Introduction
Although the incidence of hypertensive intracerebral
hemorrhage (HICH) has declined recently, the disability rate and
mortality at long-term follow up thereof are on the increase
(1). Previous clinical report
findings showed that the mortality rate of intracerebral hemorrhage
(ICH) was 34–43.9% at 90 days follow-up (2) and 47% after 1 year (3). Effective management of acute ICH,
particularly its poor prognosis, requires a profound understanding
of risk factors involved, including age, volume and location of
hematoma, extension to intraventricular hemorrhage or not, blood
sugar, blood pressure (BP), temperature and consciousness (4–6). It is
important to identify early warning signals of death of HICH and
take proactive interventions.
The present study aimed to examine key risk factors
that provided early prediction of death, with the aim of early
intervention to reduce mortality. The medical records of 128 HICH
patients admitted to the Department of Xuzhou Central Hospital
(Xuzhou, China) were retrospectively examined. Association of
mortality and survival with age, BP, hematoma volume and
neurological scores of the patients were analyzed.
Materials and methods
Patient information
A total of 128 patients, aged ≥18 years, had
spontaneous ICH confirmed by computed tomography (CT) within 6 h
onset and elevated systolic BP ≥150–220 mmHg. Inclusion criteria
for the study were: ≥18 years of age, spontaneous HICH within 6 h
confirmed by CT, and elevated systolic BP of ≥150 mmHg. Exclusion
criteria for the study were: clear evidence that the HICH was
secondary to a structural cerebral abnormality (e.g., arteriovenous
malformation, intracranial aneurysm, or tumour) or under treatment
with the use of a thrombolytic agent, or with a pre-planned
decompressive neurosurgical intervention.
Analyses of clinical records
BP records
BP was recorded for each patient at the following
time points: i) emergency, ii) admission, ii) once every 6 h during
the first 24 h after admission, and iv) twice per day after 24 h
and within 1 week.
Measurement of hematoma volume
Standard CT scanning was performed at admission, and
at 24±3 h after admission. Hematoma volume was measured manually by
the ABC/2 method using Philip Brilliance 64-slice CT (Philips
Medical Systems, Eindhoven, The Netherlands).
Neurological deficit scoring
The neurological functions of the patients were
evaluated at admission, 24 h after admission, 1 week after
admission or discharge using the Glasgow Coma Scale (GCS), National
Institutes of Health Stroke Scale (NIHSS) scoring scale.
Evaluation of neurological function recovery
The modified Rankin scale (mRS) was utilized to
assess the neurological function recovery state of the patients
after stroke (grading scores, 0–6). The scores used were: 0, no
symptoms at all; 1, with symptoms but without obvious disabilities,
able to complete all of their normal duties and day-to-day
activities; 2, mild disability, failed to complete all of their
normal duties and activities, but were able to handle their
personal business without external assistance; 3, moderate
disability, some assistance required, albeit able to walk by
themselves; 4, severe disability, unable to walk without assistance
or carry out daily functions; 5, complete disability, completely
bedridden, suffer from gatism, and requiring continuous care and
nursing; 6, death. The mRS scores were recorded at follow up on day
28 and 90.
Statistical analysis
Statistical analysis was performed using SPSS 19
software (IBM Corp., Armonk, NY, USA). Numerical data were
subjected to the Kolmogorov-Smirnov normality test. Normal
distribution was assessed using the t-test, and abnormal
distribution was detected using the non-parametric rank sum test
(Wilcoxon two-sample test). Ranked data were analyzed by the
Chi-square test. Correlation and independent risk factors of death
were analyzed by calculating the correlation index and logistic
binary regression using Spearman's correlation.
Results
Patient characteristics
The average age of the patients included in the
present study was 64.28±14.04 years. The results showed that of the
128 subjects studied, 15 patients succumbed within 90 days and the
mortality of HICH was 11.7%. Of the 15 patients, 1 succumbed within
24 h, accounting for 6.7% of the total death toll, while 6 patients
succumbed within 1 week, accounting for 40% of the total death
toll. The remaining 8 patients succumbed between 1 week and 90
days. The average age for these patients was 64.28±14.04 years.
Clinicopathological characteristics
and mRS scores
Clinical characteristics such as age, gender,
hematoma volume and location, and BP at different time points were
analyzed for patients that succumbed as well as survivors (Table I). Mortality was closely associated
with age (P<0.001) although not with gender. Mortality was
significantly associated with BP at 30 min, 45 min and 6 h after
admission (P<0.05), although not on admission 180.33±17.17 vs.
