Introduction
Bell's palsy is a form of temporary facial nerve
paralysis that occurs primarily in young adults. Bell's palsy is a
condition usually without aura symptoms and its exact
pathophysiology remains to be elucidated (1). The onset of Bell's palsy is sudden and
symptoms, including inability to lift the eyebrow or close the eyes
(1), usually peak within a few days.
The diagnosis, prognosis and curative effect evaluation of Bell's
palsy depend primarily on the clinical symptoms. The severity of
the disease is classified according to the Facial Nerve Grading
System 2.0 (NGS2.0), which classifies the severity of Bell's palsy
as grades I–VI (2). Imaging support
is required to observe the real facial nerve. Physicians are
frequently unable to make a definitive judgment regarding the
necessity of treatment continuation for patients in grade I or II.
Without access to appropriate and effective methods of evaluation,
physicians depend on their clinical experiences to form such a
judgment.
Hagino et al (3) demonstrated that for the facial
paralysis patients participating in his study, only 15 cases of
mastoidectomy and facial nerve canal operation manifested facial
nerve edema. However, the degree and the details of nerve edema
were not identified.
The prognostic value of magnetic resonance imaging
(MRI) remains at the level of a differential diagnosis, such as
exclusion of intracranial lesions. MRI results are often referred
to as being contrast-enhanced rather than physical nerve swelling
(4,5). In Bell's palsy, facial nerve
enhancement in the facial canal is the characteristic MR change
(6). Bell's paralysis with
incomplete recovery in the mastoid segment of the facial nerve was
better enhanced than the labyrinthine and geniculate ganglion
section in MRI (7,8). High-resolution temporal bone computed
tomography (CT) is also able to identify temporal bone fractures
that violate the facial nerve canal (9). However, there are a few challenges in
using CT and MRI techniques to reveal the extracranial facial
nerve. Additionally, CT and MRI are unable to efficiently evaluate
the facial nerve edema. Thus, to support the clinical diagnosis
ultrasonography (US) should be combined with CT and MRI.
Prior to the advancement of imaging methods,
electrophysiological methods were used to evaluate the outcomes in
the treatment of facial nerve diseases, such as Bell's palsy
(10). Electromyography, facial
nerve conduction and blink reflexes were among the tests used for
these evaluations (10). These
methods had high sensitivity in the diagnosis of early neuritis,
but their low specificity impeded the prediction of treatment
effectiveness.
Since Fornage (11)
reported on the sonographic evaluation of peripheral nerves two
decades ago, high-frequency ultrasonography (HFUS) has evolved
rapidly. HFUS is a useful tool for observing the facial nerves in
patients with facial paralysis. In recent years, a variety of
evaluation techniques and US devices have been reported (12). HFUS is a non-invasive method that is
cost-effective and is capable of demonstrating the lesion structure
together with the course of the affected nerve (13).
In the present study, US was employed to observe the
normal and abnormal facial nerve. This method was compared to
well-known electrophysiological techniques, such as facial nerve
M-wave detection. The results showed that HFUS is able to provide a
more convenient and effective method for diagnostic, prognostic and
treatment evaluation purposes for patients suffering from Bell's
palsy.
Materials and methods
General
Informed consent was obtained from the patients and
their families. The present study was approved by the Ethics
Committee of Capital Medical University (Beijing, China). A total
of 104 healthy volunteers, 40 patients with acute onset of Bell's
palsy and 30 patients who underwent three-month routine therapy for
their disease were included in the present study. Healthy
volunteers and patients underwent HFUS examination and VII nerve
conduction.
All 104 healthy volunteers met the criteria for
inclusion in the control group (CG) (mean age, 31; range, 15–57; 55
males). The experimental group A (EGA) included 40 patients (mean
age, 28; range, 18–48; 28 males) with acute onset of unilateral
Bell's palsy, with all the patients being examined within one week
from onset of the symptoms. Each patient's facial nerve was
measured clearly. Each patient was examined by an experienced
neurologist who confirmed the diagnosis and classified the severity
of the disease according to the Facial NGS2.0 grading system.
Results from the examination of EGA classified the patients between
grade III and VI (1). The
experimental group A (EGB) included 30 patients (mean age, 30;
range, 18–60; 15 males). All the patients in the EGB, who had
undergone three-month of routine therapy, were evaluated and
classified as grade I and II.
The exclusion criteria for this study included
idiopathic facial nerve disease and peripheral nerve lesions caused
by complications of diabetes, stroke, traumatic brain injury,
otitis media or tumor. Additionally, prior to the
electrophysiological examinations, the patients did not use any
medication that potentially affected the results.
HFUS examination
HFUS examinations were conducted using a MHz
linear-array probe (C12-5, Philips iU22, Philips, Amsterdam, The
Netherlands) by an experienced operator. As the study progressed,
every effort was made to scan volunteers in a completely supine
position, allowing for better visualization on the small nerve
structures. The facial nerve in the longitudinal view was
identified at the mastoid region at the point from which it emerged
from the stylomastoid foramen. At this site, it traversed
anteriorly into the parotid gland substance prior to dividing into
five branches (10). In the first
step, the left end of the probe was aimed at the stylomastoid
foramen, and subsequently, the probe was tilted outward and away
from the parotid gland. The shallow hypoechoic muscle was revealed
in the acoustic window. The region between the stylomastoid foramen
exit and the parotid gland was visualized.
