Introduction
Immunoglobulin A nephropathy (IgAN) is a type of
immune complex-mediated glomerulonephritis that is pathologically
characterized by the deposition of IgA immune complexes in the
mesangium of the kidney (1). IgAN is
the most common form of primary glomerulonephritis worldwide,
particularly in China (2). In
addition, 15–20% of patients with IgAN develop end-stage renal
failure (ESRD) within 10 years, and 30–40% within 20 years
(3–5). Proteinuria is regarded as the most
severe risk factor for unfavorable renal prognosis, and its
reduction is an important therapeutic goal in clinical practice
(6).
Optimized supportive therapy is the key strategy for
patients with IgAN who are at risk of progression, with
renin-angiotensin-system (RAS) inhibitors being the most common
treatment. However, the optimal immunosuppressive treatment
strategy for patients with IgAN suffering from moderate to severe
proteinuria remains uncertain. According to the guidelines outlined
by the clinical practice guideline for glomerulonephritis (7), patients with IgAN who suffer from
persistent proteinuria of >1 g/day following 6 months of
treatment with RAS inhibitors are recommended to undergo
corticosteroid therapy. There is an ongoing debate over the
efficacy and safety of immunosuppressive agents other than
glucocorticoid monotherapy in patients with IgAN who present with
moderate to severe proteinuria; specifically, the use of relatively
novel agents, including leflunomide (LET) and mycophenolate mofetil
(MMF).
Increasing attention has been paid to the role of
immunosuppressive agents in the treatment of patients with IgAN.
The present report aimed to generate a meta-analysis from the most
up-to-date studies regarding the safety and efficacy of various
immunosuppressive therapeutic strategies for the treatment of
patients with IgAN, in order to provide comprehensive current
information to nephrologists to aid decision making. China has the
largest population worldwide, and has a high incidence of IgAN. In
order to exclude interferences that may be due to the ethnicity of
patients, the present meta-analysis was performed using studies
involving Chinese patients exclusively.
Methods
Information sources and search
strategy
All randomized controlled trials (RCTs) that
assessed the efficacy and safety of various immunosuppressive
agents in the treatment of Chinese patients with IgAN between 1990
and September 2013 were included in the present meta-analysis.
Numerous databases were searched for eligible RCTs, including:
PubMed, Excerpta Medica database, the Cochrane Library, Chinese
National Knowledge Infrastructure, Wanfang, Weipu, Chinese
Biomedical Literature Database and Qinghuatongfang. The following
medical terms and phrases were used for the search: ‘Immunoglobulin
A nephropathy’; ‘IgA nephropathy’; ‘Berger disease’;
‘glomerulonephritis’; ‘RCT’; ‘controlled clinical trial’ and
‘immunosuppressive therapy’. Only RCTs published in either English
or Chinese were considered to be eligible. The title and abstract
of the search results were analyzed by two independent
investigators. According to the inclusion criteria, reference lists
from all identified articles were also searched.
Inclusion criteria
The following inclusion criteria were used in the
present meta-analysis: (i) Prospective RCTs comparing various
immunosuppressive agents; (ii) the selected patients with IgAN were
Chinese, whether adults or children; (iii) diagnosis of IgAN was
performed via renal biopsy; and (iv) the study was published in
English or Chinese.
Exclusion criteria
Studies were excluded if they: (i) Assessed
secondary types of IgAN or patients who were not Chinese; (ii) were
designed without randomization, such as retrospective studies and
descriptive studies; (iii) included the use of traditional Chinese
medicine, with its unknown additional effects on immunosuppressive
agents and uncertain doses of active components; (iv) only assessed
corticosteroids; or (v) failed to exclude patients with other
systemic diseases, such as lupus, Henoch-Schonlein purpura and
rheumatoid arthritis.
Study selection
All of the studies included in the present
meta-analysis were independently assessed by two reviewers, in
accordance with the set criteria, through retrieved abstracts and,
if necessary the full texts. Disagreements were resolved in
consultation with a third reviewer until a consensus was
reached.
Data extraction
Data were independently extracted from each study by
two reviewers using a predesigned review form (Microsoft Office
Excel 2010; Microsoft Corporation, Redmond, WA, USA); disagreements
were resolved in discussion with a third reviewer until a consensus
was reached.
The review form included the following data: First
author and unit of each study; year of publication; country of
publication; journal; patient characteristics, including age,
gender and ethnicity; interventions, including type of
immunosuppressive agents, dose and usage; and the methodology of
the RCTs. Baseline, final proteinuria, serum creatinine and serum
albumin levels, and the type of outcome (complete remission,
partial remission and total effective rate) were recorded. In
addition, the presence of side effects, including: Elevated levels
of liver enzymes; hypertension; diabetes; glaucoma; cataracts;
leukocytopenia and infection, were recorded.
Assessments of methodological
quality
The respective qualities of the RCTs were
independently assessed by two authors, using the scoring system
developed by Jadad et al (8).
The quality scoring system was as follows: (i) Generation of random
sequences (2=appropriate, computer generated random numbers or
similar methods; 1=unknown, randomized trials but did not describe
the method of random distribution; 0=inappropriate, adopted the
method of alternate distribution such as single and double); (ii)
randomization concealment (2=appropriate, clinicians and patients
matched by unpredictable assigned sequence method; 1=unknown, only
stating the use of a random number table or other random allocation
scheme; 0=description not clear); (iii) blinding method
(2=appropriate, using the identical placebo or similar methods;
1=not clear, statement for blinding method, but no description
available; 0=inappropriate, did not adopt the method of double
blinding, or inappropriate blinding such as tablets and
injections); (iv) withdrawal (1=described the withdrawal or exit
and indicated the reasons; 0=did not describe the withdrawal or
exit or the reasons thereof).