174.72±16.13 mmHg (P=0.211). Mortality was also associated with
hematoma volume but not with the location. The average age of
patients who succumbed was significantly higher than that of the
survivors (75.53 vs. 62.79 years, P=0.001). Higher mRS scores,
signifying worse recovery of neurological functions following
cerebral hemorrhage, were primarily found in senior patients aged
over 64.25 years (r=0.375, P<0.001. Fig. 1). By contrast, the effect of gender
on the mRS score was not significant.
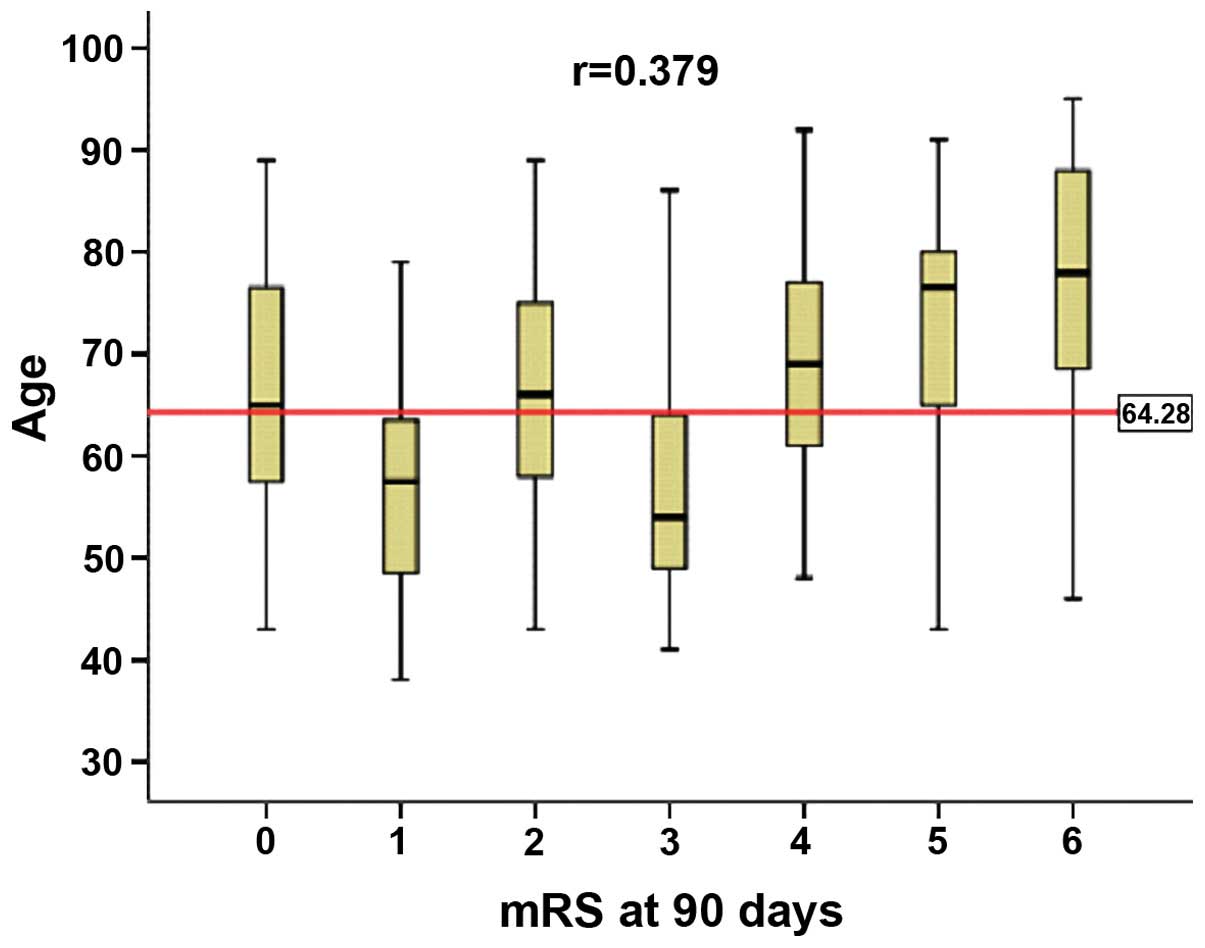 | Figure 1.Association between the modified
Rankin scale (mRS) scores at 90 days and the age of hypertensive
intracerebral hemorrhage. Data are presented as mean, media and
standard deviation. The mRS scores were calculated as 0–6 with 0
indicating no symptoms and 6 indicating mortality. Age, mean,
media, standard deviation and min-max, respectively, at mRS 0 were
(66.92, 65.00, 13.761, 43 and 89); mRS 1 (56.35, 57.50, 10.876, 38
and 79); mRS 2 (65.31, 66.00, 12.925, 43 and 89); mRS 3 (58.23,
54.00, 12.624, 41 and 86); mRS 4 (68.16, 69.00, 12.013, 48 and 92);
mRS 5 (72.80, 76.50, 13.522, 43 and 91); and mRS 6 (75.53, 78.00,
15.175, 46 and 95); Age average was 64.28 years. |
 | Table I.Clinical characteristics of patients
who succumbed and survivors. |
Table I.
