Introduction
Currently, among the various treatment methods for
liver alveolar echinococcosis (LAE), radical surgical excision has
been the most effective (1).
Clinical follow-up results have shown that complete resection of
LAE lesion regions during surgery can ensure nonrecurrence and
achieve radical cure (2). Hepatic
surgical radical resection usually requires the resection range to
be 1 cm over the lesion edge (2);
however, since LAE lesions usually have no envelope, and the
boundaries between the lesions and the normal liver tissues may not
be obvious, surgical LAE lesion resection based on visual
observation can leave residual lesions, resulting in the high
likelihood of disease recurrence following surgery (3). A diagnosis of LAE and LAE lesion
boundaries should be confirmed preoperatively. For patients with
LAE that cannot undergo radical liver resection, evaluating such
biological characteristics as the proliferation and invasion of
lesions is important so that an appropriate alternative treatment
plan can be made (4).
A previous study (5)
showed that the increased uptake of
18F-fluorodeoxyglucose (18F-FDG) was not only
associated with tumor cell proliferation and increased
hypoxia-induced glycolysis, but also with gene mutations of various
tumors during signal transduction, which resulted in the inhibition
of apoptosis and oxidative phosphorylation and the activation of
glycolysis; therefore, in vivo molecular imaging examination
can determine the biological target regions of tumors, and the
imaging can be used to guide clinical diagnosis and treatment.
Another study confirmed that the biological behavior of LAE lesions
was similar to that of tumors, including peri-LAE lesion neovessels
and hepatocellular apoptosis (6). In
terms of chronic inflammation, 18F-FDG accomplished the
imaging diagnosis primarily by identifying the notably increased
energy demand of active-energy metabolism inflammatory cells and
proliferated fibroblasts, among others. Reuter et al
(7) used positron emission
tomography (PET) to follow the metabolic changes of
18F-FDG inside LAE lesions and found that when the edge
of the focal liquefaction zone retained the sparse reduction of
radionuclides, the lesion would be relatively stable; however, when
radionuclides on the edge of the focal liquefaction zone progressed
from sparse reduction to local accumulation, the invasive progress
of the lesions increased, and the ‘proliferation and infiltration
band’ on the lesion edges or the active lesion region was
recognized as important indicator of the development of lesions.
Peri-LAE tissue-bioactive regions that exhibited different energy
metabolisms and redefined biological boundaries of the lesions
revealed the clinical significance of recognizing the LAE
infiltration mechanism in the surrounding hepatic tissues.
As a treatment option for advanced LAE, autologous
liver transplantation (ALT) has been increasingly carried out in
recent years. Effectively determining the indications and surgical
timing for LAE-ALT and detecting post-ALT recurrence and metastasis
as early as possible is the significant clinical value of ALT as a
treatment for LAE (8). In
vitro, hepatic resection plus ALT could be an ideal choice for
end-stage LAE radical treatment. 18F-FDG PET/computed
tomography (CT) can reveal more biological characteristics of the
lesions, the tissue cells of the lesion limbic region and the
associated development and outcomes. Multiple factors post-ALT can
easily lead to LAE recurrence and distant metastasis.
18F-FDG PET/CT can assist in the early detection and
assessment of post-ALT hepatic functional status, as well as the
recurrence or risk of metastasis. The present study reviews the
clinical data, radiological information and follow-up associated
with the use of 18F-FDG PET/CT in 8 LAE-ALT patients,
with the aim of evaluating its value in preoperative evaluation and
prognosis determination.
Materials and methods
Clinical data
Eight patients with LAE, who were treated in the
First Affiliated Hospital of Xinjiang Medical University (Urumqi,
China) between December 2010 and December 2013, were selected for
this study. The patients included 3 males and 5 females with an
average age of 36±14 years. This study was conducted in accordance
with the Declaration of Helsinki and with approval from the Ethics
Committee of Xinjiang Medical University. Written informed consent
was obtained from all participants. All patients underwent
echinococcosis serological examination, with 8 positive results
that were confirmed by postsurgical echinococcosis
histopathological examination. All patients were treated by in
vitro hepatic resection plus ALT. The LAE diagnoses in this
study were between benign and malignant, growth was relatively slow
and the compensatory hyperplasia of the healthy liver met the need
for transplantation. The normal liver mass in these patients with
LAE was 860±350 g after a half year of rest.