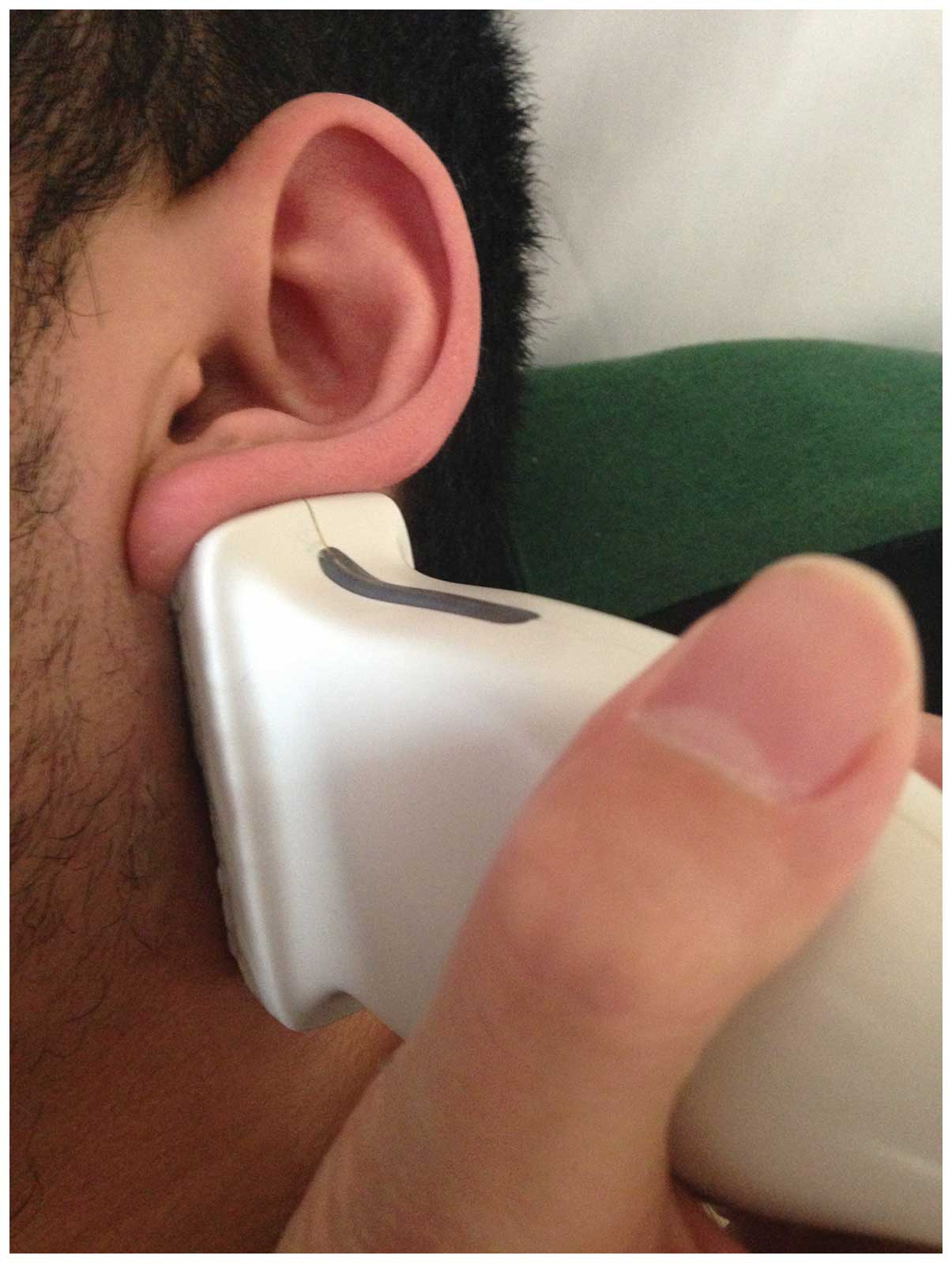
Fig. 1 shows the US
examination method in longitudinal planes in a volunteer. The
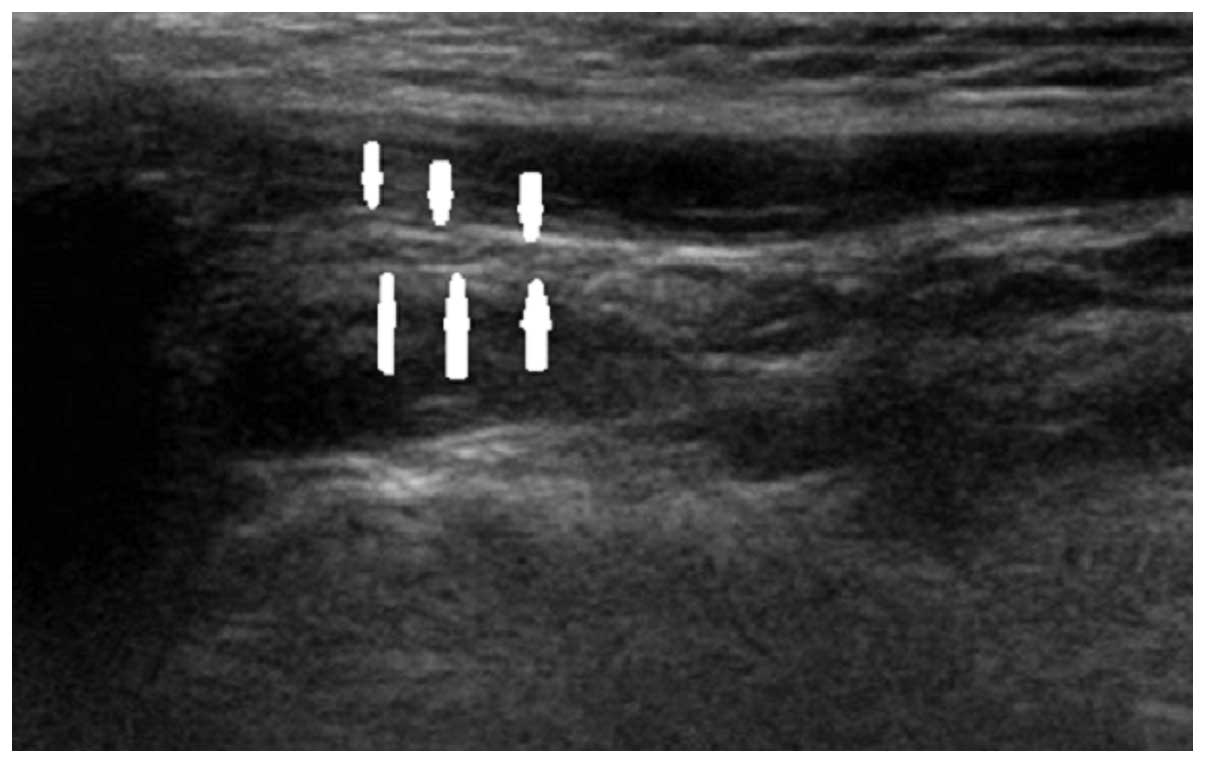
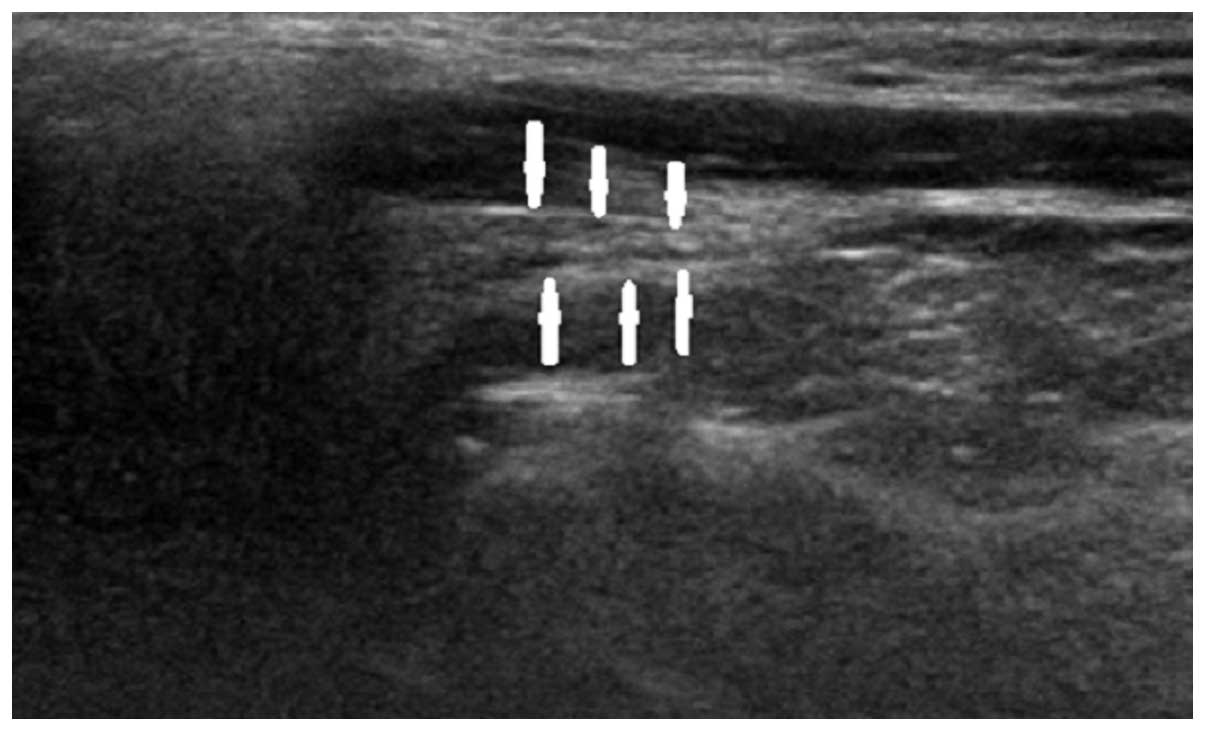
normal facial nerve in the longitudinal plane had a relatively
hyperechoic sheath compared to the surrounding muscles, exhibiting
a linear fascicular appearance with an oval hypoechoic structure
and the spot echo surrounding the hyperechoic film strip in the
transverse sonogram. By contrast, the abnormal facial nerve was
often swollen with decreased echo, hyperechoic sheath and the
fascicular pattern was obscure. Patients in EGA were scanned within
the first week of the onset of their symptoms. Patients in EGB were
scanned when they had a favorable outcome three months subsequent
to clinical presentation. Figs.
2–4 show longitudinal sonograms
of the normal and affected facial nerve in 1 week and 3 months,
respectively.
From the most proximal to the distal visualized
portions, the average surface area and diameter of facial nerve
cross-sections was measured. The average length as well as the
depth from the skin, visible length and the facial nerve to facial
artery ratio of the same location were also measured.
