Introduction
Aging is not only a natural phenomenon which is an
inevitable biological process, but is also associated with diverse
chronic diseases, including cancer, Parkinson's and cardiovascular
diseases (1,2). With the increasing elderly population
in the world, aging has already become an important public issue
(3). Oxidative stress is considered
to be significant in the pathophysiology of various diseases,
including the aging process. Reactive oxygen species (ROS) damage
cellular lipids, proteins and DNA, inhibiting their normal
functions. An excess of ROS can result in cell aging and death
(1,2,4).
Numerous studies have discovered that oxidative stress occurs
during the pathogenesis of age-associated diseases (4–8).
D-galactose (D-gal)-treated mice have been
demonstrated to display similar symptoms to those aging naturally
(9). D-gal injection has since been
widely used to establish a model for anti-aging research (10–13).
D-gal, a reducing sugar, is a naturally occurring substance in the
body, which is completely metabolized at normal concentrations.
However, at higher concentrations it is converted to aldose,
hydrogen peroxide and galactose oxidase, thus speeding up the
generation of superoxide anion and oxygen-derived free radicals
that impair the function of macromolecules and cells (14). Various studies have indicated that
D-gal accelerates aging in mice, rats, houseflies and human fetal
lung fibroblast and has been used as an aging model since 1985
(15). Studies with D-gal-induced
mice have shown that D-gal-induced oxidative stress causes
cognitive impairment, neurotoxicity, tissue injury and inflammation
(16,17).
The use of herbal medicines has increased globally
due to their lower adverse effects, price and good efficacy in the
majority of human illnesses (18,19). In
recent years, numerous traditional Chinese medicines have been
found to possess potent anti-aging activities and have attracted
considerable interest as potential candidates for the development
of novel anti-aging therapies (20,21).
Rhein, one of the major bioactive constituents of the rhizome of
rhubarb (Rheum palmatum Linn. or R. tanguticum
Maxim), is a widely used traditional Chinese herb with broad
pharmacological effects, including antidiabetic activity (22,23),
anti-inflammation and inhibition of interleukin-1-induced
chondrocyte activation (24).
However, due to its water insolubility, the efficacy of rhein is
limited in vivo. Rhein lysinate (RHL) is the salt of lysine
and rhein that is water soluble and so may be administered in
vivo in drinking water.
A previous study from our laboratory demonstrated
that RHL possessed an anti-aging effect in vitro, which may
be associated with its anti-oxidative properties (25). However, no studies to date have
addressed the effect of RHL on the aging process in vivo.
The aim of the present study was, therefore, to use of the
D-gal-induced aging model mice in order to investigate the
anti-aging effects of RHL in vivo and explore the underlying
anti-aging molecular mechanisms.
Materials and methods
Chemicals and reagents
Rhein (purity, 98%) was purchased from Nanjing
Qingze Medicine Ltd. (Nanjing, Jiangsu, China), while lysine was
purchased from Beijing Solarbio Science and Technology Co. Ltd.
(Beijing, China). RHL was synthesized at the Oncology Department of
the Institute of Medicinal Biotechnology, Chinese Academy of
Medical Sciences and Peking Union Medical College (Beijing, China;
patent no. 2008100890258). Polyclonal rabbit anti-human Sirtuin 1
(SIRT1; 1:1,000; cat. no. 2493s), polyclonal rabbit anti-human p16
(1:1,000; cat. no. 4824), monoclonal rabbit anti-human p21
(1:1,000; cat. no. 2947s), monoclonal rabbit anti-human β-actin
(1:1,000; cat. no. 12620) primary antibodies, and secondary
antibodies against rabbit (1:5,000; cat. no. 7074s) or mouse
(1:5,000; cat. no. 7076s) IgG were purchased from Cell Signaling
Technology (Danvers, MA, USA). The prestained protein marker,
p7708V, was purchased from New England Biolabs Ltd. (Beijing,
China). All other chemicals were of standard analytical grade.
Animals and drug administration
A total of 40 male Kun-Ming mice (age, 7 weeks;
weight, 32±2 g) were purchased from the Institute of Laboratory
Animal Science (Chinese Academy of Medical Sciences, Beijing,
China). All procedures conducted with animals were approved by our
institutional review board (Animal Experiments Ethics Board,
Beijing Hospital, Beijing, China). The mice were housed and
maintained at 22±2°C with 12 h light/dark cycles and a relative
humidity of 40–60%. Food was provided ad libitum throughout
the study. Following one-week of acclimatization to the home cage,
the mice were randomly divided into four groups, each comprising 10
mice, as follows: i) Model group, in which the mice were injected
subcutaneously with D-gal at a dose of 100 mg/kg/day, and
simultaneously given distilled water by intragastric gavage; ii)
control group, in which the mice were injected subcutaneously with
the same volume of normal saline, and simultaneously given
distilled water by intragastric gavage; iii) RHL (25 mg/kg/day)
group, in which the mice were injected subcutaneously with D-gal at
a dose of 100 mg/kg/day, and simultaneously given RHL (25
mg/kg/day) by intragastric gavage; and iv) RHL (50 mg/kg/day)
group, in which the mice were injected subcutaneously with D-gal at
a dose of 100 mg/kg/day, and simultaneously given RHL (50
mg/kg/day) by intragastric gavage. During the study, the
performance and body weight of the mice were recorded every day.