18F-FDG-PET/CT imaging
protocol and image interpretation
The Discovery VCT PET/CT (GE Healthcare
Bio-Sciences, Pittsburgh, PA, USA) was used with an
18F-FDG tracer produced by Cyclotron (GE Healthcare
Bio-Sciences) that had a radiochemical purity of >95%. Prior to
the PET/CT examination, patients fasted for 4–6 h to achieve a
fasting blood glucose of <7 mmol/l. Patients were intravenously
injected with 18F-FDG (7.4 MBq/kg body weight); 30 min
after the injection, the patients drank 1,000 ml water. Following
bladder emptying 1 h later, the patients drank another 300 ml water
to fill the gastrointestinal tract. The PET/CT imaging
followed.
The positioning image was performed first to
determine the scanning range, which was from the top of the cranium
to the mid-upper segment of the thighbone. The CT image acquisition
parameters were as follows: Voltage, 120 kV; tube current, 260 mA;
detector collimation, 64×0.625 mm; layer thickness, 3.75 mm;
interlayer spacing, 3.75 mm; 0.6 msec/rotation; detector pitch,
0.983; and scanning time, 20–30 sec. The patient was told to
breathe calmly. The three-dimensional PET acquisition was performed
with the same scanning range as the CT, generally with 6–8 bed
positions. Data for each bed position were collected for 3 min.
Following collection, the CT data were applied to perform
attenuation corrections for the PET images. The ordered subset
expectation maximization iterative method was used to reconstruct
the images of the cross-sectional, coronal and sagittal planes, as
well as the PET-CT fusion images. All patients were treated with
standard 18F-FDG-PET/CT acquisition (i.e., PET/CT
acquisition was performed 1 h after 18F-FDG injection;
the delayed 18F-FDG-PET/CT acquisition was performed 2 h
after 18F-FDG injection).
The early and delayed images of the patients were
collected; image diagnosis was carried out by experienced
physicians in the Department of Nuclear Medicine at the First
Affiliated Hospital of Xinjiang Medical University, and the PET, CT
and PET/CT fusion images were independently analyzed. First, image
quality was determined by visual analysis, allowing normal
physiological uptake, as well as normal variations and artifacts,
to be standardized. Next, the abnormal radiopharmaceutical
accumulation and biological borders of the lesions, and the numbers
and measurements of the maximum standardized uptake value (SUVmax)
accumulated in these lesions were recorded. According to the
anatomical information provided by the same CT machine, the
position of each lesion was then precisely identified.
All patients underwent surgery, and pathological
sectioning was performed on the diseased tissues; a pathologist
analyzed the slices.
Statistical analysis
SPSS 16.0 software (SPSS, Inc., Chicago, IL, USA)
was used for the statistical analysis, and the measurement data are
expressed as the mean ± standard deviation. The biological
boundaries of LAE and the ALT-SUVmax were measured, and the data
were analyzed by two-sample t-tests. The diagnostic performance
analysis of the 18F-FDG-PET/CT LAE biological boundary
was determined by the χ2 test, with P<0.05 considered
to be a significant difference.
Results
Preoperative LAE-ALT assessment
The 8 patients with LAE had a total of 17 hepatic
lesions, among which 12 were in the right lobe, 5 were in the left
and 3 were extrahepatic. The mean LAE lesion diameter was 6.85±4.35
cm.
The LAE liver morphologies were disordered, the
ratio of hepatic lobes was imbalanced, the outlines were
incomplete, partial liver structures were missing, the
radioactivity distribution inside the liver parenchyma was uneven
and there were various degrees of disease involvement in the
hepatic boundaries. Ten LAE lesions exhibited points of
calcification that all were located in the central part of the
lesions. Five patients exhibited obvious abnormalities in the porta
hepatis, and 3 exhibited extrahepatic bile duct involvement. The
lesions were all mixed space-occupying lesions and most had
irregular shapes. The LAE lesion boundaries were unclear and
infiltrated tissues; the peri-lesion boundaries could be outlined
through the radiopharmaceutical intake. Focal radiopharmaceutical
distribution presented as an enhanced distribution of radioactivity
on the partial edges, suggesting that the focal lesions were in the
proliferative growth or LAE active period (Fig. 1).
