Introduction
In recent years, high-fat diet due to the
improvement of standards of living have led to an increase in the
morbidity of non-alcoholic fatty liver disease (NAFLD),
particularly in younger patients in countries such as China
(1–3). NAFLD is a medical condition
characterized by a series of hepatic pathological changes including
simple steatosis, non-alcoholic steatohepatitis and cirrhosis
(4–6).
Hepcidin, secreted by hepatic cells, is a key
regulator of the absorption of iron into the blood circulation in
human. The association between disturbances in iron metabolism and
the manifestation of NAFLD is well established (7). Since Hepcidin regulates iron
absorption, its increased level can accelerate the development of
the disease. Nuclear factor (NF)-κB is known to play a crucial role
in the transformation from simple steatosis to steatohepatitis
(8). The excitation of toll-like
receptor 4 (TLR4) activates NF-κB through a cascade of signal
transduction, which, in turn, promotes the release of transforming
growth factor-β (TGF-β), ultimately inducing the necrosis of liver
cells, inflammation and the formation of fibrosis (9).
The aim of the present study was to investigate the
manner in which Hepcidin in NAFLD is regulated by the TLR4/NF-κB
signaling pathway by observing changes in Hepcidin expression in
NAFLD rats treated with pathenolide, an established NF-κB
inhibitor.
Materials and methods
Animals
Sixty male Sprague-Dawley rats (200±20 g) were
obtained from the Animal Center of Wuhan University (Wuhan, China)
were used for the study. Following an acclimatization period, the
rats were randomly divided into the control, NAFLD and intervention
groups (n=20 animals per group). The rats of the control group
received standard laboratory diet, the NAFLD group received
high-fat diet (standard laboratory diet + 2% cholesterol + 10% lard
+ 2.5% vegetable oil) and the intervention group received high-fat
diet and was also administered with 10 µmmol/2 ml of pathenolide
intraperitoneally. The rats were maintained and fed for 28 days.
After 28 days, the rats were sacrificed by cervical dislocation
under ether anesthesia and a small portion of hepatic tissue from
the right lobe of the liver was excised.
Chemicals
Pathenolide was purchased from Sigma-Aldrich China,
Inc. (Shanghai, China). Rabbit anti-human TLR4 antibody, goat
anti-mouse NF-κB antibody and rabbit anti-rat Hepcidin antibody
were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). Rabbit anti-goat immunoglobulin G (IgG) was purchased from
Wuhan Boster Biological Technology, Ltd. (Wuhan, China). The
quantitative polymerase chain reaction (qPCR) kit was purchased
from Takara Biomedical Technology Co., Ltd. (Beijing, China).
Histological analysis
The excised tissues were fixed in 10% formalin,
embedded in paraffin, and cut into 4 µm sections. The microsections
were stained with hematoxylin and eosin. The stained sections were
observed under a microscope (Olympus CX31-LV320; Olympus, Tokyo,
Japan) and the changes recorded. Fat dying was analyzed by oil red
staining in which the liver cell nucleus became hyacinthine and the
lipid droplets red.
Evaluation of liver
histopathology
The degree of fat degeneration was evaluated by
taking into account the ratio of the number of lipid droplets in
the hepatic lobule/total number of cells, which was scored as: 0
(−), <1/3 (+), 1/3–2/3 (++), >2/3(+++), and =1 (++++).
Quantification of TLR4 and NF-κB
Total protein from hepatic tissue was extracted from
cell lysate using TRIzol reagent (Invitrogen-Life Technologies,
Carlsbad, CA, USA). Protein concentration was determined by
Coomassie Brilliant Blue Staining method. Protein (20 µg) was
loaded and resolved using a 10% SDS-PAGE and transferred to a
nitrocellulose membrane. The membrane was blocked with 5% non-fat
skim milk at room temperature (25°C). The membrane was then
incubated with primary antibodies such as rabbit anti-human TLR4
polyclonal antibody and goat anti-rat NF-κB (dilution 1:500) for 12
h. Subsequently, horseradish peroxidase conjugated rabbit anti-goat
IgG (dilution 1:1,000) was added and the membrane was incubated for
1 h at room temperature. Enhanced chemiluminescence reagent (Boster
Biological Technology, Ltd., Wuhan, China) was used to detect
hybridization signal and Quantity One® software (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) was used to analyze the
relative amount of TLR4 and NF-κB proteins. β-actin served as the
internal reference.