The mice were sacrificed following 8 weeks of treatment. Blood was
collected through cardiac puncture and centrifuged at 1,500 × g for
15 min at 4°C to obtain plasma. Plasma was stored at −70°C until
assays were performed. The liver and kidney tissues were
immediately collected, weighed and homogenized (4°C; 3,000 × g for
15 min) for biochemical and histological analyses. The organ index
was measured using the following equation: Organ index (mg/g) =
organ weight (mg)/body weight (g) (26).
Biochemical and histological
analysis
The antioxidant activities of superoxide dismutase
(SOD) and glutathione peroxidase (GSH-Px), and the levels of
malondialdehyde (MDA) in the blood and tissues were determined
using SOD, GSH-Px and MDA kits, according to the manufacturer's
instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing,
Jiangsu, China). Subsequent to fixing in 10% formalin for 24 h,
tissue samples were progressively dehydrated in different
concentrations of ethanol, hyalinized in xylene, embedded in
paraffin, sliced into thin sections (5 µm), dewaxed and stained
with hematoxylin and eosin (Beijing Solarbio Science and Technology
Co. Ltd.). Sections were examined using an Olympus CK40 microscope
(Olympus, Tokyo, Japan). Cross sections were selected from three
plates per sample.
Western blot analysis
Western blot analysis was employed to detect the
protein expression levels of SIRT1, p16 and p21. The tissues were
treated with a lysis buffer and a mixture of phosphatase inhibitors
(Roche, Indianapolis, IN, USA). Samples (30 µg) were fractionated
by 10% SDS-PAGE. Once the proteins were transferred to a
polyvinylidene difluoride membrane, the membrane was incubated in a
blocking buffer containing bovine serum albumin (1%) and Tween-20
(0.1% v/v) in phosphate-buffered saline at room temperature for 1
h. The membrane was then incubated overnight at 4°C with the
appropriate primary antibodies and then incubated with the
appropriate secondary antibodies at room temperature for 2 h. Each