Data analysis and statistical
methods
Statistical analyses were performed using Review
Manager software (version 5.0.2; The Cochrane Collaboration,
Oxford, UK). Two-sided P-values were obtained via the χ2
test and P<0.05 was considered to indicate a statistically
significant difference. Dichotomous outcome data were analyzed
using the odds ratio, 95% confidence intervals (CI), and the
standardized mean difference (SMD). 95% CI was used as a summary
estimator for continuous outcomes. The heterogeneity of the trial
results was investigated visually by examination of the plots and
statistically via the heterogeneity I2 values. In order
to reveal possible publication bias, funnel plots of study size vs.
effect size were visually assessed for the total clinical remission
rate.
Results
Study characteristics
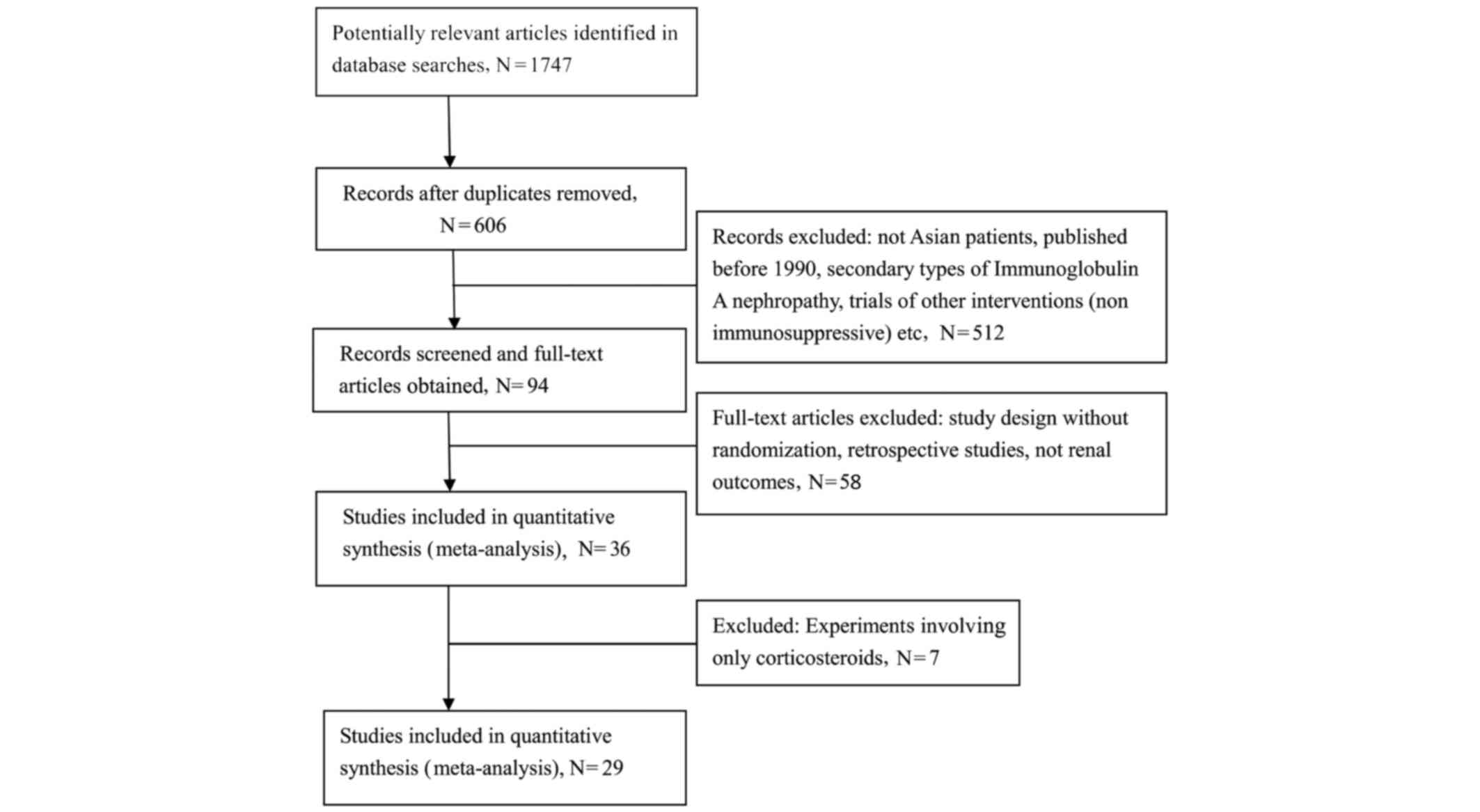
The combined search identified 1,747 articles, of
which 1,653 were excluded during the initial review. The main
reasons for the exclusion of eligible RCTs were: Non-randomization,
evaluation of other interventions (e.g. non-immunosuppressive
agents), and a lack of renal outcomes of interest. Furthermore,
animal and basic research studies were excluded, in addition to a
number of review articles on the topic. The full-text versions of
the remaining 94 articles were analyzed and a further 64 articles
were excluded for similar reasons. Overall, 29 studies (9–37),
including 1,466 patients, were included in the present
meta-analysis (Fig. 1). The
following comparisons were analyzed: MMF (or plus steroid) vs.
steroid therapy alone (n=6); azathioprine (AZA) (or plus steroid)
vs. steroid (n=5); LET (or plus steroid) vs. CTX (or plus steroid)
(n=4); CTX (or plus steroid) vs. steroid (n=2). In 13 studies, LET
was involved in the comparison. The characteristics of the included
RCTs are shown in Table I.
 | Table I.Characteristics of the included
trials. |
Table I.