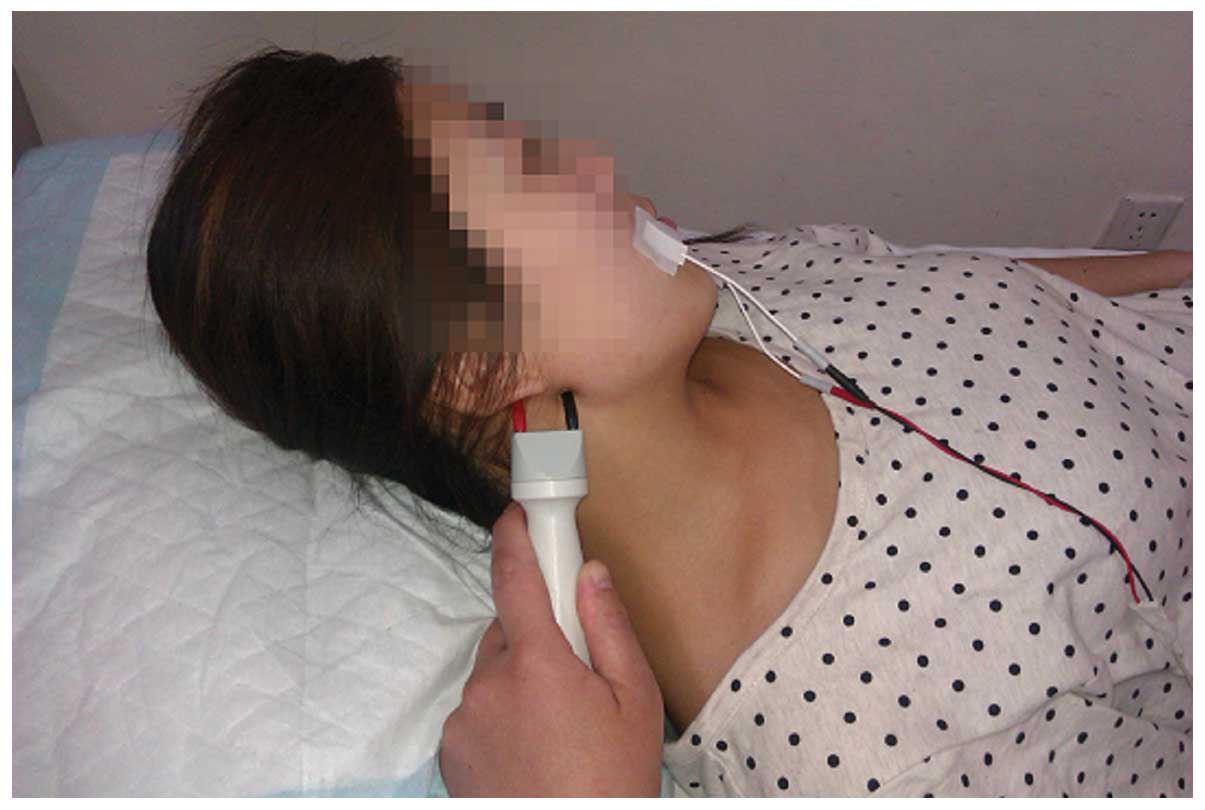
Nerve conduction
Electromyograph and evoked potential measurement
(Keypoint; Dantec Dynamics A/S, Kongeriget, Denmark) was performed
following marking of the body surface according to US observation.
Subsequently, the facial nerve M-wave detection was selected.
Electrophysiological detection was performed at room temperature
(20–30°C). Patients were in the supine position, with eyes closed.
Surface electrodes were placed on the orbicularis oculi muscle and
the orbicularis oris muscle. Reference electrodes were placed on
the chin and the ground wire was placed on the arm. For patients
with visible facial nerve at the surface, stimulation was applied.
The sensitivity was 0.2 mV/div and the scanning speed was 5 ms/div.
The initial intensity of the stimulus induced a maximum compound
muscle action potential (CMAP), which was increased to achieve a
stronger stimulation of 10-30%. The stimulation time was 0.1 msec
and the analysis time was 20 msec. CMAP amplitude and distal motor
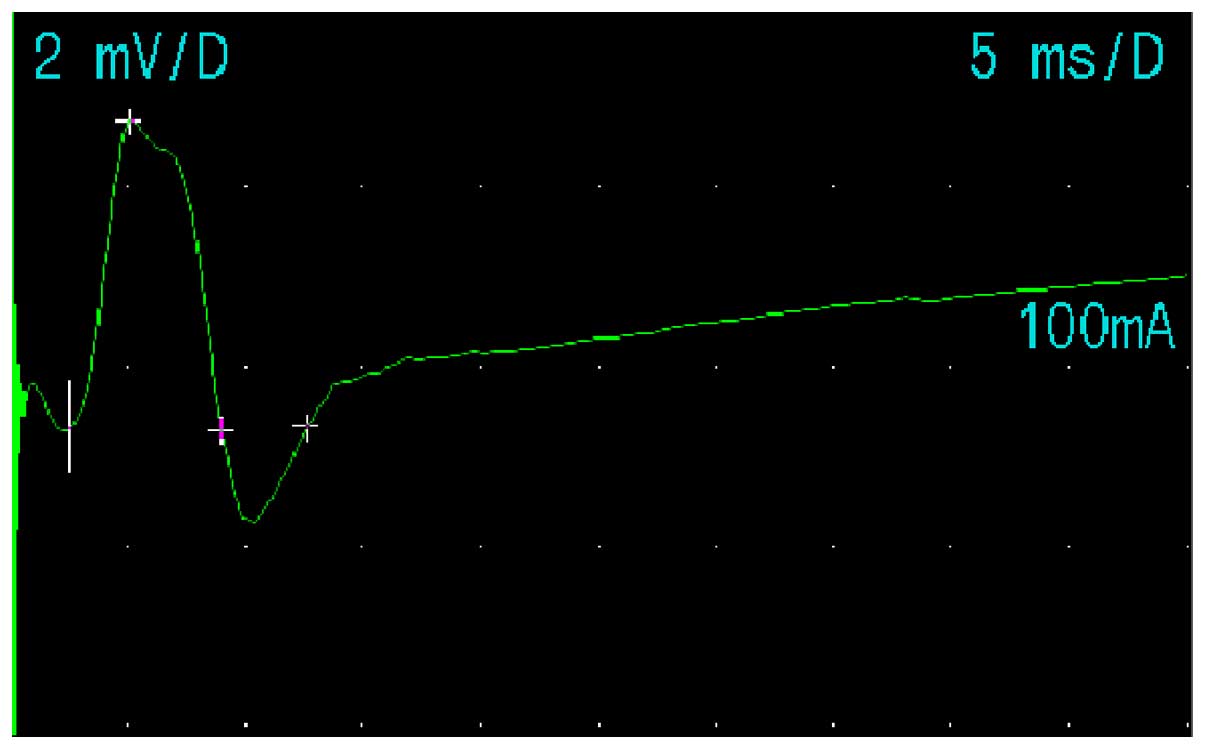
latency of the facial nerve were observed. Fig. 5 shows the method for nerve conduction
of the left facial nerve in the volunteers of the present study.