membrane was developed using an enhanced ChemiImager 5500
chemiluminescence system (Alpha Innotech Corporation, Miami, FL,
USA).
Statistical analysis
Data are presented as the mean ± standard deviation.
Treatment effects were compared using the Student t-test and
differences between the means were considered to be statistically
significant when P≤0.05.
Results
Behavior observations and organ
index
No statistically significant differences (P>0.05)
in food intake and body weight change were observed between the
mice in the different groups (data not shown). Prior to being
sacrificed, no mice died during the experimental procedure. Mice of
the model group with D-gal demonstrated evident symptoms of aging,
including slow movement, a lag in response, listlessness, and
withered and lackluster fur. In addition, organ indexes of the
liver and kidney of the model group were significantly lower
(P<0.05) compared with those of the control group, as shown in
Table I. However, RHL administration
may improve those organ indexes (P<0.05).
 | Table I.Organ index of mice in each group
(mg/g). |
Table I.
Organ index of mice in each group
(mg/g).
| Group | Liver | Kidney |
|---|
| Control | 46.86±5.43 | 9.27±0.89 |
| Model |
39.24±4.12a |
7.64±0.62a |
| RHL 25 mg/kg |
44.76±4.89b |
8.34±0.66b |
| RHL 50 mg/kg |
45.64±3.65b |
9.01±0.78b |
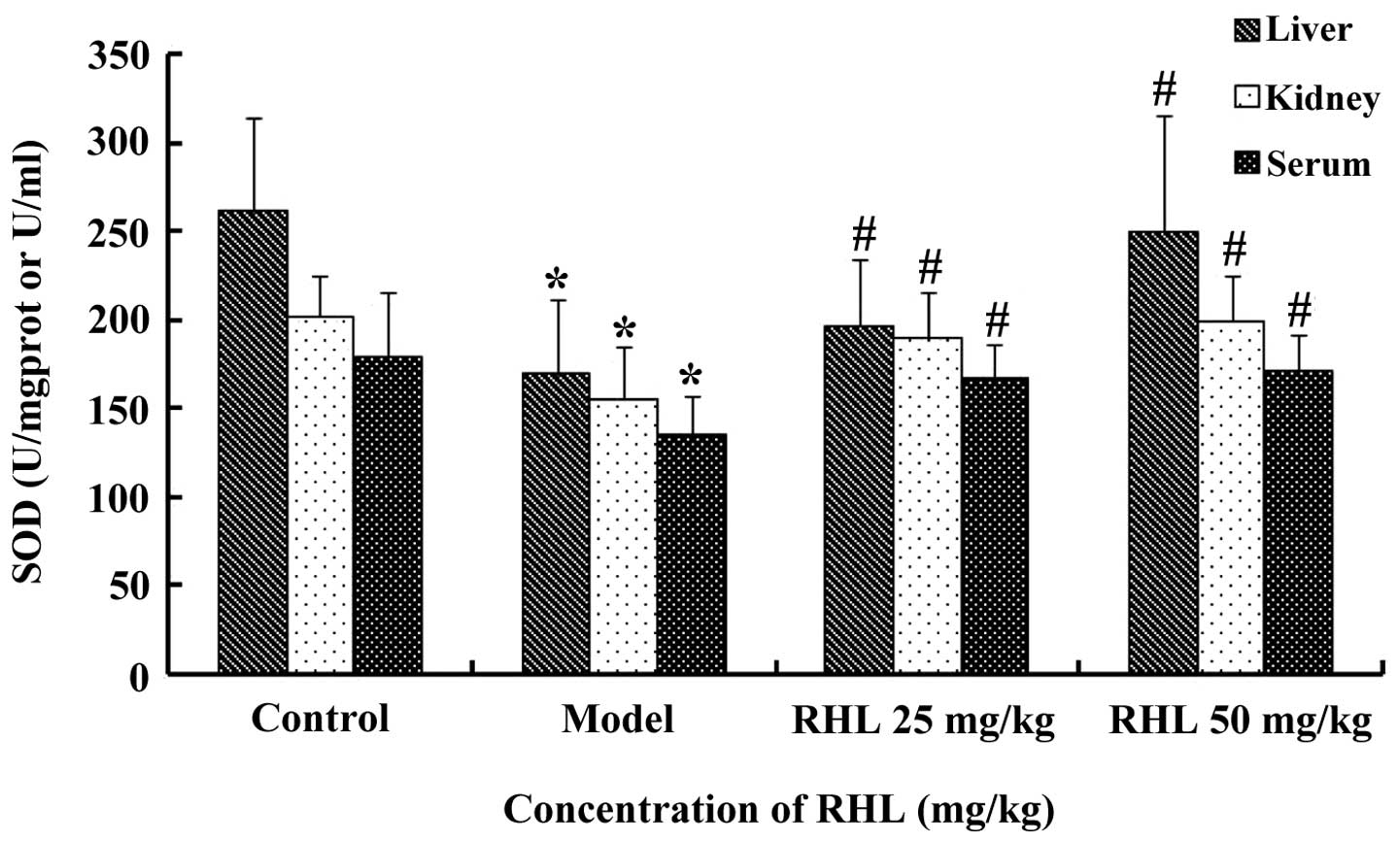
Effects of RHL on the SOD activity in
aging mice
The model group receiving D-gal showed significantly
lower SOD activity (P<0.05) in the liver compared with that of
the control mice. However, treatment of the aging mice with RHL
significantly increased the SOD activity (P<0.05) following 8
weeks of treatment. In particular, the high-dose RHL group
demonstrated higher SOD activity than that of the model group
(P<0.05). However, no statistically significant differences
(P>0.05) were observed between the low- and high-dose RHL groups
(Fig. 1). The results from the