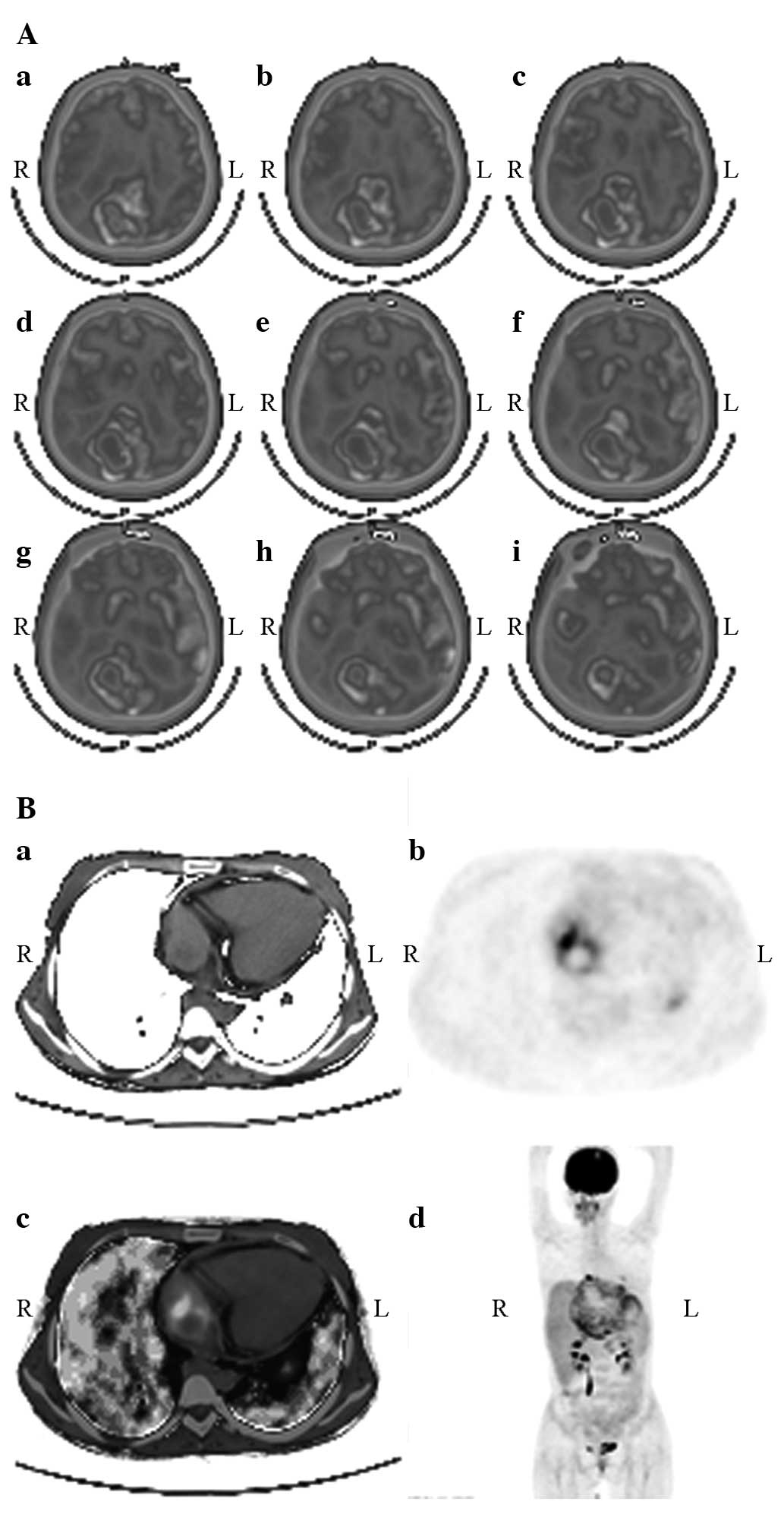
 | Figure 1.Whole-body positron emission
tomography (PET)/computed tomography (CT) imaging. (A) (a) Hepatic
right lobe exhibited a mixed space-occupying lesion with multiple
scattered, spotty calcification lesions and regions of liquefactive
necrosis, as detected by CT; (b) A region with significant
radiopharmaceutical distribution was noted at the right anterior
segment of hepar, as detected by PET; (c) A region with significant
radiopharmaceutical distribution was noted at the junction of the
hepatic right and left lobes; the shape was irregular and there was
high radiopharmaceutical up take, as detected by PET/CT fusion
imaging; (d) A region with significant radiopharmaceutical
distribution was noted at the hepatic right lobe, as detected by
MIP. (B) (a) The hepatic right lobe exhibited a mixed
space-occupying lesion with multiple scattered, spotty
calcification lesions and regions of liquefactive necrosis, as
detected by CT; (b) The radiopharmaceutical accumulation was
significantly enhanced, as compared with the earlier image, and
standardized uptake value (SUV) was increased, as detected in the
PET delayed image; (c) A region with significant
radiopharmaceutical distribution was noted at the junction of the
hepatic right and left lobes, and the radiopharmaceutical
accumulation was significantly enhanced, as detected in the PET/CT
fusion delayed image; (d) The radiopharmaceutical accumulation was
significantly enhanced, as compared with the earlier image, and SUV
was increased at the hepatic right lobe, as detected in the MIP
delayed image. (C) Between the coagulation of necrotic and normal
liver tissue, fibroblast proliferation and lymphocyte, plasma cell
and eosinophil infiltration could be observed, forming the
inflammatory fibrous tissue band (HE staining). MIP, maximum
intensity projection. |
The radiopharmaceutical intake in the peri-lesion
tissues was significant, possessing the characteristics of
radiopharmaceutical-outlined borders and exhibiting
border-increased radiopharmaceutical intake. The SUVmax value of
the patients was 3.57±1.21 and exhibited the characteristics of
biological activity (Fig. 1).