Characteristics of the included
trials.
| Study
(reference) | Number of
patients | Mean age (years) | Length (months) | Baseline proteinuria
(g/day) | Initial dose of
immunosuppressive agents (per day) | Quality grade |
|---|
| AZA (or plus steroid)
vs. steroid |
| Huang S
(9) | 36 | 30.8/30.8 | 9 |
1.78±1.11/1.82±1.23 | 2 mg/kg AZA | 3 |
| Kuang B
(10) | 64 |
18.0–68.0a | 6 |
0.39±0.09/0.38±0.09 | 2 mg/kg AZA | 1 |
| Li YN
(11) | 42 |
18.0–65.0a | 12 |
1.41±1.29/1.32±0.91 | 1.5 mg/kg AZA | 3 |
| Ma FL
(12) | 80 | 37.1/37.5 | NC |
1.80±1.17/1.83±1.52 | 2 mg/kg AZA | 2 |
| Xu H
(13) | 70 | 38.4/35.2 | 18 |
38.42±12.80/35.26±13.27 | 2 mg/kg AZA | 4 |
| MMF (or plus steroid)
vs. steroid |
| Tang SC
(18) | 40 | 42.1/43.3 | 8 |
1.80±0.21/1.87±0.28 | 2 g (weight, ≥60kg)
or 1.5 g (weight, <60kg) MMF | 3 |
| Chen XM
(14) | 62 | 28.0/29.0 | 24 |
3.20±1.70/2.90±1.50 | 1.0 g (weight.
<50kg) or 1.5 g (weight, >50kg) MMF | 2 |
| Chen XSh
(15) | 39 | 42.7/38.6 | 12 | NC | 1.5 g MMF | 3 |
| Guo QY
(16) | 34 | 8.7/9.4 | 12 |
0.97±0.34/1.13±0.23 | 800–1000
mg/m2 MMF | 2 |
| Huang Z
(17) | 38 | 34.0/32.0 | 2 |
6.71±5.53/5.89±4.90 | 0.5–2 gMMF | 2 |
| Wang WM
(19) | 40 | 40.0/39.4 | 12 |
1.75±1.60/1.51±0.92 | 1.0–1.5 g MMF
(weight, <50kg is 1.0g) | 3 |
| LET (or plus
steroid) vs. steroid |
| Lou TQ
(25) | 55 | 33.0/33.0 | 6 |
1.30±0.70/1.60±0.80 | 60 mg LET | 4 |
| Yang FY
(30) | 82 | 36.6/38.2 | 6 |
2.60±1.40/2.50±1.20 | 50 mg LET | 4 |
| Hu RH
(22) | 45 | 42.7/42.7 | 9 |
2.16±0.64/2.08±0.73 | 50 mg LET | 3 |
| Zhang Y
(32) | 42 | 16–68a | 6 | 2.85±0.80/NC | 50 mg LET | 3 |
| Wang L
(29) | 36 | 36.3/34.2 | 6 |
2.38±1.31/2.42±1.28 | 50 mg LET | 2 |
| Fu Q
(21) | 37 | 32.6/36.5 | 1 |
2.56±0.90/2.48±0.82 | 50 mg LET | 3 |
| Li T
(24) | 60 | 36.4/37.7 | 6 |
2.40±1.30/2.50±1.40 | 50 mg LET | 2 |
| Cao LO
(20) | 36 | 40.0/38.0 | 6 |
2.4(1.4–4.0)/2.6(1.7–3.1) | 40 mg LET | 3 |
| Huang
YX (23) | 62 | 43.5/43.5 | 6 |
2.30±1.30/2.20±1.20 | 40 mg LET | 4 |
| Pu L
(26) | 60 | 36.2/37.4 | 6 |
2.10±0.94/2.08±1.03 | 20 mg LET | 3 |
| Zhang
LW (31) | 30 | 43.5/42.8 | 6 |
2.43±1.32/2.24±1.14 | 50 mg LET | 3 |
| Sun MD
(28) | 36 | 37.0/37.0 | 3 | 2.68
±0.68/2.66±0.69 | 50 mg LET | 3 |
| Shen P
(27) | 42 | 32.5/31.4 | 6 |
3.16±1.42/3.08±1.52 | 50 mg LET | 3 |
| CTX (or plus
steroid) vs. steroid |
| Wang WM
(19) | 40 | 39.5/39.4 | 12 |
1.74±1.06/1.51±0.92 | 0.5–0.75
g/m2 CTX | 3 |
| Liu Y
(33) | 32 | NC | 12 |
1.55±0.44/1.38±0.62 | 8–12 mg/kg CTX | 3 |
| LET (or plus
steroid) vs. CTX (or plus steroid) |
| Guo M
(34) | 60 | NC | 28 |
5.12±1.08/5.14±1.34 | 50 mg LET; 8–12
mg/kg CTX | 3 |
| Zhong
ShH (37) | 56 | NC | 7 |
5.16±1.32/5.10±1.07 | 50 mg LET; 8–12
mg/kg CTX | 3 |
| Zhang
HF (36) | 40 | 43.7/43.7 | 4 | NC | 50 mg LET; 8–12
mg/kg CTX | 4 |
| Sun ZhX
(35) | 70 | 35.7/35.7 | 12 |
2.61±1.38/2.54±1.28 | 50 mg LET; 0.75
g/m2 CTX | 3 |
Effects of interventions
AZA (or plus steroid) vs. steroid
Five RCTs (9–13) involving 292 patients compared the
administration of AZA (or plus steroids) with steroid therapy. AZA
(2–5 mg/kg/day) was administered for 6–24 months. Prednisolone
(0.8–1.0 mg/kg/day) was administered for 4–8 weeks and subsequently
tapered off within one year (9–13).