kidney and serum followed the same pattern.
Effects of RHL on the GSH-Px activity
in aging mice
Mice of the model group receiving D-gal showed
significantly lower activities of GSH-Px (P<0.05) in the liver
compared with the control mice. However, treatment of the aging
mice with RHL significantly increased the activities of GSH-Px
(P<0.05) following 8 weeks of treatment (Fig. 2). The results from the kidney and
serum were similar.
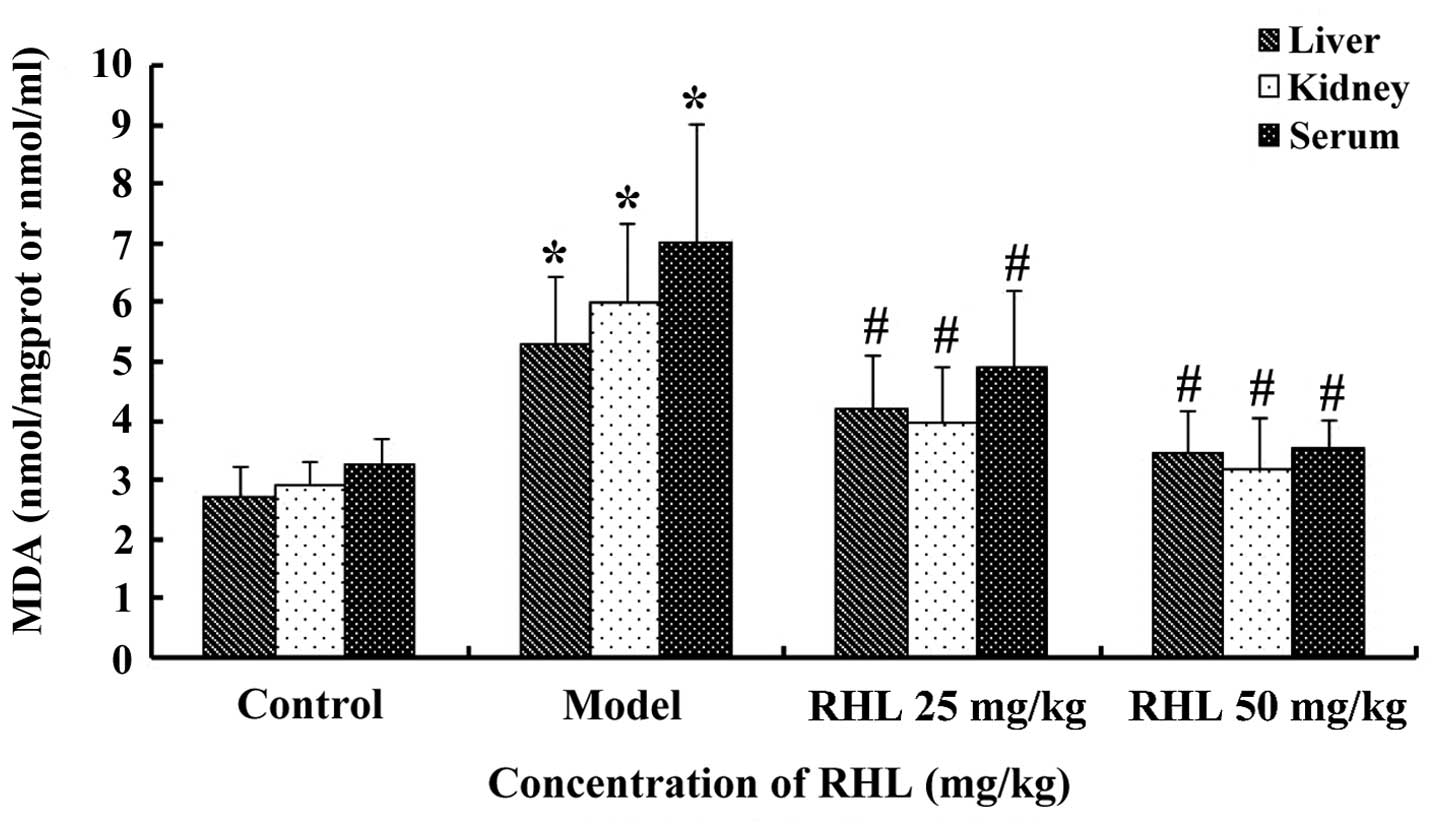
Effects of RHL on the MDA content in
aging mice
The MDA content in the liver of the model group was
significantly greater (P<0.05) than that of the control group,
which indicated that the aging animal model had been successfully
established. The MDA content of the RHL groups was much smaller
(P<0.05) compared with that of the model group. In particular,
the MDA content in the high-dose group was lower compared with that
of the control group, but there were no statistically significant
differences (P>0.05) between the low- and high-dose RHL groups
(Fig. 3). Similar results were
obtained for MDA in the kidney and serum.
Effects of RHL on liver and kidney
morphological alterations
The morphological features of hematoxylin and
eosin-stained liver sections are presented in Fig. 4. The hepatocytes of D-gal-treated
mice exhibited extensive hepatic edema and some degrees of
ballooning degeneration, and the cytoplasm color of hepatocytes
became lighter as opposed to that of the control mice. Notably, RHL
treatment was able to attenuate liver injury induced by D-gal in
mice. The different doses of RHL demonstrated excellent
liver-protecting activity, while hepatic edema was largely
controlled. Similarly, the renal tubular epithelial cells of
D-gal-treated mice exhibited edema (Fig.
5). The cell membrane penetrability of the epithelial cells was
increased and the cytoplasm color became shallow and even
transparent. Therefore, RHL may mitigate renal tubular edema, thus
protecting the kidney.