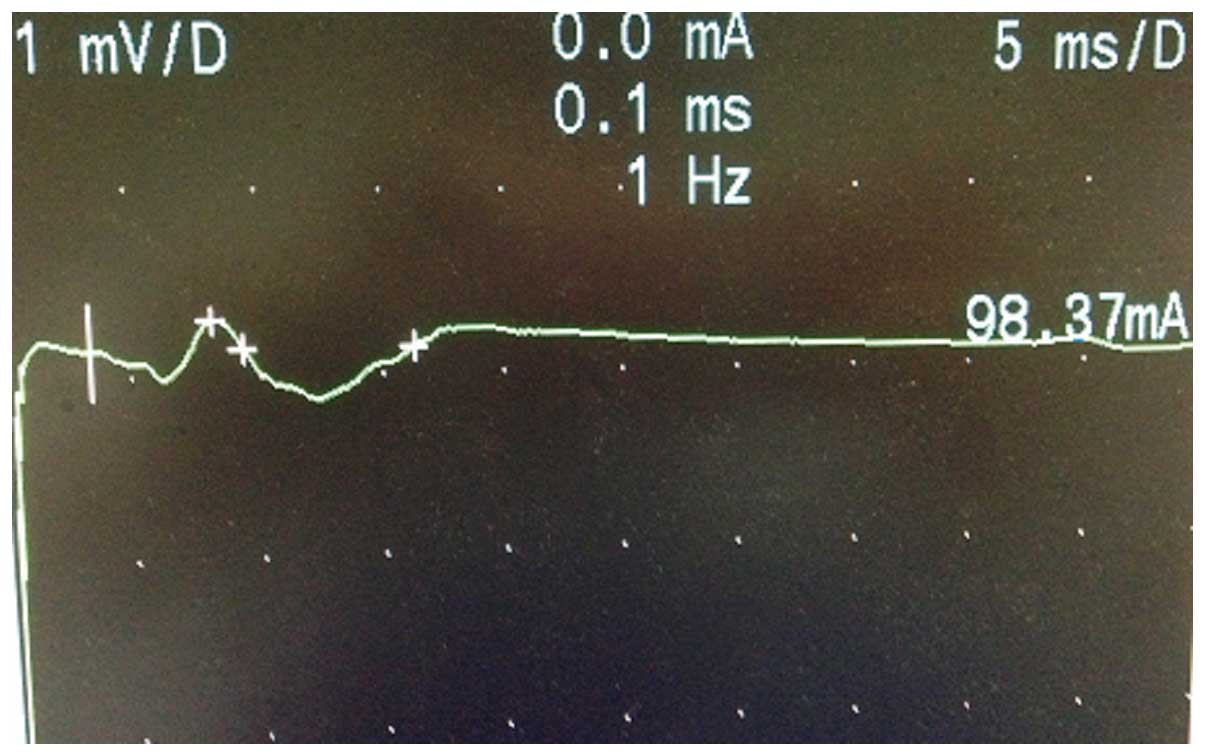
Figs. 6 and 7 show the M-wave of the facial nerve in the
normal and abnormal facial nerve subjects.
Statistical analysis
Data are presented as mean ± standard deviation. The
measurement data for the facial nerves were used for the paired
t-test. The differences between groups were analyzed using one-way
ANOVA and numerical data were analyzed using the χ2
test. The SPSS statistical software (SPSS, Inc., Chicago, IL, USA)
package was used for the statistical analysis. P<0.05 indicated
statitistically significant differences.
Results
Results of the present study revealed that the mean
normal facial nerve diameter was 0.16±0.03 cm and the
cross-sectional area (CSA) was 0.05±0.01 cm2. The visual
length and depth from the skin were 1.01±0.12 and 0.85±0.13 cm,
respectively. The ratio (facial nerve to facial artery ratio) of
the normal average right facial nerve was 1.06±0.27.