qPCR quantification of Hepcidin
mRNA
Total RNA was extracted using TRIzol reagent. The
isolated RNA was quantified spectrophotometrically at a wavelenghth
of 260 nm. qPCR analysis was conducted as per the manufacturer's
instructions (Takara Biomedical Technology Co., Ltd.). Obtained
amplicons were subjected to agarose gel (Sigma-Aldrich Inc.,
Shanghai, China) electrophoresis. qPCR data analysis was performed
by normalization of data to β-actin as the standard to determine
the absolute quantity of starting Hepcidin gene expression. The
primer sequences used to analyze the Hepcidin gene are shown in
Table I.
 | Table I.Primer sequences used for quantitative
polymerase chain reaction. |
Table I.
Primer sequences used for quantitative
polymerase chain reaction.
| Gene | Primer sequence | Amplicon (bp) |
|---|
| Hepcidin | Upstream:
5′-TGTCTCCTGCTTCTCCTCCTTG-3′ | 291 |
|
| Downstream:
5′-GGAGGGCAGGAATAAATAATGG-3′ |
|
| β-actin | Upstream:
5′-CCACAGCTGAGAGGGAAATC-3′ | 108 |
|
| Downstream:
5′-TCTCTTCCCACTCACGGGTTGIL-3′ |
|
Statistical analysis
Data were analyzed using SPSS 15.0 statistical
software (SPSS, Inc., Chicago, IL, USA) and presented as mean ±
standard deviation. P<0.05 was considered to indicate a
statistically significant difference.
Results
Visual screening of liver
The shape, texture and color of the liver in the
control group appeared normal. However, in the NAFLD group, the
volume of the liver had increased with an obtuse margin and the
section appeared faint yellow and was slightly greasy. The
appearance of liver in the intervention group was similar to the
control group.
Histopathology of liver
In the control group, the structure of the hepatic
lobule and hepatic cords appeared normal and the cells were
arranged in an orderly manner. At the center of the cells, the
nucleus was intact and the cytoplasm was well distributed without
the lipid droplet (Fig. 1A). In the
NAFLD group, the structure of the hepatic lobule was irregular.
Diffuse and numerous round fat vacuoles of varying size with
hydropic degeneration and inflammatory cell infiltration were
observed. A number of cells contained large red fat vacuoles
(Fig. 1B). In the intervention
group, a few microvesicular fat droplets were evident (Fig. 1C).
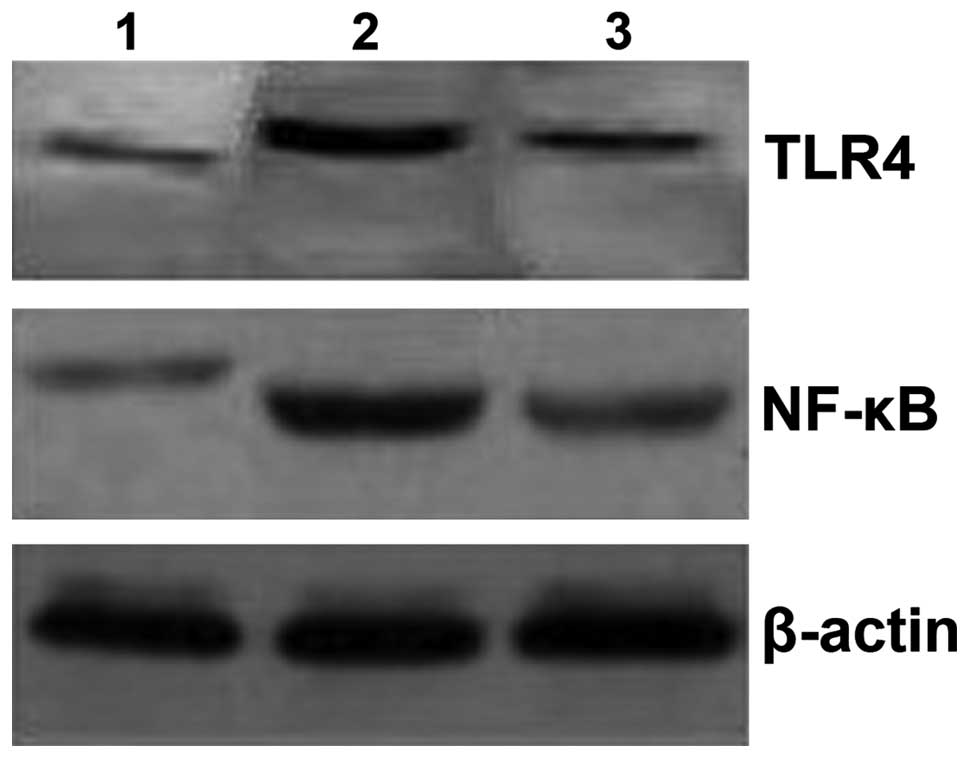
Downregulation of TLR4 and NF-κB
proteins
The levels of TLR4 and NF-κB in the NAFLD group were
significantly higher when compared with the control group
(P<0.05; Table II and Fig. 2). TLR4 and NF-κB levels in the
intervention group were significantly decreased (P<0.05) in
comparison with the NAFLD group as shown in Table II and. Fig. 2
 | Table II.The relative amount of TLR4 and NK-κB
proteins in the control, NAFLD and intervention groups. |
Table II.