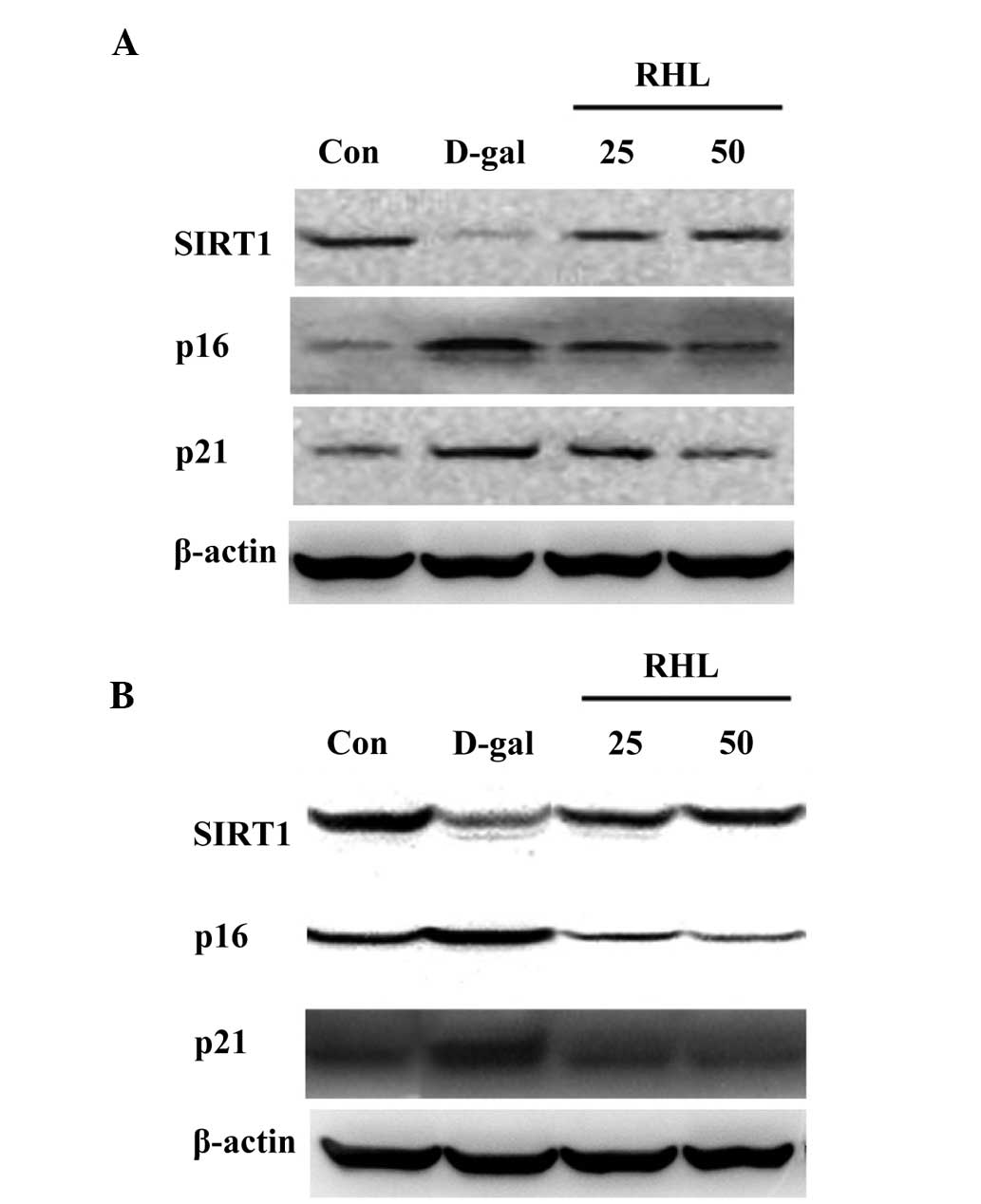
Effects of RHL on the expression of
proteins associated with aging
According to the results of western blot analysis,
the expression levels of p16 and p21 increased in the liver and
kidney (P<0.05), while SIRT1 expression decreased in
D-gal-treated mice (P<0.05). Compared with the model group, the
expression of p16 and p21, which was elevated in D-gal-treated
mice, was inhibited in the 25 mg/kg and 50 mg/kg RHL groups
(P<0.05) (Fig. 6). RHL also
upregulated the level of SIRT1 expression compared with
D-gal-treated mice (P<0.05).
Discussion
From a biological perspective, aging is an
inevitable spontaneous process and a complicated natural phenomenon
(27,28). Although certain mechanisms of aging
have been proposed, theories linking aging and cellular oxidative
stress have received more support (29,30).
With the improvement of living standards all over
the world, individuals are increasingly concerned about their
appearance and health (3).
Postponing body aging has become a key topic of concern for
numerous people (3). The processes
that delay and/or reverse visible signs of aging are termed as
anti-aging. Numerous scientists and pharmaceutical companies have
attempted to develop drugs to reduce the speed of human aging, but
no effective drug has been discovered to date. In the last decade,
the importance of folk medicine and herbal medicines has been
revisited and has resulted in the development of various effective
drugs for anti-aging. The majority of anti-aging herbs have
antioxidant components and reduce free radicals which are
by-products of abnormal body metabolism in the elderly.
Rhein, one of the major bioactive constituents of
the rhizome of rhubarb (Rheum palmatum Linn. or R.
tanguticum Maxim), has received attention for its anti-aging
effects in vitro (25). RHL
is a novel compound, which was synthesized by our team. Compared
with rhein, RHL is easily dissolved in water (25,31).
In the present study, to the best of our knowledge,
the effects of RHL on oxidative stress and aging-associated gene
expression have been investigated for the first time in an animal
model, using chronic administration of D-gal to induce aging. The
model groups in the experiments demonstrated clear differences with
other groups in their daily behavior, pathological sections and
biochemical indexes. In addition, no mouse succumbed due to the
D-gal model; therefore, the aging model was created successfully.
The results revealed a significant anti-aging effect for RHL in
D-gal-induced aging mice.
The kidney and liver are important organs for
detoxification; however, their functions gradually decline due to
age-associated structural atrophy (12). The present results indicated that the
kidney and liver were atrophied in D-gal-induced aging mice.
However, RHL may be able to increase these organ indexes.