Patients receiving AZA demonstrated significantly increased
complete response (CR)/partial response (CR) proteinuria remission
rates [CR/PR; relative risk (RR), 3.43; 95% confidence interval
(CI), 1.92–6.12, P<0.0001] (Fig.
2), as compared with the steroid therapy alone.
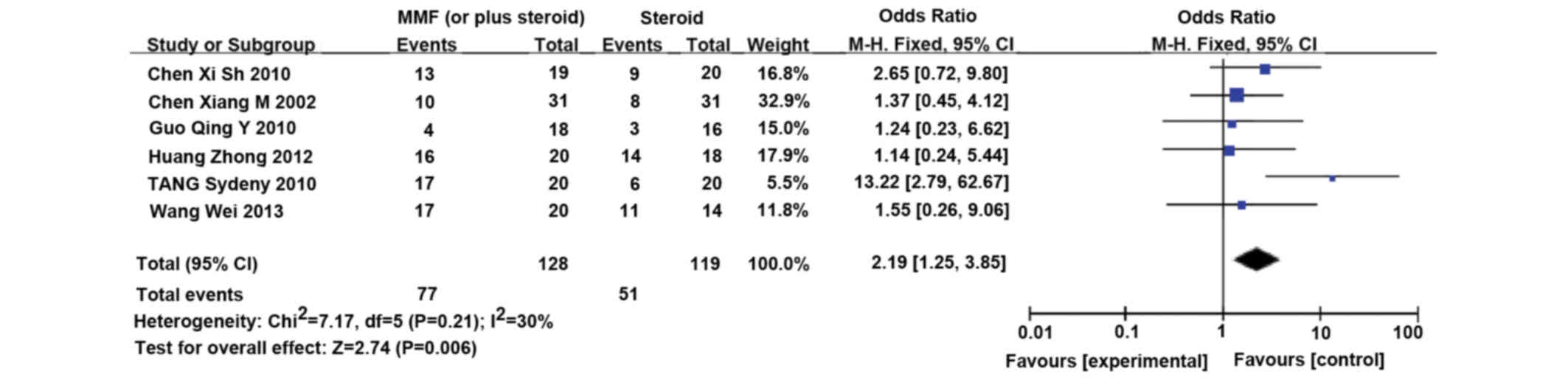
MMF (or plus steroid) vs. steroid
Six RCTs (14–19)
involving 253 patients compared MMF (or plus steroid) with steroid
therapy alone. MMF (1.0–2.0 g/day) was orally administered for 3–6
months, and gradually tapered thereafter. The immunosuppressive
treatment lasted for <12 months (14–19).
Patients receiving MMF demonstrated significantly increased CR/PR
proteinuria remission rates (CR/PR; RR, 2.19; 95% CI, 1.25–3.85,
P=0.006) (Fig. 3), as compared with
the steroid therapy alone.
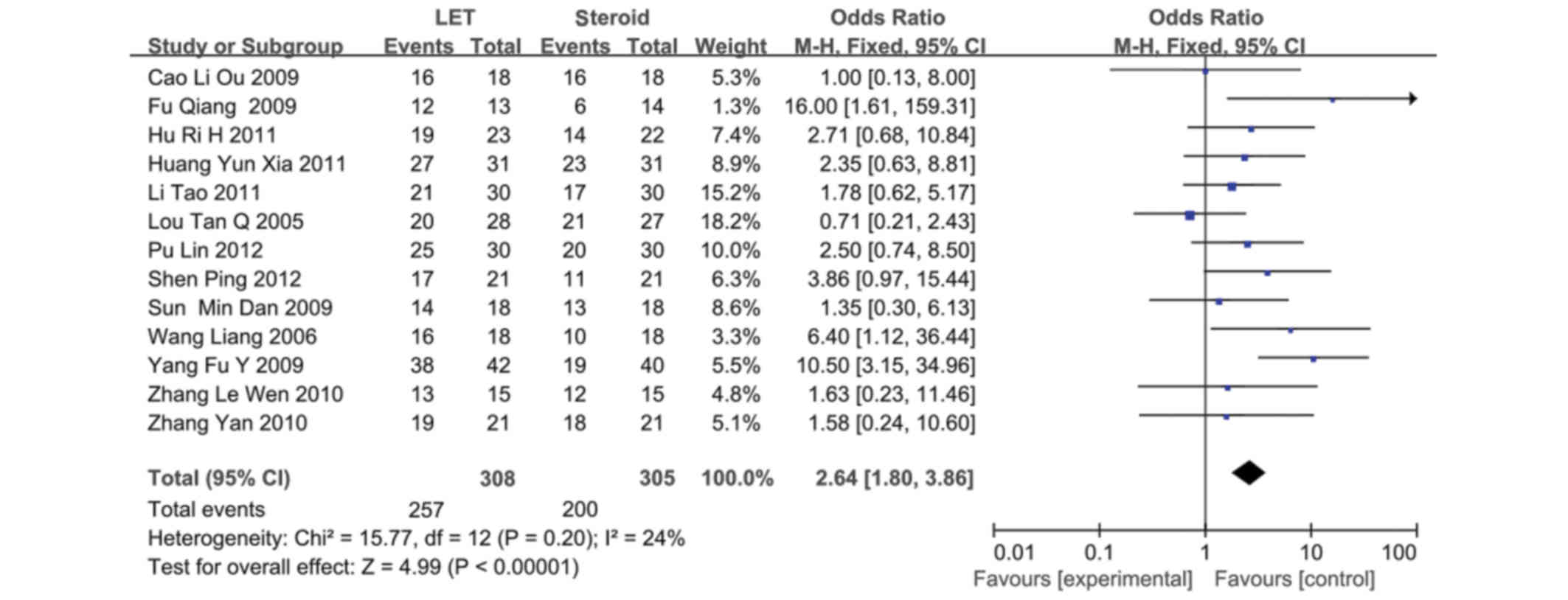
LET (or plus steroid) vs. steroid
A total of 13 RCTs (20–32)
involving 623 patients compared LET (or plus steroid) with steroid
therapy alone. LET (50 mg/day) was orally administered for 3 days,
reduced to 20–30 mg/day for 3 months, and subsequently tapered
(20–32). LET demonstrated a marked advantage on
CR/PR proteinuria remission, as compared with the steroid therapy
(CR/PR; RR, 2.64; 95% CI, 1.80–3.86; P<0.00001) (Fig. 4).