Clinical characteristics of patients
who succumbed and survivors.
| Characteristics | Dead (n=15) | Survivors
(n=113) | P-value |
|---|
| Age, years | 75.53 (15.18) | 62.79 (13.25) | 0.001a |
| Male |
10
(66.7%) |
73
(64.7%) | 0.876 |
| Hematoma volume,
ml |
91.67
(24.96–133.66) |
32.33
(13.78–49.67) | 0.044a |
| Location |
|
|
|
| Basal
ganglia |
9
(60.0%) |
63
(55.8%) | 0.107 |
|
Thalamus |
2
(13.3%) |
15
(13.3%) | 0.165 |
|
Lobar |
– |
5
(4.4%) | – |
|
Cerebella |
1
(6.7%) |
13
(11.5%) | 0.341 |
|
Brain-stem |
3
(20.0%) |
11 (9.7%) | 0.167 |
|
Intraventricular
extension |
– |
6
(5.3%) | – |
| Blood pressure |
|
|
|
|
Admission |
180.33
(17.17) |
174.72
(16.13) | 0.211 |
| 15
min |
169.60
(16.92) |
164.90
(17.29) | 0.324 |
| 30
min |
176.47
(14.46) |
160.10
(20.01) | 0.003a |
| 45
min |
166.33
(13.36) |
156.01
(18.64) | 0.014a |
| 1 h |
158.80
(15.17) |
153.00
(20.20) | 0.286 |
| 6 h |
150.60
(18.46) |
139.95
(18.50) | 0.038a |
| 12 h |
151.21
(16.22) |
141.47
(17.79) | 0.053 |
| 18 h |
149.69
(13.64) |
142.58
(19.23) | 0.198 |
| 24 h |
147.31
(17.58) |
143.36
(16.58) | 0.421 |
| GCS
scoreb |
|
|
|
|
Admission |
14
(11–14) |
13 (12–14) | 0.991 |
| 24 h |
10 (5–13) |
13 (12–15) | 0.007a |
| NIHSS
scorec |
|
|
|
|
Admission |
13 (7–19) | 10
(4–18) | 0.286 |
| 24 h |
22 (9–28) |
8 (4–15) | 0.011a |
Systolic BP
The systolic pressure of the HICH patients was
monitored for 1 week after admission or up to death (Fig. 2). The results showed that the BP for
patients who died versus those who survived during our observation
period was similar at emergency (181.87±18.30 vs. 184.39±18.65
mmHg) and on admission (180.33±17.17 vs. 174.72±16.13 mmHg).
However, a significant difference was observed for BP between
patients who died and survivors measured at 30 min, 45 min and 6 h
(Table I and Fig. 2).
GCS and NIHSS scores
The results revealed that death was associated with
the hematoma volume following onset and within 24 h, but was
irrelevant to the hematoma location (P>0.05) (Table II). Death occurring during 90 days
was irrelevant to the NIHSS score on admission, but relevant to the
neurological score within 24 h and 7 days after admission (Table III). The average GCS scores were
9.29±4.07 vs. 12.76±2.25 and 9.56±3.40 vs. 13.42±2.26 for patients
who died within 24 h and 1 week, respectively. The NIHSS score for
patients who died were 19.00±10.88 vs. 10.20±8.08 and 23.56±12.98
vs. 8.39±8.22 within 24 h and 1 week, respectively.
 | Table II.Correlation of hematoma volume between
dead and survivors with location. |
Table II.