Radiopharmaceutical fading did not occur in the 2-h delayed images,
whereas the radioactivity distribution was more enhanced than the
previous 18F-FDG uptake. The SUVmax in the delayed
images was 4.19±1.70 overall, and each lesion exhibited various
degrees of increased SUV (Fig.
2).
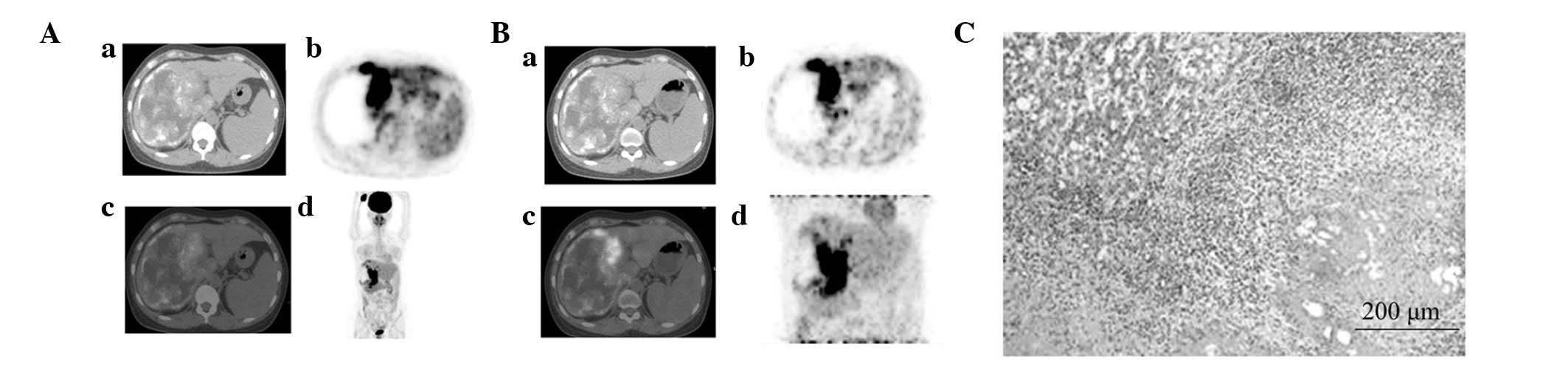
 | Figure 2.Comparison of whole-body positron
emission tomography (PET)/computed tomography (CT) images (A) prior
to and (B) following autologous liver transplantation. (A) (a)
Hepatic right lobe exhibited a mixed space-occupying lesion with
multiple scattered, spotty calcification lesions and regions of
liquefactive necrosis, as detected by CT; (b) A cycle with
significant and uneven radiopharmaceutical distribution was noted
at the the hepatic right lobe, as deteceted by PET; (c) A cycle
with significant and uneven radiopharmaceutical distribution was
noted at the hepatic right lobe, the shape was irregular and there
was high radiopharmaceutical uptake, as detected by PET/CT fusion
imaging; (d) A lump-like and uneven significant radiopharmaceutical
distribution was noted at the hepatic lobes, as detected by MIP.