CTX (or plus steroid) vs. steroid
Two RCTs (19,33)
involving 72 patients compared CTX (or plus steroid) treatment with
steroid therapy alone. CTX (0.5–0.75 g/m2) was
intravenously administered in monthly pulses for six months,
followed by quarterly pulses for the next six months. The
immunosuppressive therapy lasted for 12 months in total (19–33).
Patients receiving CTX (or plus steroid) demonstrated markedly
increased reductions in urinary protein excretion levels by the end
of treatment, as compared with patients treated with steroid
therapy exclusively (SMD, 0.91; 95% CI, 0.41–1.41; P=0.0004)
(Fig. 5).
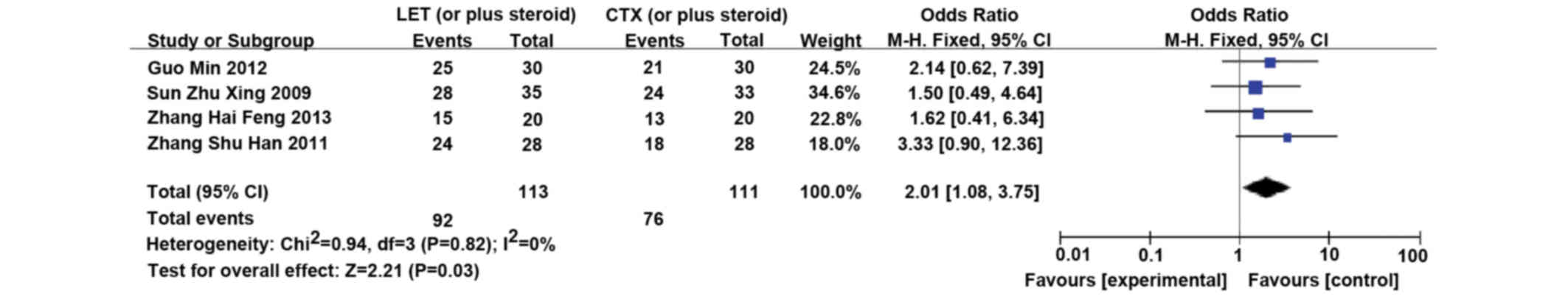
LET (or plus steroid) vs. CTX (or plus
steroid)
Four RCTs (34–37)
involving 226 patients compared LET (or plus steroid) with CTX (or
plus steroid). LET (50 mg/day) was orally administered for 3 days,
tapered, and subsequently reduced to 20 mg/day for 3 months
(34–37). LET was significantly more effective
in inducing remission, as compared with CTX (CR/PR; RR, 2.01; 95%
CI, 1.08–3.75; P=0.03) (Fig. 6).
Side effects
Although it is challenging to statistically analyze
the side effects in a single comparison, the major side effects for
each agent were recorded. A total of 143 patients were treated with
AZA, and adverse events were reported in 79 patients, including:
Myelosuppression (n=5; 6.3%), liver dysfunction (n=6; 7.6%), and
digestive symptoms (n=8; 0.12%). Infection was the most frequent
side effect of AZA (14/79, 17.7%). A total of 16 patients (14.8%)
treated with MMF demonstrated digestive symptoms; whereas a further
4 patients (3.7%) exhibited elevated liver enzymes. A total of
13/267 patients (4.7%) treated with LET demonstrated elevated liver
enzymes, and a further 10 patients (3.7%) exhibited digestive
symptoms.
No obvious nephrotoxicity directly related to the
administration of immunosuppressive agents was demonstrated. In the
‘LET (or plus steroid) vs. CTX (or plus steroid)’ comparison, 9 and
13 patients treated with LET and CTX, respectively, demonstrated
liver dysfunction; whereas 1 and 3 patients receiving CTX and LET,
respectively, demonstrated digestive symptoms. Furthermore,
leukocytopenia was detected in 6 patients treated with CTX, whereas
5 individuals treated with LET demonstrated alopecia.