Correlation of hematoma volume between
dead and survivors with location.
|
| First CT
(emergence) | Second CT
(following-up first CT 24±3 h) |
|---|
|
|
|
|
|---|
| Characteristics | Dead | Survivors | Dead | Survivors |
|---|
| Hematoma volume
(ml)a | 36.93
(3.18–78.42) | 27.94
(10.63–45.25) | 63.63
(3.86–84.21) | 31.05
(20.25–53.18) |
| Correlation
index | 0.331 | 0.331 | 0.534 | 0.534 |
| P-value | 0.000 | 0.000 | 0.001 | 0.001 |
 | Table III.Spearman correlation between dead at
90 days and the neurological scores. |
Table III.
Spearman correlation between dead at
90 days and the neurological scores.
| Scores time | Admission,
n=128 | 24 h, n=127 | 7 days, n=122 |
|---|
| Neurological
scores | GCS | NIHSS | GCS | NIHSS | GCS | NIHSS |
| Correlation
index | 0.058 | 0.112 | −0.287 | 0.273 | −0.319 | 0.317 |
| P-value | 0.513 | 0.209 |
0.001a | 0.002a |
0.000a | 0.000a |
Results from the multifactor regression analysis
revealed that of the risks factors examined, age and hematoma
volume were independent predictors of death [age odds ratio
(OR)=1.082, 95% confidence interval (CI): 1.018–1.149, p=0.011;
hematoma volume OR=1.011, 95% CI: 1.000–1.023, p=0.046].
Discussion
The aim of the present study was to investigate
early warning signals of death in patients with acute HICH. Of the
128 patients with acute HICH included in this study, mortality was
0.8% within 24 h, 4.7% within 1 week, increasing to 11.7% in the
follow up at 90 days Mortality was irrelevant to gender, but
closely relevant to age. The average ages of the dead and surviving
patients were 75.53±15.18 vs. 62.79±13.25 (p<0.05). This result
indicated that the older the patients, the higher the mortality,
which was consistent with literature reports showing patients
>75 had a higher mortality (7).
An increase in age leads to degeneration of the function of tissues
and organs within the body, thus, compensation ability becomes
exacerbated. As for the blood vessel itself, the vascular wall of
elderly patients was more vulnerable to degenerative changes: their
lipid became hyaline-degenerated, fibrous protein became necrotic,
and segmental aortic structure was broken, in such a manner that
their vascular compliance became exacerbated (5). These degenerative changes contributed
to high mortality of acute HICH in senior patients.
High BP was an important factor that affected the
onset and prognosis of cerebral hemorrhage. However, at which point
BP should be regulated remains controversial (8–10).
Baños-González et al reported poor prognosis
for cerebral hemorrhage patients with too low or too high systolic
pressure, and it was preferable to maintain the BP at 100–159 mmHg
(11). If the BP for acute HICH
patients with onset within 6 h was proactively under control at an
early stage, particularly if the 1-h systolic pressure was within
140 mmHg, patients had a better clinical prognosis than those
following the conventional depressurization standard protocol
(>180 mmHg) (13). By contrast,
our data showed that high systolic BP recorded, following emergent
treatment (184 mmHg) and on admission (175 mmHg) (P=0.772 and
P=0.375, respectively), was not significantly correlated with death
for the HICH patients. It is possible that the BP in patients with
acute cerebral hemorrhage increased temporarily, however, the level
of BP at this point was not influences at the 90-day prognosis of
patients.
Tetri et al (13) reported that despite their higher BP
values at admission, subjects with untreated hypertension showed
improved survival and more frequently favorable outcome after
BP-lowering therapy than other patients The findings may be
attributed to the young age of their recruited patients and did not
have cardiovascular and cerebrovascular diseases, history of
anticoagulant drugs and diabetes mellitus. In this case, the level
of BP following admission may not provide early warning to the
prognosis of cerebral hemorrhage. Such findings were consistent
with the results of our study. Our study also found that patients
who died had significantly higher BP at 30, 45 and 60 min after
admission than those survived (all P<0.05). The higher the BP
was, the higher the mortality was (r=0.263, p=0.003). Therefore,
regulation of BP within 6 h after admission was crucial. From 6 to
24 h after admission, BP was controlled at 143.21±17.76. Other
studies on the safety of early active antihypertensive therapy on
acute HICH also demonstrated similar findings (12,14–16). If
BP was proactively reduced at an early stage, hematoma enlargement
within 24 h could be controlled, and consequently clinical
prognosis could be improved.