Table I summarizes
results obtained from all the patients. Some results of two sides
of HFUS and M-wave in each group were significantly different. The
CSA of the facial nerve was not perfectly round. The CSA was not
calculated using the usual formula, but individual tracings were
obtained to calculate the area using integration. The CSA of the
facial nerve was drawn and the automatic measurement.
 | Table I.HFUS and M-wave detection of the
facial nerves about CG, EGA and EGB. |
Table I.
HFUS and M-wave detection of the
facial nerves about CG, EGA and EGB.
|
| CG | EGA | EGB |
|---|
|
|
|
|
|
|---|
| Characteristics | Left | Right | p-value | AS | US | p-value | AS | US | P-value |
|---|
| Diameter, cm | 0.16±0.02 | 0.16±0.03 | 0.027 | 0.20±0.02 | 0.14±0.02 | 0.000 | 0.19±0.03 | 0.14±0.02 | 0.000 |
| CSA,
cm2 | 0.05±0.01 | 0.05±0.01 | 0.006 | 0.09±0.01 | 0.04±0.01 | 0.000 | 0.07±0.01 | 0.04±0.01 | 0.000 |
| Depth, cm | 0.86±0.02 | 0.84±0.13 | NS | 0.85±0.02 | 0.82±0.03 | NS | 0.83±0.03 | 0.84±0.12 | NS |
| VL, cm | 0.99±0.14 | 1.01±0.12 | NS | 1.02±0.10 | 1.11±0.12 | NS | 1.10±0.08 | 1.10±0.07 | NS |
| Hyperechioc | 97 | 96 | NS | 4 | 38 | 0.000 | 27 | 29 | NS |
| Hypoechioc | 1 | 2 | NS | 36 | 2 |
| 3 | 1 |
|
| Clear | 96 | 96 | NS | 10 | 36 | 0.000 | 16 | 28 | 0.000 |
| Obscure | 2 | 2 | NS | 30 | 4 |
| 14 | 2 |
|
| Delitescence,
msec | 3.13±0.06 | 2.94±0.06 | NS | 3.66±0.66 | 2.84±0.06 | 0.000 | 2.94±0.38 | 3.03±0.28 | NS |
| Amplitude, mV | 2.25±0.11 | 2.24±0.12 | NS | 1.43±0.75 | 2.35±0.22 | 0.000 | 2.22±0.89 | 2.40±0.26 | NS |
| Length, cm | 11.1±1.21 | 10.0±1.86 | NS | 12.4±1.32 | 10.4±1.24 | NS | 12.5±1.02 | 11.4±1.24 | NS |
We found significant differences in nerve diameters
in different groups (P<0.05). We also found significant
differences in CSA, definition, echogenicity, delitescence and
amplitude (P<0.05). The nerve diameters in the two experimental
groups were greater than the CG. Additionally, nerve diameters in
EGA were larger than those in EGB. These results suggested that
facial nerve edema improved following treatment (Table II).
 | Table II.Comparison of P-values between CG,
EGA and EGB. |
Table II.
Comparison of P-values between CG,
EGA and EGB.
|
Characteristics | CG with EGA | CG with EGB | EGA with EGB | CG, EGA and
EGB |
|---|
| Diameter | 0.000 | 0.000 | 0.026 | 0.000 |
| Definition | 0.000 | 0.000 | 0.015 | 0.000 |
| Echogenicity | 0.000 | NS | 0.000 | 0.000 |
| Delitescence | 0.011 | NS | 0.000 | 0.012 |
| Amplitude | 0.028 | NS | 0.000 | 0.023 |
In the EGA group, no significant correlation was
identified for the severity grading nerve, diameter, CSA,
delitescence and amplitude. A statistically significant correlation
was observed for severity grading in the EGB during HFUS
examinations, but the delitescence and amplitude had no statistical
significance (Tables III and
IV). The echogenicity of the nerve
was restored, although the diameter and the definition of the nerve
remained different in the EGB. The diameter and the definition of
patients with severity grade II were different compared to grade I.
Furthermore, there was no significant correlation among abnormal US
diameter, delitescence and amplitude.
 | Table III.Comparison of severity grading for
EGA at HFUS and M-wave detection. |
Table III.
Comparison of severity grading for
EGA at HFUS and M-wave detection.
| Grade | III | IV | V–VI | P-value |
|---|
| Diameter, mm | 0.21±0.02 | 0.20±0.04 | 0.21±0.02 | NS |
| Definition,
clear/obscure | 8/2 | 4/4 | 4/18 | NS |
| Echogenicity,
hyper/hypo | 6/4 | 3/5 | 2/20 | NS |
| Delitescence,
msec | 3.78±0.64 | 3.75±0.43 | 3.57±0.74 | NS |
| Amplitude, mV | 1.01±0.18 | 1.09±0.22 | 1.75±0.87 | NS |
| Number | 10 | 8 | 22 |
|
 | Table IV.Comparison of severity grading for
EGB at HFUS and M-wave detection. |
Table IV.
Comparison of severity grading for
EGB at HFUS and M-wave detection.
| Grade | I | II | p-value |
|---|
| Diameter, mm | 0.16±0.02 | 0.21±0.02 | 0.000 |
| Definition,
clear/obscure |
8/3 |
8/11 | 0.04 |
| Echogenicity,
hyper/hypo | 10/1 | 17/2 | NS |
| Delitescence,
msec | 2.86±0.43 | 2.98±0.36 | NS |
| Amplitude, mV | 2.07±0.70 | 2.14±0.92 | NS |
| Number | 11 | 19 |
|
Discussion
HFUS examination in normal and abnormal nerves. The
HFUS method has proved its efficacy over a wide range of nerve
types as well as a wide range of ages and respective body sizes
(13). The current approach for
localizing and assessing the severity of nerve injuries involves
accurate clinical history, physical examination and
electrodiagnostic studies (14).