Publication bias and sensitivity
analysis
Publication bias was examined using funnel plots,
which did not show any significant visual asymmetry (Fig. 7). Furthermore, in order to assess the
robustness of the meta-analysis results, a sensitivity analysis
focusing on the patients and the quality of the RCTs was conducted.
Analysis was performed by excluding low quality RCTs. As outlined
in Table I, the quality scores of
all included RCTs were not high; therefore, any RCTs scoring less
than 3 points were excluded (n=7). This sensitivity analysis did
not substantially change the results of the comparisons.
Discussion
IgAN, which was initially identified by Berger and
Hinglais in 1968 (1), is the most
common form of primary glomerular nephritis in Asia. The
immunological mechanisms associated with the development and
progression of IgAN suggest that immunosuppressive therapies may
have a beneficial role in the treatment of IgAN and associated
proteinuria. Although previous studies have demonstrated that the
administration of glucocorticoids may reduce the risk of ESRD in
patients with IgAN (38,39), the optimal immunosuppressive
treatment strategy for patients with IgAN who suffer with moderate
to severe proteinuria remains uncertain. In the present
meta-analysis, a comprehensive literature search with limited
restrictions in publication language was performed in order to
compare the efficacy and safety of immunosuppressive agents such as
CTX, LET, MMF and AZA, which are widely used in the treatment of
Chinese patients with IgAN.
A total of 1,466 patients from 29 studies (9–37) were
included in the present meta-analysis. As too few studies reported
long-term outcomes, the present meta-analysis only reviewed
short-term parameters in order to evaluate the efficacy of the
respective treatments, including the final urinary protein
excretion and therapeutic remission (complete or partial) of the
participants. The most frequently used definition for ‘partial
remission’ of proteinuria was 0.3–2.0 g/day or a decrease of 50%.
Complete remission of proteinuria was defined as <0.3 g/day and
serum albumin >35 g/l with normal renal function. However, these
definitions may be heterogeneous.
Notably, the patients demonstrated an improved
treatment response to AZA administration, as compared with steroid
treatment alone (RR, 3.43; 95% CI; 1.92–6.12; P<0.0001). In
addition, following treatment with MMF therapy, the Chinese
patients with IgAN demonstrated significantly increased CR/PR
remission rates (CR/PR; RR, 2.19; 95% CI, 1.25–3.85; P=0.006), as
compared with the administration of steroid alone. In the analysis
of ‘LET (or plus steroid) versus steroid’, the administration of
LET demonstrated an improved response, as compared with steroid
treatment alone. Tolerable side effects were demonstrated in
patients administered AZA, MMF and LET. Only two studies involving
72 patients compared CTX plus steroid and steroid therapy alone;
therefore further high quality RCTs are required in the future to
determine the effects of CTX. Four studies were included in the
analysis of ‘CTX (or plus steroid) versus LET (or plus steroid)’;
LET administration was significantly effective in inducing
remission of proteinuria and was associated with a lower incidence
of adverse reactions, as compared with CTX.
Some findings of the present study were consistent
with the results of previous studies in patients of various
ethnicities. Several RCTs have demonstrated that, when combined
with steroid treatment, CTX was able to reduce urinary protein
levels and conserve kidney function in patients with IgAN (19,33).
Yoshikawa et al (40)
demonstrated that the administration of AZA plus steroid therapy
resulted in the increased complete remission of proteinuria in 80
juvenile patients newly diagnosed with IgAN. However, the efficacy
of MMF administration in patients with IgAN differs among
ethnicities. In a Belgian study that assessed MMF vs. placebo in 34
patients, Maes et al (41)
demonstrated an average proteinuria level of 1.8 g/day; whereas no
differences in the reduction of proteinuria or preservation of
glomerular filtration rate were demonstrated. Similarly, in a North
American study, Frisch et al (42) demonstrated that a 1-year regimen of
MMF vs. placebo in patients with IgAN with an initial average
proteinuria level of 2.7 g/day provided no benefits over 24 months.
The efficacy and safety of LET in the treatment of patients with
IgAN in other ethnicities is unknown, and no relevant RCTs were
found. Due to the heterogeneity of the results from previous
studies, the optimal immunosuppressive therapeutic strategy for the
treatment of patients with IgAN remains controversial. The reasons
for this heterogeneity require further investigation, however, the
following may be considered contributing factors: Ethnicity
differences; variations in the levels of therapeutic agent
achieved; limited number of trials and small sample sizes; and
suboptimal methodological quality. The results of the present
meta-analysis demonstrated the potential of immunosuppressive
agents, including AZA, CTX, MMF and LET, as a short-term (6–12
months) therapeutic strategy for the treatment of proteinuria in
patients with IgAN.
The present meta-analysis had various strengths.
Firstly, in an attempt to minimize bias, rigorous methods were used
and only randomized trials were included. Secondly, as compared
with previous meta-analyses, a greater number of studies were
included and attempts were made to tabulate the occurrence of
adverse events. It remains premature to recommend the routine use
of AZA, CTX, MMF and LET immunosuppressive agents as treatment for
patients with IgAN, for various reasons. The data analyzed in the
present meta-analysis were obtained from short-term studies with
small sample sizes, which were generally conducted in a single
center. Furthermore, as the analysis was conducted on pooled data
from published papers, individual patient and original data were
not available, which prevented a more detailed analysis from being
completed, which may have yielded more comprehensive results.