Aging model mice are characterized by an increased
concentration of ROS and a significant reduction in their
antioxidant defenses (32). ROS are
chemically reactive oxygen-derived molecules. A growing body of
evidence suggests that accumulation of ROS in biological systems
causes oxidative damage to tissues, affecting cellular integrity
and function. Oxidative damage caused by ROS has frequently been
associated with the pathogenesis of various diseases, and ROS are
considered to be important causative factors in the aging process
(33).
Free radicals derived from oxygen exert detrimental
effects on humans, including peroxidation of membrane lipids,
enzyme inactivation, DNA fragmentation and activation of apoptosis
(34). MDA is a major biomarker that
is observed during the final stages of lipid peroxidation initiated
by excessive ROS. In addition, supplementation with antioxidants
has been reported to be beneficial with respect to slowing down the
aging process (35).
As part of the antioxidant defense systems, a group
of enzymes, including SOD and GSH-Px, function as superoxide anion
and hydrogen peroxide scavengers to prevent ROS-induced damage. The
MDA, SOD and GSH-Px levels are, therefore, indicators of oxidative
stress status. The present results indicated that RHL markedly
diminished oxidative stress in the aged mice by increasing the
activities of SOD and GSH-Px in the liver, kidney and serum and
decreasing the content of MDA, supporting the mechanism of action
of RHL and the theory of oxidative stress in aging. Thus, it can be
deduced that RHL inhibits aging partly by reducing the level of
ROS.
The expression levels of SIRT1, p21 and p16 are
closely associated with mammalian aging. SIRT1 can increase
deacetylation of p53 and SIRT1 is a nicotinamide adenine
dinucleotide-dependent deacetylase that slows aging in lower
organisms and inhibits the development of aging-associated diseases
in mammals. SIRT1 affects a variety of biological functions,
including DNA repair, energy metabolism, tumor suppression and
mitochondrial homeostasis. There is increasing evidence that
elevated SIRT1 activity can have beneficial effects on aging and
aging-associated diseases in mammals (36–38).
These effects may be associated with SIRT1-mediated modulation of
DNA and metabolic damage (36,37).
Roles for SIRT1 in preventing endothelial cells from replicative
senescence or stress-induced premature senescence have been
reported in previous years (39,40).
These anti-aging effects were associated with the effects of
deacetylation of liver kinase B1 or p53 by SIRT1. SIRT1 was also
demonstrated to protect human umbilical cord fibroblasts from
replicative senescence by promoting the transcription of telomerase
reverse transcriptase (41).
The p16 gene, a cyclin-dependent kinase
inhibitor, is considered to play an important role in tumor growth
suppression and cell senescence (42,43).
Expression of p16 notably increases with aging in the majority of
rodents and in human tissues. The accumulation of p16 contributes
to senescence by negatively regulating the cell cycle in
vitro and in vivo (44–46).
Another inhibitor of cell cycle progression, p21,
increases with age and contributes to the impaired cellular
regeneration of an aging organism. p21 deficiency partially
prevented age-induced decline in cell proliferation and tissue
function (47). In the present
study, RHL was found to markedly decrease p16 and p21 expression
levels and increase SIRT1 expression. These results suggested that
RHL may modulate age-associated gene expression.
In conclusion, the results obtained in the current
study demonstrated that RHL can reduce the aging effects induced by
D-gal injection in mice. This effect may be mediated, at least
partly, through enhancing antioxidant activity, scavenging free
radicals and modulating aging-associated gene expression. These
data suggest that RHL has anti-aging effects, and has development
potential as an anti-aging medicine.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (no. 81001439) and the
General Program of Natural Science Foundation of Hebei Province of
China (no. H2012401030).
References
|
1
|
Balaban RS, Nemoto S and Finkel T:
Mitochondria, oxidants and aging. Cell. 120:483–495. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Peiris H, Dubach D, Jessup CF, Unterweger
P, Raghupathi R, Muyderman H, Zanin MP, Mackenzie K, Pritchard MA
and Keating DJ: RCAN1 regulates mitochondrial function and
increases susceptibility to oxidative stress in mammalian cells.
Oxid Med Cell Longev. 2014:5203162014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rattan SI: Aging is not a disease:
Implications for intervention. Aging Dis. 5:196–202. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Butterfield DA, Di Domenico F and Barone
E: Elevated risk of type 2 diabetes for development of Alzheimer
disease: A key role for oxidative stress in brain. Biochim Biophys
Acta. 1842:1693–1706. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yu W, Bonnet M, Farso M, Ma K, Chabot JG,
Martin E, Torriglia A, Guan Z, McLaurin J, Quirion R and Krantic S:
The expression of apoptosis inducing factor (AIF) is associated
with aging-related cell death in the cortex but not in the
hippocampus in the TgCRND8 mouse model of Alzheimer's disease. BMC
Neurosci. 15:732014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jo-Watanabe A, Ohse T, Nishimatsu H,
Takahashi M, Ikeda Y, Wada T, Shirakawa J, Nagai R, Miyata T,
Nagano T, Hirata Y, et al: Glyoxalase I reduces glycative and
oxidative stress and prevents age-related endothelial dysfunction
through modulation of endothelial nitric oxide synthase
phosphorylation. Aging Cell. 13:519–528. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jomova K, Vondrakova D, Lawson M and Valko
M: Metals, oxidative stress and neurodegenerative disorders. Mol
Cell Biochem. 345:91–104. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jomova K and Valko M: Advances in
metal-induced oxidative stress and human disease. Toxicology.