The results of the present study have shown that
mortality was closely associated with the volume of hematoma
following onset and 24 h after onset, but irrelevant to the
hematoma location. We performed binary logistic regression analysis
on age, gender, hematoma volume on admission, level of
consciousness and BP, and found that age and hematoma volume were
independent risk factors of death. The larger the hematoma volume
was, the higher the mortality was. This result was consistent with
previous findings (17). Methods
that may intervene in the enlargement of hematoma include BP
adjustment, encephaledema and relieving of intracranial
hypertension, surgical resection, anticoagulant therapy and
hemostasis therapy (18,19). A systematic review (20) identified that ventricular drainage
combined with defibrinogen therapy on patients with extension to
ventricles effectively prevents hydrocephalus and improves patient
quality of life. Previous findings have shown that conservative
treatment by operation and medications had no difference on the
short-term outcomes for young and elderly patients with cerebral
hemorrhage. However, in the long term, the prognosis of operative
treatment on the youth was better than those receiving conservative
treatment (21).
The GCS and NIHSS scores identified on admission
were not significantly correlated with death. This result indicated
that the level of consciousness on admission was not predictive of
death. However, mortality was relevant to the neurological score
recorded at 24 h and 1 week after admission. If the GCS score was
<9, the NIHSS scores were >19 and >24 within 24 h and 1
week, respectively, high mortality was observed (data not shown;
GCS: 12±3, r=-0.622; NIHSS: 11±8, r=0.707). Disturbance of
consciousness on admission was one of the most important factors
for death of cerebral hemorrhage (3,22).
However, the level of consciousness is likely to be influenced by a
hematoma spot (supratentorial or subtentorial), hematoma volume,
with extension to ventricles or not, and the time from onset to
admission (23). In particular,
hematoma occurring in the brain stem affected the ascending
reticular activating system, and thus influenced the level of
consciousness, resulting in somnolence and coma. Additionally,
larger hematoma may cause more obvious mass effect, leading to more
severe subsequent hematoma. These factors may also affect the level
of patient consciousness, which may explain the reason for the
consciousness level not clearly predicting death.
In conclusion, the results of the present study have
shown that patient age and hematoma volume were independent risk
factors of death of acute HICH, and may serve as important early
warning signals for cerebral hemorrhage. Proactive control and
management of hematoma may reduce the mortality of HICH.
References
|
1
|
Zhang Y, Yi B, Ma J, Zhang L, Zhang H,
Yang Y and Dai Y: Quercetin promotes neuronal and behavioral
recovery by suppressing inflammatory response and apoptosis in a
rat model of intracerebral hemorrhage. Neurochem Res. 40:195–203.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sia SF, Tan KS and Waran V: Primary
intracerebral haemorrhage in Malaysia: in-hospital mortality and
outcome in patients from a hospital based registry. Med J Malaysia.
62:308–312. 2007.PubMed/NCBI
|
|
3
|
Nilsson OG, Lindgren A, Brandt L and
Säveland H: Prediction of death in patients with primary
intracerebral hemorrhage: A prospective study of a defined
population. J Neurosurg. 97:531–536. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen HS, Hsieh CF, Chau TT, Yang CD and
Chen YW: Risk factors of in-hospital mortality of intracerebral
hemorrhage and comparison of ICH scores in a Taiwanese population.
Eur Neurol. 66:59–63. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stein M, Luecke M, Preuss M, Boeker DK,
Joedicke A and Oertel MF: Spontaneous intracerebral hemorrhage with
ventricular extension and the grading of obstructive hydrocephalus:
the prediction of outcome of a special life-threatening entity.