The relative amount of TLR4 and NK-κB
proteins in the control, NAFLD and intervention groups.
| Group | TLR4 | NK-κB |
|---|
| Control | 0.24±0.17 | 0.92±0.14 |
| NAFLD |
1.53±0.18a |
1.63±0.12a |
| Intervention |
0.31±0.11b |
1.01±0.09b |
Downregulation of Hepcidin by
pathenolide
Hepcidin was found to be overexpressed in the NAFLD
group as compared to the control group (P<0.05) (Fig. 3). Hepcidin was significantly
downregulated by pathenolide in the intervention group as compared
to the NAFLD group (P<0.05).
Discussion
Hepcidin is a key regulatory factor of iron
homeostasis (10,11) in which iron absorption and recycling
is affected by the degradation of ferroprotein, thereby
downregulating iron concentration in intestinal mucosa cells and
macrophage cells and hepatocytes (12). Currently, iron overload has been
identified in several types of chronic diseases due to imbalances
in iron homeostasis. Previous findings suggested that during the
transformation from simple hepatic steatosis into non-alcoholic
steatohepatitis, the iron deposition in the liver gradually
increases, showing a significant positive correlation with the
severity of disease (13,14).
NF-κB is a crucial transcriptional regulator that
regulates genes involved in the induction of inflammation. The
adipose tissue is the main source of cytokines. However, the
condition of liver cell steatosis promotes the release of various
cytokines that have a strong proinflammatory effect such as tumor
necrosis factor-α, while TGF-β plays an important role through the
signaling pathway of NF-κB (15–18).
Since inflammation during liver steatosis is capable of
upregulating TLR4/NF-κB, which in turn leads to the overexpression
of Hepcidin, this may be a possible reason for the accumulation of
excess iron in the liver of NAFLD patients (19,20).
The present study has demonstrated that the NAFLD
group exhibited typical steatosis. However, following intervention
with the NF-κB inhibitor, Pathenolide, the pathological changes
attributed to NAFLD had reversed to normal. Similarly, the
expression of TLR4,/NF-κB proteins and Hepcidin mRNA in NAFLD was
higher than that of the control group. However, the expression of
TLR4/NF-κB proteins and Hepcidin mRNA were decreased close to the
values of the control, indicating that, inhibiting the TLR4/NF-κB
pathway can repress the expression of Hepcidin, thus reverting
pathological characteristic changes of NAFLD.
In conclusion, inhibition of the activation of the
TLR4/NF-κB pathway using pathenolide, downregulated the expression
of Hepcidin. This findings is crucial in the prevention and
treatment of steatohepatitis and repression of the pathological
process of liver fibrosis in NAFLD.
