the
nervous system. Annu Rev Physiol. 16:349–370. 1954. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Giraud R, Siegenthaler N, Arroyo D and
Bendjelid K: Impact of epinephrine and norepinephrine on two
dynamic indices in a porcine hemorrhagic shock model. J Trauma
Acute Care Surg. 77:564–569; quiz 650–651. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yamashima T: Jokichi Takamine (1854–1922),
the samurai chemist, and his work on adrenalin. J Med Biogr.
11:95–102. 2003.PubMed/NCBI
|
|
17
|
Grisanti LA, Perez DM and Porter JE:
Modulation of immune cell function by α(1)-adrenergic receptor
activation. Curr Top Membr. 67:113–138. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Straub RH, Bijlsma JW, Masi A and Cutolo
M: Role of neuroendocrine and neuroimmune mechanisms in chronic
inflammatory rheumatic diseases-the 10-year update. Semin Arthritis
Rheum. 43:392–404. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cosentino M and Marino F: Adrenergic and
dopaminergic modulation of immunity in multiple sclerosis: Teaching
old drugs new tricks? J Neuroimmune Pharmacol. 8:163–179. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Black PH: The inflammatory consequences of
psychologic stress: Relationship to insulin resistance, obesity,
atherosclerosis and diabetes mellitus, type II. Med Hypotheses.
67:879–891. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Capellino S, Cosentino M, Wolff C, Schmidt
M, Grifka J and Straub RH: Catecholamine-producing cells in the
synovial tissue during arthritis: Modulation of sympathetic
neurotransmitters as new therapeutic target. Ann Rheum Dis.
69:1853–1860. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Grisanti LA, Woster AP, Dahlman J, Sauter
ER, Combs CK and Porter JE: α1-Adrenergic receptors positively
regulate toll-like receptor cytokine production from human
monocytes and macrophages. J Pharmacol Exp Ther. 338:648–657. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Johnson JD, Campisi J, Sharkey CM, Kennedy
SL, Nickerson M, Greenwood BN and Fleshner M: Catecholamines
mediate stress-induced increases in peripheral and central
inflammatory cytokines. Neuroscience. 135:1295–1307. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guha M and Mackman N: LPS induction of
gene expression in human monocytes. Cell Signal. 13:85–94. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bauernfeind FG, Horvath G, Stutz A,
Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks
BG, Fitzgerald KA, et al: Cutting edge: NF-kappaB activating
pattern recognition and cytokine receptors license NLRP3
inflammasome activation by regulating NLRP3 expression. J Immunol.
183:787–791. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Takeuchi O and Akira S: Pattern
recognition receptors and inflammation. Cell. 140:805–820. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
He Q, You H, Li XM, Liu TH, Wang P and
Wang BE: HMGB1 promotes the synthesis of pro-IL-1β and pro-IL-18 by
activation of p38 MAPK and NF-κB through receptors for advanced
glycation end-products in macrophages. Asian Pac J Cancer Prev.
13:1365–1370. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Von Elm E, Altman DG, Egger M, Pocock SJ,
Gøtzsche PC and Vandenbroucke JP: STROBE Initiative: The
strengthening the reporting of observational studies in
epidemiology (STROBE) statement: Guidelines for reporting
observational studies. J Clin Epidemiol. 61:344–349. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Relja B, Höhn C, Bormann F, Seyboth K,
Henrich D, Marzi I and Lehnert M: Acute alcohol intoxication
reduces mortality, inflammatory responses and hepatic injury after
haemorrhage and resuscitation in vivo. Br J Pharmacol.
165:1188–1199. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Relja B, Hortstmann JP, Kintradowitz K,
Jurida K, Schaible A, Neunaber C, Oppermann E and Marzi I: Nlrp1
inflammasome is downregulated in trauma patients. J Mol Med (Berl).
Aug 02–2015.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Martinon F, Mayor A and Tschopp J: The
infammasomes: Guardians of the body. Annu Rev Immunol. 27:229–265.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen YL, Huang YL, Lin NY, Chen HC, Chiu
WC and Chang CJ: Differential regulation of ARE-mediated TNFalpha
and IL-1beta mRNA stability by lipopolysaccharide in RAW264.7
cells. Biochem Biophys Res Commun. 346:160–168. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sirenko OI, Lofquist AK, DeMaria CT,
Morris JS, Brewer G and Haskill JS: Adhesion-dependent regulation
of an A+U-rich element-binding activity associated with AUF1. Mol
Cell Biol. 17:3898–3906. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mariathasan S and Monack DM: Inflammasome
adaptors and sensors: Intracellular regulators of infection and
inflammation. Nat Rev Immunol. 7:31–40. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen S and Sun B: Negative regulation of
NLRP3 inflammasome signaling. Protein Cell. 4:251–258. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
He Y, Franchi L and Núñez G: TLR agonists
stimulate Nlrp3-dependent IL-1β production independently of the
purinergic P2X7 receptor in dendritic cells and in vivo. J Immunol.
190:334–339. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Netea MG, Nold-Petry CA, Nold MF, Joosten
LA, Opitz B, van der Meer JH, van de Veerdonk FL, Ferwerda G,
Heinhuis B, Devesa I, et al: Differential requirement for the
activation of the inflammasome for processing and release of
IL-1beta in monocytes and macrophages. Blood. 113:2324–2335. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Latz E, Xiao TS and Stutz A: Activation
and regulation of the inflammasomes. Nat Rev Immunol. 13:397–411.
2013. View
Article : Google Scholar : PubMed/NCBI
|
