(B) (a) The density of autologous transplanted liver was uniform,
as detecetd by CT; (b) Radiopharmaceutical distribution was
uniform, as detected by PET; (c) Radiopharmaceutical distribution
was uniform in autologous transplanted liver, as detected by PET/CT
fusion imaging; (d) Radiopharmaceutical distribution was uniform in
autologous transplanted liver, as detected by MIP. MIP, maximum
intensity projection. |
The patients underwent surgical excision of the
lesions, and the resected lesions were sent to the pathology
department for slicing and staining. The results of the
pathological diagnoses were as follows: i) Hepatic
steatosis/hepatic sinusoid dilatation and congestion; ii)
inflammatory band around the liver cells; iii) echinococcosis with
Echinococcosis multilocularis; iv) calcified lesions of
echinococcosis; v) fibrous inflammatory band/infarct zone; vi)
small bile duct hyperplasia; vii) inflammatory band; and viii)
normal/fibrous inflammatory band/echinococcosis region.
The SUV of the LAE-normal liver areas was 0.95±0.19
overall. Among the 8 patients with LAE, 3 exhibited a significant
extrahepatic radiopharmaceutical uptake area; specifically, the
gyrus rectus of the right brain, right atrium and both lungs
exhibited biologically active LAE lesions (Fig. 3). The SUVmax value was 2.05±0.72 and
the SUVmax of the delayed imaging was 3.15±0.83, indicating LAE
extrahepatic invasion and a proliferating or active period of
LAE.
Diagnostic PET/CT LAE test
analysis
Pathological diagnosis was performed on the 17
lesions, and SPSS 17.0 software was used to determine the
statistical analyses of the biopsy results. The PET/CT images,
PET/CT-based LAE biological borders and pathological lesion
boundaries determined the diagnostic test analyses with a
corresponding fourfold table. The statistical analyses of the
pathological results and PET/CT diagnostic results are shown in
Table I.
 | Table I.Statistics of PET/CT diagnosis and
pathological diagnosis. |
Table I.
Statistics of PET/CT diagnosis and
pathological diagnosis.
|
| Pathological
diagnosis (standard diagnosis) |
|
|---|
|
|
|
|
|---|
| PET/CT diagnosis | + | − | Total |
|---|
| + | 11 | 2 | 13 |
| − | 1 | 3 | 4 |
| Total | 12 | 5 | 17 |
Post-ALT assessment
Assessment of liver survival status following
LAE-ALT
The SUVmax value of patients 1 month after LAE-ALT
was 1.23±0.78; the values after 3 and 6 months were 1.15±0.67 and
0.85±0.35, respectively. Compared with the normal liver SUV value
(0.95±0.19), the 1-month SUV value exhibited a statistically
significant difference. All LAE-ALT patients were followed for 1–39
months, and no new occurrence of extrahepatic LAE metastasis was
found.
Biological activities of post-LAE-ALT
extrahepatic metastatic lesions
The SUVmax value of the extrahepatic metastatic
lesions 3 months after LAE-ALT was 1.85±0.62 and the delayed
imaging SUVmax at the same time-point was 2.95±0.79. These values
exhibited no significant difference from the preoperative
values.
Discussion
Since LAE cannot be easily detected in the early
clinical stage, Echinococcus parasites have typically
already widely invaded intrahepatically and extrahepatically when
obvious liver function impairment is found (8,9). As a
result, the radical resection rate associated with LAE has been low
(10), and, if the condition is not
treated, the 10-year mortality rate can be as high as 93%. LAE
causes much more harm to the human body than does cystic hydatid
disease, and thus it has been referred to as the ‘worm cancer’
(11).
Currently, among the LAE treatment methods, only
radical surgical excision has shown notable efficacy. Clinical
follow-up results have revealed that the complete removal of LAE
lesions can ensure no recurrence and radical cure (12). For those patients in whom radical
liver resection is not an option, evaluating the proliferation and
invasion of lesions is important for an appropriate treatment
plan.