References
|
1
|
Rinella ME: Nonalcoholic fatty liver
disease: a systematic review. JAMA. 313:2263–2273. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Targher G, Chonchol MB and Byrne CD: CKD
and nonalcoholic fatty liver disease. Am J Kidney Dis. 64:638–652.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Granér M, Nyman K, Siren R, Pentikäinen
MO, Lundbom J, Hakkarainen A, Lauerma K, Lundbom N, Nieminen MS and
Taskinen MR: Ectopic fat depots and left ventricular function in
nondiabetic men with nonalcoholic fatty liver disease. Circ
Cardiovasc Imaging. 8:e0019792015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fargion S, Porzio M and Fracanzani AL:
Nonalcoholic fatty liver disease and vascular disease:
state-of-the-art. World J Gastroenterol. 20:13306–13324. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu H, Jin M, Han D, Zhou M, Mei X, Guan Y
and Liu C: Protective effects of aerobic swimming training on
high-fat diet induced nonalcoholic fatty liver disease: Regulation
of lipid metabolism via PANDER-AKT pathway. Biochem Biophys Res
Commun. 458:862–868. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Paradies G, Paradies V, Ruggiero FM and
Petrosillo G: Oxidative stress, cardiolipin and mitochondrial
dysfunction in nonalcoholic fatty liver disease. World J
Gastroenterol. 20:14205–14218. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Haap M, Machann J, von Friedeburg C,
Schick F, Stefan N, Schwenzer NF, Fritsche A, Häring HU and Thamer
C: Insulin sensitivity and liver fat: role of iron load. J Clin
Endocrinol Metab. 96:E958–E961. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao CY, Yan L, Wang YD, Wang W, Zhou JY
and Zhen Z: Role of resistin in inflammation of hepatocytes in
nonalcoholic steatohepatitis. Zhonghua Gan Zang Bing Za Zhi.
17:683–687. 2009.(In Chinese). PubMed/NCBI
|
|
9
|
Rivera CA, Adegboyega P, van Rooijen N,
Tagalicud A, Allman M and Wallace M: Toll-like receptor-4 signaling
and Kupffer cells play pivotal roles in the pathogenesis of
non-alcoholic steatohepatitis. J Hepatol. 47:571–579. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Aboul-Enein A, El-Beshlawy A, Hamdy M,
Shaheen I, El-Saadany Z, Samir A and El-Samie HA: Peripheral
expression of hepcidin gene in Egyptian β-thalassemia major. Gene.
564:206–209. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Boumaiza M, Ezzine A, Jaouen M, Sari MA
and Marzouki MN: Molecular characterization of a novel hepcidin
(HepcD) from Camelus dromedarius. Synthetic peptide forms
exhibit antibacterial activity. J Pept Sci. 20:680–688. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ganz T: Hepcidin and iron regulation, 10
years later. Blood. 117:4425–4433. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mitsuyoshi H, Yasui K, Harano Y, Endo M,
Tsuji K, Minami M, Itoh Y, Okanoue T and Yoshikawa T: Analysis of
hepatic genes involved in the metabolism of fatty acids and iron in
nonalcoholic fatty liver disease. Hepatol Res. 39:366–373. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tsuchiya H, Ashla AA, Hoshikawa Y, Matsumi
Y, Kanki K, Enjoji M, Momosaki S, Nakamuta M, Taketomi A, Maehara
Y, et al: Iron state in association with retinoid metabolism in
non-alcoholic fatty liver disease. Hepatol Res. 40:1227–1238. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Csak T, Ganz M, Pespisa J, Kodys K,
Dolganiuc A and Szabo G: Fatty acid and endotoxin activate
inflammasomes in mouse hepatocytes that release danger signals to
stimulate immune cells. Hepatology. 54:133–144. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Amacher DE: Progress in the search for
circulating biomarkers of nonalcoholic fatty liver disease.
Biomarkers. 19:541–552. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hassan K, Bhalla V, El Regal ME and
A-Kader HH: Nonalcoholic fatty liver disease: a comprehensive
review of a growing epidemic. World J Gastroenterol.
20:12082–12101. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wynn TA: Cellular and molecular mechanisms
of fibrosis. J Pathol. 214:199–210. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kelley CE, Brown AJ, Diehl AM and Setji
TL: Review of nonalcoholic fatty liver disease in women with
polycystic ovary syndrome. World J Gastroenterol. 20:14172–14184.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fitzpatrick E and Dhawan A: Noninvasive
biomarkers in non-alcoholic fatty liver disease: current status and
a glimpse of the future. World J Gastroenterol. 20:10851–10863.
2014. View Article : Google Scholar : PubMed/NCBI
|