However, such diagnostic tests do not reveal the exact location or
the cause of the lesions. Additionally, they do not provide spatial
information concerning nerves and surrounding structures.
High-resolution US offers several advantages over other existing
techniques. HFUS is faster, has superior spatial resolution and is
more dynamic, therefore it is an optimal option for patients
(15). A clear understanding of
neural anatomy is crucial in designing a successful clinical
therapy. Appropriate treatment can be designed and planned only
when there is a clear image with regard to the function and
distribution of nerve bundles and the anatomical relationship with
the adjacent structures. For surgeons, identification of the
location and the tract of the facial nerve anatomy is
imperative.
For ultrasound radiologists, detection of the
correct images of facial nerve and avoidance of the interferences
from tendons, muscles and parotid gland is important. US
examinations are operator-dependent and require experience in
superficial soft-tissue structures, which have a relatively long
learning curve. The present study examined the feasibility of
performing measurements on normal and abnormal facial nerves by
ultrasound radiologists without facial nerve expertise. Parameters
such as diameter, CSA, depth from the skin and visible length were
used as references in the absence of nerve electrophysiological
cases. Stylomastoid foramen is a significant characteristic for
identifying facial nerves. Tendons and nerves are difficult to
identify as they are linked together. Thus, technicians should move
the probe gently to identify the facial nerve entering the parotid
gland. Kele (16) reported that in
individuals in good physical condition, cranial nerves, such as the
vagal and accessory nerves, can be visualized regularly. The nerves
have cable-like structures and appear on the transversal sections
as round to oval hyperechoic structures. They are surrounded by an
echogenic rim representing the epifascicular epineurium and the
perineurial fatty tissue. The sonographic echo pattern (texture) is
known as ‘honeycomb-shaped’ (17).
As mentioned earlier, Hagino et al (3) in his study established that facial
paralysis patients manifested facial nerve edema in only in few
(15) cases of mastoidectomy and
facial nerve canal operation.
In the present study, we found significant
differences between the two sides of normal nerve diameters. In
several volunteers along with the age, even with the exclusion of
idiopathic facial nerve disease and peripheral nerve lesions caused
by complications from diabetes, stroke, traumatic brain injury,
otitis media or tumor, patients inevitably encounter cavities,
periodontitis and other oral diseases or even issues with chewing.
These issues may be detected in electrophysiology or have a direct
effect on the nerve itself. However, electrophysiology does not
detect lesions that are evident with HFUS. Nevertheless, the
differences between the two methods were <0.02 cm, indicating
that there was no clinical significance.
HFUS identifies patients with grading
II
We also analyzed Bell's palsy according to Facial
Nerve Grading. The patients were generally classified as grade III
and above; therefore, we assigned grades III–VI for the patients in
the EGA. The patients were treated actively and continued to be
treated when clinical symptoms persisted three months following the
initial therapy. Patients considered the disease cured following
amelioration of grade from VI to I or II. In these cases, it was
necessary to judge objectively whether to continue patient
treatment. Evaluation of the curative effects for grade I and II
patients had more clinical significance. There was no statistical
significance in neural function detection in patients as grade I or
II. The US findings are clinically important as they can provide
accurate guidance to patients and physicians with regard to whether
to continue the treatment for facial paralysis.
The nerve diameters in the two experimental groups
were greater than the CG. In the EGA, nerve diameters were larger
than those in the EGB. These results suggested that facial nerve
edema had the tendency to improve following treatment. The
echogenicity of the nerve was restored, although there were
differences in the diameters and definitions of nerve in the EGB.
The diameter and definition of patients with grade II severity were
different to those with grade I. Consequently, the patients with
grade II were judged to be eligible to continue their
treatment.
A previous report revealed that the measurement of
facial nerve diameter was an appropriate predictor for good
prognosis (with positive predictive value of 100%). However, it was
not a useful predictor in the case of an unsatisfactory prognosis
(negative predictive value of 77%). HFUS was highly correlated with
clinical grade outcomes (18). Our
results are important because we examined the diameter and CSA, and
analyzed the characteristics of facial nerve images, such as
definition and echogenicity. The results from the current study
also confirmed that US detection correlated well with
House-Brackemann Facial Nerve Grading.
US combined with electrophysiological and
MRI support clinical diagnosis
The neural electrophysiological verification of HFUS
measurement of the facial nerve can effectively confirm the results
obtained by ultrasound and thus ensure its accuracy. We selected
the M-wave of nerve electrophysiology because the operation was
simple and the observation results were obvious (19). In comparison to M-wave between CG,
EGA and EGB, the delitescence and amplitude were statistically
significant for severity grades III to VI in the EGA. Thus, the
results of M-wave determined facial paralysis with high sensitivity
and were closely associated with clinical symptoms. We found no
statistical significance for grading in the EGA and EGB, suggesting
that US was a superior technique compared to electrophysiological
studies in outcome prediction.
MRI scans may be used for imaging purposes for the
facial nerve from the brainstem to the fundus of the internal
auditory canal (8). MRI normally
visualizes soft tissues well and is better suited for intracranial
lesions. MRI may actually reveal lesions and vascular rather than
structural changes visible with US (3). US combined with CT and MRI may support
clinical diagnosis.