According to the distribution and distribution
characteristics of positron radiopharmaceuticals, the associations
and pathological relationships with other suspicious lesions can be
diagnosed and, thus, the positive boundary edges between the LAE
lesions and normal liver tissues can be more accurately exhibited
(13). Within the 8 LAE cases in
this study, there were 17 lesions, with 12 on the right lobe and 5
on the left. The overall LAE lesion diameter was 6.85±4.35 cm. The
radiopharmaceutical uptake around the LAE lesions was significant
and exhibited increased radiopharmaceutical-outlined borders. The
overall SUV value was 3.57±1.21 and indicated bioactive
characteristics. The 2-h delayed image did not indicate fading
radiopharmaceutical uptake, and the radioactivity distribution was
more enhanced than the previous 18F-FDG uptake. The SUV
of the delayed image was 4.19±1.70 overall, and each lesion
exhibited different degrees of increased SUV. Postoperative
pathology confirmed the diagnosis of LAE and an inflammatory tissue
band, similar to that described by Caoduro et al (14).
Following the study of LAE for 30 years, Wen et
al (15) successfully completed
the LAE-ALT procedure. Due to limited intrahepatic lesions and no
extrahepatic metastasis, 90% of patients with early LAE can be
cured, but most patients are in the end stages of disease when
diagnosed; therefore, only 35% of patients have the option of
radical surgery.
As a result of the late diagnosis of LAE, liver
transplantation has become the only treatment method for end-stage
LAE. LAE recurrence and metastasis are key factors that influence
the long-term outcomes of liver transplantation (16). A lower dose of immunosuppressive
drugs following transplantation, as well as the long-term, systemic
administration of anti-LAE drugs, is important to prevent and
reduce LAE recurrence and metastasis. In vitro liver
resection plus ALT is an ideal choice for end-stage LAE radical
treatment (17,18). The significance of the evaluation
efficacy of PET/CT in LAE-ALT has made the biological
characteristics of LAE lesions clear prior to surgery; it is now
possible to evaluate whether invasion has occurred in other organs
prior to surgery.
In order to determine the probabilities of the
occurrence post-ALT recurrence and metastasis of LAE, PET/CT can
simultaneously enable observation of the morphology and metabolism
of all body tissues and reveal the biological characteristics of
the transplanted liver. In this study, the SUVmax value of liver
surviving 1 month after LAE-ALT was 1.23±0.78, and that of liver
surviving after 3 and 6 months was 1.15±0.67 and 1.03±0.06,
respectively. Compared with that of normal liver, the 1-month
SUVmax value exhibited a statistically significant difference,
suggesting that the post-ALT surviving liver exhibited dynamic
18F-FDG metabolic changes. In the first month the
18F-FDG uptake indicated high metabolic changes, but
these stabilized 2 months later, which was similar to the
18F-FDG metabolism of the normal liver. All LAE-ALT
patients were followed up for 1–39 months, with the exception of 1
patient with LAE extrahepatic metastasis, and this reflects the
ideal radical surgical treatment towards end-stage LAE.
Among the 8 patients in this study, 3 exhibited
significant extrahepatic radiopharmaceutical uptake on the gyrus
rectus of the right side of the brain, the right atrium or both the
lungs, which showed bioactive LAE lesions (SUVmax, 2.05±0.72;
delayed imaging SUVmax, 3.15±0.83), indicating that ALT did not
change the biological activities of the extrahepatic lesions of
LAE.
Controversy remains, both in China and elsewhere,
about the selection of indications and timing of LAE-ALT.
Bresson-Hadni et al (19,20)
believed that patients with end-stage LAE that are unable to
undergo this surgical treatment should be added to the liver
transplantation waiting list. It has also been suggested that
preoperative brain metastasis should be classified as a
contraindication to surgery, whereas lung metastasis has not been
classified as a contraindication (21). From the results of the current study,
ALT should still be considered the ideal treatment method, even in
the presence of extrahepatic metastasis. As the SUVmax value of the
post-ALT extrahepatic metastatic lesions was 1.85±0.62 and the
delayed imaging SUVmax exhibited no significant difference from the
value prior to transplantation, this study could provide an
important basis for selecting the indications and surgical timing
of LAE-ALT.
Acknowledgements
This study was supported by the Natural Science
Foundation of Xinjiang Uygur Autonomous Region (grant nos.
2012211A0 and 2012211A081).
References
|
1
|
Silva MA, Mirza DF, Bramhall SR, Mayer AD,
McMaster P and Buckels JA: Treatment of hydatid disease of the
liver. Evaluation of a UK experience. Dig Surg. 21:227–233. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parsak CK, Demiryurek HH, Inal M, et al:
Alveolar hydatid disease: Imaging findings and surgical approach.