In conclusion, based on the Chinese patients and
short duration RCTs examined, the administration of AZA, MMF, LET
and CTX, demonstrated superior potency in inducing the remission of
proteinuria in patients with IgAN with tolerable adverse effects,
as compared with steroid treatment. In addition, as compared with
CTX, LET administration demonstrated a lower incidence of adverse
reactions. Furthermore, the results of the present meta-analysis
support the need for a large, high-quality multicenter trial in
order to ascertain whether immunosuppressive treatments may be
effective in a broad population of patients with high-risk IgAN,
specifically to determine their effects in kidney failure.
Acknowledgements
The present meta-analysis was supported by the Jiang
Xi Natural Science Foundation Program (grant no. 20122BAB215004)
and the National Natural Science Foundation for Innovative Research
Groups of China (grant no. 81260120). The authors of the present
study acknowledge Professor Haiping Mao, Dr Li Fan and Dr Rong R of
the First Affiliated Hospital of Sun Yat-sen University for their
support in data analyses.
Note added at revision of article
Subsequently to the publication of the above
article, an interested reader drew to our attention that the
article described the use of the drug acetazolamide for IgA
nephropathy on a number of occasions in the text, whereas the
references refer to the drug azathioprine. The authors confirmed
that, owing to an oversight on their part, all the references to
“acetazolamide” in their paper (abbreviated as ‘AZA’) should have
been written as “azathioprine”. Given the nature of this error,
note that the drug name for ‘AZA’ has been corrected in the article
itself, and this is a revised version of the original article.
References
|
1
|
Berger J and Hinglais N: Intercapillary
deposits of IgA-IgG. J Urol Nephrol (Paris). 74:694–695. 1968.(In
French). PubMed/NCBI
|
|
2
|
Bartosik LP, Lajoie G, Sugar L and Cattran
DC: Predicting progression in IgA nephropathy. Am J Kidney Dis.
38:728–735. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
D Amico G: Natural history of idiopathic
IgA nephropathy: Role of clinical and histological prognostic
factors. Am J Kidney Dis. 36:227–237. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Alamartine E, Sabatier JC, Guerin C,
Berliet JM and Berthoux F: Prognostic factors in mesangial IgA
glomerulonephritis: An extensive study with univariate and
multivariate analyses. Am J Kidney Dis. 18:12–19. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bogenschütz O, Bohle A, Batz C, Wehrmann
M, Pressler H, Kendziorra H and Gärtner HV: IgA nephritis: On the
importance of morphological and clinical parameters in the
long-term prognosis of 239 patients. Am J Nephrol. 10:137–147.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Reich HN, Troyanov S, Scholey JW and
Cattran DC: Toronto Glomerulonephritis Registry: Remission of
proteinuria improves prognosis in IgA nephropathy. J Am Soc
Nephrol. 18:3177–3183. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Beck L, Bomback AS, Choi MJ, Holzman LB,
Langford C, Mariani LH, Somers MJ, Trachtman H and Waldman M: KDOQI
US commentary on the 2012 KDIGO clinical practice guideline for
glomerulonephritis. Am J Kidney Dis. 62:403–441. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jadad AR, Moore RA, Carroll D, Jenkinson
C, Reynolds DJ, Gavaghan DJ and McQuay HJ: Assessing the quality of
reports of randomized clinical trials: Is blinding necessary?
Control Clin Trials. 17:1–12. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang S: Clinical observation of
Benazepril combined with azathioprine on IgA nephropathy. Guo Ji Yi
Yao Wei Sheng. 20:53–55. 2009.
|
|
10
|
Kuang B: Clinical observation of
Benazepril combined with azathioprine on IgA nephropathy. ZhongGuo
Xian Dai Yi Sheng. 19:6–7. 2010.
|
|
11
|
Li YN: Curative effect analysis of
Benazepril combined with azathioprine on IgA nephropathy along with
the amount of proteinuria. Hua Bei Mei Tan Yi Xue Yuan XueBao.
4:471–472. 2011.
|
|
12
|
Ma FL: Clinical research oil azathioprine
combined with benazepril treating IgA nephropathy. Xian Dai Zhen
Duan Yu Zhi Liao. 6:653–654. 2012.
|
|
13
|
Xu H: Azathioprine and prednisone,
valsartan Clinical observation on treatment of IgA nephropathy. Zhe
Jiang Yi Xue. 3:227–229. 2013.
|
|
14
|
Chen XM, Chen P, Cai G, Wu J, Cui Y, Zhang
Y, Liu S and Tang L: A randomized control trial of mycophenolate
mofeil treatment in severe IgA nephropathy. Zhonghua Yi Xue Za Zhi.
82:796–801. 2002.(In Chinese). PubMed/NCBI
|
|
15
|
Chen XSh: Clinic Effect of Mycophenolate
Mofetil combined Valsartan on IgA Nephropathy. Zhong Guo Yi Yao Zhi
Nan. 20:53–54. 2010.
|
|
16
|
Guo QY: Efficacy of pediatric IgA
nephropathy mycophenolate mofetil combined hormone therapy. Guang
Dong Yi Xue. 7:902–903. 2010.
|
|
17
|
Huang Zh: Efficacy of focal proliferative
sclerosing 38 cases of patients with IgA nephropathy. He Nan Yi Xue
Yan Jiu. 4:437–439. 2012.
|
|
18
|
Tang SC, Tang AW, Wong SS, Leung JC, Ho YW
and Lai KN: Long-term study of mycophenolate mofetil treatment in
IgA nephropathy. Kidney Int. 77:543–549. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang WM: Clinical study on treatment of
IgA nephropathy with renal insufficiency by corticosteroid,
corticosteroid combined with cyclophosphamide and corticosteroid
combined with mycophenolate mofetil. Shang Hai Jiao Tong Da Xue Xue
Bao. 2:162–167, 173. 2013.
|
|
20
|
Cao LO: Leflunomide in combination with
medium/low dose of prednisolone in treatment of progressive IgA
nephropathy. Shanghai Yi Xue. 9:791–795. 2009.
|
|
21
|
Fu Q: Effects of leflunomide combined with
hormone therapy for lg a nephropathy. Shan Xi Yi Xue Za Zhi.