Limitations
Limitations were found in evaluating peripheral
nerves. US examinations were operator-dependent and required
experience in the superficial soft-tissue structures that had a
relatively long learning curve. The number of cases in the EGA and
EGB were limited. M-wave was only one of the numerous
electrophysiological techniques and we did not employ other
techniques, such as blink reflex and F-wave.
In conclusion, HFUS as an adjunct to neural
electrophysiology is highly useful in the establishment of the
normal values of facial nerve. HFUS is also useful in the process
of evaluation and prognosis of Bell's palsy. HFUS confirmed that
facial nerve edema improved following treatment and was useful in
the determination of whether patients with NGS2.0 grading II
required additional treatment. HFUS also proved itself to be
superior to electrophysiological studies in outcome prediction.
Acknowledgements
This study was supported in part by the Department
of Neurology and Traditional Chinese Medicine in Beijing Chaoyang
Hospital, Capital Medical University (Beijing, China).
Glossary
Abbreviations
Abbreviations:
|
HFUS
|
high-frequency ultrasonography
|
|
CSA
|
cross-sectional area
|
|
CG
|
control group
|
|
EGA
|
experimental group A
|
|
EGB
|
experimental group B
|
|
NGS2.0
|
Nerve Grading System 2.0
|
|
EMG
|
electromyography
|
|
NC
|
nerve conduction
|
|
MWD
|
M-wave detection
|
|
CMAP
|
compound muscle action potential
|
|
DML
|
distal motor latency
|
References
|
1
|
Gupta S, Mends F, Hagiwara M, Fatterpekar
G and Roehm PC: Imaging the facial nerve: a contemporary review.
Radiol Res Pract. 2013:2480392013.PubMed/NCBI
|
|
2
|
Vrabec JT, Backous DD, Djalilian HR,
Gidley PW, Leonetti JP, Marzo SJ, Morrison D, Ng M, Ramsey MJ and
Schaitkin BM: Facial Nerve Disorders Committee: Facial Nerve
Grading System 2.0. Otolaryngol Head Neck Surg. 140:445–450. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hagino K, Tsunoda A, Tsunoda R and
Kishimoto S: Measurement of the facial nerve caliber in facial
palsy: implications for facial nerve decompression. Otol Neurotol.
32:686–689. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Saatçi I, Sahintürk F, Sennaroğlu L,
Boyvat F, Gürsel B and Besim A: MRI of the facial nerve in
idiopathic facial palsy. Eur Radiol. 6:631–636. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kress B, Griesbeck F, Stippich C, Bähren W
and Sartor K: Quantitative analysis of MRI intensity in of the
major petrosal nerve in patients with idiopathic facial paralysis.
Nervenarzt. 75:124–127. 2004.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kinoshita T, Ishii K, Okitsu T, Okudera T
and Ogawa T: Facial nerve palsy: evaluation by contrast-enhanced MR
imaging. Clin Radiol. 56:926–932. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nakata S, Mizuno T, Naganawa S, Sugiura M,
Yoshida T, Teranishi M, Sone M and Nakashima T: 3D-FLAIR MRI in
facial nerve paralysis with and without audio-vestibular disorder.
Acta Otolaryngol. 130:632–636. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Murphy TP: MRI of the facial nerve during
paralysis. Otolaryngol Head Neck Surg. 104:47–51. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mu X, Quan Y, Shao J, Li J, Wang H and
Gong R: Enlarged geniculate ganglion fossa: CT sign of facial nerve
canal fracture. Acad Radiol. 19:971–976. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sittel C and Stennert E: Prognostic value
of electromyography in acute peripheral facial nerve palsy. Otol
Neurotol. 22:100–104. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fornage BD: Peripheral nerves of the
extremities: imaging with US. Radiology. 167:179–182. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vakharia KT, Henstrom D, Lindsay R,
Cunnane MB, Cheney M and Hadlock T: Color Doppler ultrasound:
Effective monitoring of the buried free flap in facial reanimation.
Otolaryngol Head Neck Surg. 146:372–376. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vlad V and Iagnocco A: Ultrasound of the
knee in rheumatology. Med Ultrason. 14:318–325. 2012.PubMed/NCBI
|
|
14
|
Yildirim AO, Oken OF, Unal VS, Esmer AF,
Gülçek M and Uçaner A: Avoiding iatrogenic radial nerve injury
during humeral fracture surgery: a modified approach to the distal
humerus. Acta Orthop Traumatol Turc. 46:8–12. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Karabay N, Toros T, Ademoğlu Y and Ada S:
Ultrasonographic evaluation of the iatrogenic peripheral nerve
injuries in upper extremity. Eur J Radiol. 73:234–240. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kele H: Ultrasonography of peripheral
nerves system. New Trends in Neurosonology and Cerebral
Hemodynamics-an Update. 1:417–421. 2012.
|
|
17
|
Suk JI, Walker FO and Cartwright MS:
Ultrasonography of peripheral nerves. Curr Neurol Neurosci Rep.
13:3282013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lo YL, Fook-Chong S, Leoh TH, Dan YF, Lee
MP, Gan HY and Chan LL: High-resolution ultrasound in the
evaluation and prognosis of Bell's palsy. Eur J Neurol. 17:885–889.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ishikawa M, Namiki J, Takase M, Kojima A
and Kawase T: F-waves of the facial muscles in healthy control
subjects and in patients with peripheral facial nerve disturbance.
Electromyogr Clin Neurophysiol. 39:167–174. 1999.PubMed/NCBI
|