Acta Chir Belg. 107:572–577. 2007.PubMed/NCBI
|
|
3
|
Gollackner B, Längle F, Aner H, et al:
Radical surgical therapy of abdominal cystic hydatid disease:
Factors of recurrence. World J Surg. 24:717–721. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hosch W, Junghanss T, Werner J and Düx M:
Imaging methods in the diagnosis and therapy of cystic
echinococcosis. Rofo. 176:679–687. 2004.(In German). PubMed/NCBI
|
|
5
|
Lee JW, Paeng J, Kang KW, et al:
Prediction of tumor recurrence by 18F-FDG PET in liver
transplantation for hepatocellular carcinoma. J Nulc Med.
50:682–687. 2009. View Article : Google Scholar
|
|
6
|
Pichler BJ, Kneilling M, Haubner R,
Braumüller H, Schwaiger M, Röcken M and Weber WA: Imaging of
delayed-type hypersensitivity reaction by PET and 18F-galacto-RGD.
J Nucl Med. 46:184–189. 2005.PubMed/NCBI
|
|
7
|
Reuter S, Grüner B, Buck AK, Blumstein N,
Kern P and Reske SN: Long-term follow-up of metabolic activity in
human alveolar echinococcosis using FDG-PET. Nuklearmedizin.
47:147–152. 2008.PubMed/NCBI
|
|
8
|
Charbonnet P, Buhler L, Sagnak E, Villiger
P, Morel P and Mentha G: Long-term follow up of patients with
alveolar echinococcosis. Ann Chir. 129:337–342. 2004.(In French).
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bauder B, Auer H, Schilcher F, Gabler C,
Romig T, Bilger B and Aspöck H: Experimental investigations on the
B and T cell immune response in primary alveolar echinococcosis.
Parasite Immunol. 21:409–421. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tang QK, Zhang Z, Li YS, Yuan CP and Zhang
DT: Analysis on treatment of liver alveolar hydatid disease. Ji
Sheng Chong Yu Gan Ran Xing Ji Bing. 4:19–20. 2006.(In
Chinese).
|
|
11
|
Wen H and Xu M: Practical Echinococcosis.
Beijing: Science Press. 226–227. 2007.
|
|
12
|
Manus DP, Zhang W, Li J and Bartley PB:
Echinococcosis. Lancet. 362:1295–1304. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bostanci B, Tetik C, Terzi C and Ozden A:
Efficiency of ultrasound in the detection of the viability of
hydatid cysts in the liver. Surg Laparosc Endosc Percutan Tech.
9:392–394. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Caoduro C, Porot C, Vuitton DA, et al: The
role of delayed 18F-FDG PET imaging in the follow-up of
patients with alveolar echinococcosis. J Nucl Med. 54:358–363.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wen H, Zuo MX, Wang XY, et al: The first
human liver transplantation of alveolar echinococcosis in China.
Zhong Hua Xiao Hua Wai Ke Za Zhi. 23:3752002.(In Chinese).
|
|
16
|
Yüksel O, Akyürek N, Sahin T, Salman B,
Azili C and Bostanci H: Efficacy of radical surgery in preventing
early local recurrence and cavity-related complications in hydatic
liver disease. J Gastrointest Surg. 12:483–489. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang L, Zhang SJ, Cao YW, et al: The
correlation between osteopontin and metastasis of hepatic
Echinococcus multilocularis infection. Zhongguo Ji Sheng
Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 29:33–36. 2011.(In
Chinese). PubMed/NCBI
|
|
18
|
Elsebaie SB, El-Sebae MM, Esmat ME, Nasr
MM and Kamel MM: Modified endocystectomy versus pericystectomy in
Echinococcus granulosus liver cysta: A randomized controlled
study and the role of specific anti-hydatid IgG4 in detection of
early recurrence. J Egypt Soc Parasitol. 36:993–1006.
2006.PubMed/NCBI
|
|
19
|
Bresson-Hadni S, Vuitton DA, Bartholomot
B, et al: A twenty-year history of alveolar echinococcosis:
analysis of a series of 117 patients from eastern France. Eur J
Gastroenterol Hepatol. 12:327–336. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bresson-Hadni S, Mignet JP, Lenys D, et
al: Recurrence of alveolar echinococcosis in the liver graft after
liver transplantation. Hepatology. 16:279–280. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Czemak BV, Akhan O, Hiemetzberger R, et
al: Echinococcosis of the liver. Abdom Imaging. 33:133–143. 2008.
View Article : Google Scholar : PubMed/NCBI
|