3:351–353. 2009.
|
|
22
|
Hu RH: IgA nephropathy leflunomide
combined with hormone therapy. J Clinical Internal Medicine.
12:837–839. 2011.
|
|
23
|
Huang YX: Leflunomide combined small dose
glucocorticoid treatment of progressive IgA nephropathy Efficacy.
Chinese Journal of Primary Medicine and Pharmacy. 18:1965–1966.
2011.
|
|
24
|
Li T: Study on effect of leflunomide
combined with glucocorticoid in treatment of progressive IgA
nephropathy. Modern J Integrated Traditional Chinese and Western
Medicine. 1:13–15. 2011.
|
|
25
|
Lou TQ: Controlled trial of leflunomide in
the treatment of immunoglobulin a nephropathy. Journal of Sun
Yat-sem University (Medical Sciences). 5:570–572. 2005.
|
|
26
|
Pu L: Efficacy of benazepril combined with
leflunomide in treatment of IgA nephropathy. Jiangsu Medical
Journal. 11:1277–1279. 2012.
|
|
27
|
Shen P: Leflunomide combined with low-dose
hormone therapy efficacy of IgA nephropathy. Chinese J General
Practice. 10:1580–1581. 2012.
|
|
28
|
Sun MD: Clinical observation of
leflunomide treatment of IgA nephropathy. Chinese J Gerontology.
16:2038–2039. 2009.
|
|
29
|
Wang L: Leflunomide combined with
prednisone treatment of progressive IgA nephropathy Chronic. Suzhou
University J Medical Science. 4:677–679. 2006.
|
|
30
|
Yang FY: Controlled studies of leflunomide
treatment of IgA nephropathy. China Practical Medical. 22:17–19.
2009.
|
|
31
|
Zhang LW: Efficacy of leflunomide combined
with hormone treatment of chronic progressive IgA nephropathy.
Chinese Journal of Modern Drug Application. 20:116–118. 2010.
|
|
32
|
Zhang Y: Efficacy of leflunomide combined
with prednisone in the treatment of advanced IgA nephropathy.
Academic J Guangzhou Medical College. 23:82–85. 2010.
|
|
33
|
Liu Y: Accompanied by moderate proteinuria
treatment of IgA nephropathy. China Medical Herald. 6:23–24.
2006.
|
|
34
|
Guo M: Compare leflunomide or
cyclophosphamide combined hormone therapy of chronic progressive
IgA nephropathy. Guide of China Medicine. 28:142–143. 2012.
|
|
35
|
Sun ZhX: Comparison of the efficacy of
treatment with leflunomide and cyclophosphamide IgA nephropathy
renal dysfunction. Suzhou University J Medical Science. 5:961–963.
2009.
|
|
36
|
Zhang HF: Leflunomide combined with
glucocorticoids contrast cyclophosphamide glucocorticoid treatment
efficacy of IgA nephropathy. J Taishan Medical College. 6:434–436.
2013.
|
|
37
|
Zhong ShH: Controlled studies of
leflunomide and cyclophosphamide-based therapy to nephrotic
syndrome of IgA nephropathy. J North China Coal Medical University.
5:593–594. 2011.
|
|
38
|
Lv J, Xu D, Perkovic V, Ma X, Johnson DW,
Woodward M, Levin A, Zhang H and Wang H; TESTING Study Group, :
Corticosteroid therapy in IgA nephropathy. J Am Soc Nephrol.
23:1108–1116. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Katafuchi R, Ninomiya T, Mizumasa T, Ikeda
K, Kumagai H, Nagata M and Hirakata H: The improvement of renal
survival with steroid pulse therapy in IgA nephropathy. Nephrol
Dial Transplant. 23:3915–3920. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yoshikawa N, Honda M, Iijima K, Awazu M,
Hattori S, Nakanishi K and Ito H; Japanese Pediatric IgA
Nephropathy Treatment Study Group, : Steroid treatment for severe
childhood IgA nephropathy: A randomized, controlled trial. Clin J
Am Soc Nephrol. 1:511–517. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Maes BD, Oyen R, Claes K, Evenepoel P,
Kuypers D, Vanwalleghem J, Van Damme B and Vanrenterghem YF:
Mycophenolate mofetil in IgA nephropathy: Results of a 3-year
prospective placebo-controlled randomized study. Kidney Int.
65:1842–1849. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Frisch G, Lin J, Rosenstock J, Markowitz
G, D'Agati V, Radhakrishnan J, Preddie D, Crew J, Valeri A and
Appel G: Mycophenolate mofetil (MMF) vs placebo in patients with
moderately advanced IgA nephropathy: A double-blind randomized
controlled trial. Nephrol Dial Transplant. 20:2139–2145. 2005.
View Article : Google Scholar : PubMed/NCBI
|