283:65–87. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen B, Zhong Y, Peng W, Sun Y and Kong
WJ: Age-related changes in the central auditory system: Comparison
of D-galactose-induced aging rats and naturally aging rats. Brain
Res. 1344:43–53. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li L, Ng TB, Gao W, Li W, Fu M, Niu SM,
Zhao L, Chen RR and Liu F: Antioxidant activity of gallic acid from
rose flowers in senescence accelerated mice. Life Sci. 77:230–240.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen HL, Wang CH, Kuo YW and Tsai CH:
Antioxidative and hepatoprotective effects of
fructo-oligosaccharide in D-galactose-treated Balb/cJ mice. Br J
Nutr. 105:805–809. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ye Y, Jia RR, Tang L and Chen F: In
vivo antioxidant and anti-skin aging activities of ethyl
acetate extraction from idesia polycarpa defatted fruit residue in
aging mice induced by D-galactose. Evid Based Complement Alternat
Med. 2014:1857162014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li YN, Guo Y, Xi MM, Yang P, Zhou XY, Yin
S, Hai CX, Li JG and Qin XJ: Saponins from Aralia
taibaiensis attenuate D-galactose-induced aging in rats by
activating FOXO3a and Nrf2 pathways. Oxid Med Cell Longev.
2014:3205132014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cui X, Zuo P, Zhang Q, Li X, Hu Y, Long J,
Packer L and Liu J: Chronic systemic D-galactose exposure induces
memory loss, neurodegeneration and oxidative damage in mice:
Protective effects of R-alpha-lipoic acid. J Neurosci Res.
84:647–654. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cui X, Wang L, Zuo P, Han Z, Fang Z, Li W
and Liu J: D-galactose-caused life shortening in Drosophila
melanogaster and Musca domestica is associated with
oxidative stress. Biogerontology. 5:317–325. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang ZF, Fan SH, Zheng YL, Lu J, Wu DM,
Shan Q and Hu B: Purple sweet potato color attenuates oxidative
stress and inflammatory response induced by D-galactose in mouse
liver. Food Chem Toxicol. 47:496–501. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhong SZ, Ge QH, Qu R, Li Q and Ma SP:
Paeonol attenuates neurotoxicity and ameliorates cognitive
impairment induced by D-galactose in ICR mice. J Neurol Sci.
277:58–64. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mao J, Huang S, Liu S, Feng XL, Yu M, Liu
J, Sun YE, Chen G, Yu Y, Zhao J and Pei G: A herbal medicine for
Alzheimer's disease and its active constituents promote neural
progenitor proliferation. Aging Cell. 2015 May 25;doi:
10.1111/acel.12356. [Epub ahead of print]. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rahimi R and Abdollahi M: Herbal medicines
for the management of irritable bowel syndrome: A comprehensive
review. World J Gastroenterol. 18:589–600. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang M and Lei Y: Delaying vascular aging
with Chinese medicine: Implications from an overview of the p53 and
miR-34s family. Chin J Integr Med. 17:635–639. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xiong D, Yu LX, Yan X, Guo C and Xiong Y:
Effects of root and stem extracts of Asparagus
cochinchinensis on biochemical indicators related to aging in
the brain and liver of mice. Am J Chin Med. 39:719–726. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Du H, Shao J, Gu P, Lu B, Ye X and Liu Z:
Improvement of glucose tolerance by rhein with restored early-phase
insulin secretion in db/db mice. J Endocrinol Invest. 35:607–612.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Malaguti C, Vilella CA, Vieira KP, Souza
GH, Hyslop S and Zollner Rde L: Diacerhein downregulate
proinflammatory cytokines expression and decrease the autoimmune
diabetes frequency in nonobese diabetic (NOD) mice. Int
Immunopharmacol. 8:782–791. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Martin G, Bogdanowicz P, Domagala F,
Ficheux H and Pujol JP: Articular chondrocytes cultured in hypoxia:
Their response to interleukin-1beta and rhein, the active
metabolite of diacerhein. Biorheology. 41:549–561. 2004.PubMed/NCBI
|
|
25
|
Lin YJ, Zhen YZ, Wei J, Liu B, Yu ZY and
Hu G: Effects of Rhein lysinate on
H2O2-induced cellular senescence of human
umbilical vascular endothelial cells. Acta Pharmacol Sin.
32:1246–1252. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sun JH, Liu YM, Cao T and Ouyang WQ:
Effect of kinetin on ovary and uterus in D-galactose-induced female
mouse model of aging. Sheng Li Xue Bao. 65:389–394. 2013.(In
Chinese). PubMed/NCBI
|
|
27
|
Li Z, Liu R, Kang X and Wang X: Study on
establishment of kidney deficient aging model and comparison with
D-galactose induced aging model. Zhongguo Zhong Yao Za Zhi.