Neurosurgery. 67:1243–1251; discussion 1252. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Davis SM, Broderick J, Hennerici M, Brun
NC, Diringer MN, Mayer SA, Begtrup K and Steiner T: Recombinant
Activated Factor VII Intracerebral Hemorrhage Trial Investigators:
Hematoma growth is a determinant of mortality and poor outcome
after intracerebral hemorrhage. Neurology. 66:1175–1181. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zia E, Engstrom G, Svensson PJ, et al:
Three-year survival and stroke recurrence rates in patients with
primary intracerebral hemorrhage. Stroke. 40:3567–3573. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Broderick JP, Adams HP Jr, Barsan W,
Feinberg W, Feldmann E, Grotta J, Kase C, Krieger D, Mayberg M,
Tilley B, et al: Guidelines for the management of spontaneous
intracerebral hemorrhage: a statement for healthcare professionals
from a special writing group of the Stroke Council, American Heart
Association. Stroke. 30:905–915. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bath P, Chalmers J, Powers W, Beilin L,
Davis S, Lenfant C, Mancia G, Neal B, Whitworth J and Zanchetti A:
International Society of Hypertension Writing Group: International
Society of Hypertension (ISH): statement on the management of blood
pressure in acute stroke. J Hypertens. 21:665–672. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hanger HC, Wilkinson T, Keeling S and
Sainbury R: New Zealand guideline for management of stroke. N Z Med
J. 117:U8632004.PubMed/NCBI
|
|
11
|
Baños-González M, Cantú-Brito C, Chiquete
E, Arauz A, Ruiz-Sandoval JL, Villarreal-Careaga J,
Barinagarrementeria F and Lozano JJ: Systolic blood pressure and
functional outcome in patients with acute stroke: a Mexican
registry of acute cerebrovascular disease (RENAMEVASC). Arch
Cardiol Mex. 81:169–175. 2011.(In Spanish). PubMed/NCBI
|
|
12
|
Anderson CS, Heeley E, Huang Y, Wang J,
Stapf C, Delcourt C, Lindley R, Robinson T, Lavados P, Neal B, et
al: INTERACT2 Investigators: Rapid blood-pressure lowering in
patients with acute intracerebral hemorrhage. N Engl J Med.
368:2355–2365. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tetri S, Huhtakangas J, Juvela S,
Saloheimo P, Pyhtinen J and Hillbom M: Better than expected
survival after primary intracerebral hemorrhage in patients with
untreated hypertension despite high admission blood pressures. Eur
J Neurol. 17:708–714. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tsivgoulis G, Katsanos AH, Butcher KS,
Boviatsis E, Triantafyllou N, Rizos I and Alexandrov AV: Intensive
blood pressure reduction in acute intracerebral hemorrhage: a
meta-analysis. Neurology. 83:1523–1529. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gould B, McCourt R, Gioia LC, Kate M, Hill
MD, Asdaghi N, Dowlatshahi D, Jeerakathil T, Coutts SB, Demchuk AM,
et al: ICH ADAPT Investigators: Acute blood pressure reduction in
patients with intracerebral hemorrhage does not result in
borderzone region hypoperfusion. Stroke. 45:2894–2899. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Grise EM and Adeoye O: Blood pressure
control for acute ischemic and hemorrhagic stroke. Curr Opin Crit
Care. 18:132–138. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Arima H, Wang JG, Huang Y, Heeley E,
Skulina C, Parsons MW, Peng B, Li Q, Su S, Tao QL, et al: INTERACT
Investigators: Significance of perihematomal edema in acute
intracerebral hemorrhage: the INTERACT trial. Neurology.
73:1963–1968. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Steiner T and Bösel J: Options to restrict
hematoma expansion after spontaneous intracerebral hemorrhage.
Stroke. 41:402–409. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Anderson CS, Huang Y, Arima H, Heeley E,
Skulina C, Parsons MW, Peng B, Li Q, Su S, Tao QL, et al: INTERACT
Investigators: Effects of early intensive blood pressure-lowering
treatment on the growth of hematoma and perihematomal edema in
acute intracerebral hemorrhage: the Intensive Blood Pressure
Reduction in Acute Cerebral Haemorrhage Trial (INTERACT). Stroke.
41:307–312. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nieuwkamp DJ, de Gans K, Rinkel GJ and
Algra A: Treatment and outcome of severe intraventricular extension
in patients with subarachnoid or intracerebral hemorrhage: a
systematic review of the literature. J Neurol. 247:117–121. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Koivunen RJ, Satopää J, Haapaniemi E,
Strbian D, Meretoja A, Mustanoja S, Silvennoinen H, Salonen O,
Niemelä M, Tatlisumak T, et al: Predictors of early mortality in
young adults after intracerebral hemorrhage. Stroke. 45:2454–2456.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
el Chami B, Milan C, Giroud M, Sautreaux
JL and Faivre J: Intracerebral hemorrhage survival: French register
data. Neurol Res. 22:791–796. 2000.PubMed/NCBI
|
|
23
|
Fan JS, Huang HH, Chen YC, Yen DH, Kao WF,
Huang MS, Huang CI and Lee CH: Emergency department neurologic
deterioration in patients with spontaneous intracerebral
hemorrhage: Incidence, predictors, and prognostic significance.
Acad Emerg Med. 19:133–138. 2012. View Article : Google Scholar : PubMed/NCBI
|
