37:2435–2438. 2012.(In Chinese). PubMed/NCBI
|
|
28
|
Prisila Dulcy C, Singh HK, Preethi J and
Rajan KE: Standardized extract of Bacopa monniera (BESEB CDRI-08)
attenuates contextual associative learning deficits in the aging
rat's brain induced by D-galactose. J Neurosci Res. 90:2053–2064.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mukherjee A and Haldar C: Melatonin
membrane receptor (MT1R) expression and nitro-oxidative stress in
testis of golden hamster, Mesocricetus auratus: An
age-dependent study. Exp Gerontol. 69:211–220. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tatone C, Di Emidio G, Vitti M, Di Carlo
M, Santini S Jr, D'Alessandro AM, Falone S and Amicarelli F:
Sirtuin functions in female fertility: Possible role in oxidative
stress and aging. Oxid Med Cell Longev. 2015:6596872015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hu GI, Liu J, Zhen YZ, Xu R, Qiao Y, Wei
J, Tu P and Lin YJ: Rhein lysinate increases the median survival
time of SAMP10 mice: Protective role in the kidney. Acta Pharmacol
Sin. 34:515–521. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nasto LA, Robinson AR, Ngo K, Clauson CL,
Dong Q, St Croix C, Sowa G, Pola E, Robbins PD, Kang J, et al:
Mitochondrial-derived reactive oxygen species (ROS) play a causal
role in aging-related intervertebral disc degeneration. J Orthop
Res. 31:1150–1157. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Datta HS, Mitra SK and Patwardhan B: Wound
healing activity of topical application forms based on Ayurveda.
Evid Based Complement Alternat Med. 2011:1343782011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Speakman JR and Selman C: The free-radical
damage theory: Accumulating evidence against a simple link of
oxidative stress to ageing and lifespan. Bioessays. 33:255–259.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Koyama H, Nojiri H, Kawakami S, Sunagawa
T, Shirasawa T and Shimizu T: Antioxidants improve the phenotypes
of dilated cardiomyopathy and muscle fatigue in mitochondrial
superoxide dismutase-deficient mice. Molecules. 18:1383–1393. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Herranz D and Serrano M: Impact of Sirt1
on mammalian aging. Aging (Albany NY). 2:315–316. 2010.PubMed/NCBI
|
|
37
|
Herranz D, Muñoz-Martin M, Cañamero M,
Mulero F, Martinez-Pastor B, Fernandez-Capetillo O and Serrano M:
Sirt1 improves healthy ageing and protects from metabolic
syndrome-associated cancer. Nat Commun. 1:32010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Herskovits AZ and Guarente L: SIRT1 in
neurodevelopment and brain senescence. Neuron. 81:471–483. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ota H, Akishita M, Eto M, Iijima K, Kaneki
M and Ouchi Y: Sirt1 modulates premature senescence-like phenotype
in human endothelial cells. J Mol Cell Cardiol. 43:571–579. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zu Y, Liu L, Lee MY, Xu C, Liang Y, Man
RY, Vanhoutte PM and Wang Y: SIRT1 promotes proliferation and
prevents senescence through targeting LKB1 in primary porcine
aortic endothelial cells. Circ Res. 106:1384–1393. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yamashita S, Ogawa K, Ikei T, Udono M,
Fujiki T and Katakura Y: SIRT1 prevents replicative senescence of
normal human umbilical cord fibroblast through potentiating the
transcription of human telomerase reverse transcriptase gene.
Biochem Biophys Res Commun. 417:630–634. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gil J and Peters G: Regulation of the
INK4b-ARF-INK4a tumour suppressor locus: All for one or one for
all. Nat Rev Mol Cell Biol. 7:667–77. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Krishnamurthy J, Torrice C, Ramsey MR,
Kovalev GI, Al-Regaiey K, Su L and Sharpless NE: Ink4a/Arf
expression is a biomarker of aging. J Clin Invest. 114:1299–1307.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Alcorta DA, Xiong Y, Phelps D, Hannon G,
Beach D and Barrett JC: Involvement of the cyclin-dependent kinase
inhibitor p16 (INK4a) in replicative senescence of normal human
fibroblasts. Proc Natl Acad Sci. 93:13742–13747. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Melk A, Schmidt BM, Takeuchi O, Sawitzki
B, Rayner DC and Halloran PF: Expression of p16INK4a and other cell
cycle regulator and senescence associated genes in aging human
kidney. Kidney Int. 65:510–520. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang X, Wu X, Tang W and Luo Y: Loss of
p16 (Ink4a) function rescues cellular senescence induced by
telomere dysfunction. Int J Mol Sci. 13:5866–5877. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li Y and Tollefsbol TO: p16 (INK4a)
suppression by glucose restriction contributes to human cellular
lifespan extension through SIRT1-mediated epigenetic and genetic
mechanisms. PLoS One. 6:e174212011. View Article : Google Scholar : PubMed/NCBI
|