Introduction
Chronic rhinosinusitis (CRS), characterized by
chronic inflammation of the nasal cavity and paranasal sinus
mucosa, remains a significant health problem with a considerable
socioeconomic burden and is increasing in prevalence and incidence.
Shi et al (1) have
demonstrated that the overall prevalence of CRS is currently 8%
(range, 4.8–9.7%) in seven cities in mainland China. At present,
the pathogenesis of CRS remains unclear. Many studies support the
hypothesis that allergens, bacterial, fungal infection and nasal
anatomic abnormality all play an important role (2–4).
CRS is commonly divided into two categories: CRS
without nasal polyps (CRSsNP) and with nasal polyps (CRSwNP), the
former being dominated by Th1, while the latter mainly manifests
Th2 responses (2,5). Nasal polyps are usually derived from
the ostiomeatal complex, particularly the uncinate mucosa. CRSwNP
can be further divided into eosinophilic or non-eosinophilic types,
according to the degree of infiltrative eosinophilia. CRSwNP in
patients from Western countries is typically eosinophilic and
Th2-focused, whereas it is mainly neutrophilic and Th1-focused in
Asian populations (6,2). CRSwNP, can be further divided into
three types, namely seromucinous, fibroinflammatory and edematous
according to the degree of tissue remodeling (7,8).
Chemokines, cytokines and other inflammatory mediators, including T
cells, eosinophils, neutrophils, and macrophages (2,9), play a
key role in mediating the migration and invasion of these
inflammatory cells, eventually leading to tissue remodeling and CRS
development (10–13).
Chemokines are able to combine with G-receptor
proteins on target cells and play an important role in the
development and regulation of the immune response by bringing
inflammatory cells into the site of inflammation (10–17). CC
chemokine ligand 19 (CCL19), also known as macrophage inflammatory
protein (MIP)-3β, is a chemokine of molecular weight 11 kDa. CCL19
has multiple effects and can act as a chemotactic signal for
various immune cells, including dendritic cells, T cells, B cells,
natural killer cells and macrophages (18–20).
Through interaction with its receptor (CC chemokine receptor 7),
CCL19 has an immunostimulatory effect, promoting contacts between
dendritic cells and T cells, and promoting antigen presentation
(21). Simultaneously, CCL19 can
also exert an immunosuppressive effect through the production of
interleukin (IL)-10, the restriction of movement of dendritic
cells, and the induction of apoptosis of mature dendritic cells
(22,23). Knockout mice for CCL19 have a
significant delay in the resolution of lung inflammation,
accompanied by the downregulation of IL-10 expression (22). In addition, clinical studies have
indicated that CCL19 is involved in allergic rhinitis, inflammatory
bowel disease and other inflammatory and immune disorders (24,25).
However, the expression and possible role of CCL19 in CRS are not
currently described. Therefore, this study aimed to analyze the
differential expression of CCL19 in normal nasal mucosa and in
different types of CRS and to explore its significance in the
pathophysiology of this condition.
Materials and methods
Subjects and samples
Samples were taken from patients undergoing
endoscopic nasal surgery at the Department of Otolaryngology-Head
and Neck Surgery of the Renmin Hospital of Wuhan University (Wuhan,
China), between June 2013 and December 2013. Tissue specimens of
CRSwNP were from 71 patients, of whom 47 were male and 24 female.
Eleven of these cases had a history of allergic rhinitis or asthma.
Tissue specimens for CRSsNP were obtained from 21 patients, of whom
11 were male and 10 were female. The normal control group included
20 cases (13 male and 7 female). The diagnosis of CRS in all cases
was made according to the recommended European diagnostic standard
EPOS2012 (26). Patients were
excluded if they had a history of autoimmune disease, the ‘aspirin
triad’, primary cilia motility dysfunction or cystic fibrosis, or
had a history of intranasal or oral corticosteroid use in the 2
weeks prior to the surgery. During the surgery, polyps were taken
from patients with CRSwNP, and a biopsy of the uncinate process
mucosa was made from patients with CRSsNP and those with nasal
septum deviation. The specimens were divided into two parts. One
was reserved in liquid nitrogen, while the other was fixed in 4%
paraformaldehyde for 24 h prior to embedding in paraffin. This
study was approved by the Ethics Committee of Renmin Hospital of
Wuhan University (approval number: 20130308). Informed consent was
obtained from every subject.
Paraffin section staining
Serial sections were made from each specimen, with a
thickness of 5 µm, for Masson trichrome, hematoxylin and eosin
(H&E) and periodic acid Schiff (PAS) staining (all Wuhan
Jiayuan Quantum Dots Co., Ltd., Wuhan, China). All stains were
performed in accordance with the manufacturer's protocol. The
analysis of nasal polyp tissue morphology and eosinophil
classification were performed as previously described in the
literature (2,8,9).
Western blot analysis to detect the
expression of CCL19 protein
The tissues were cut into pieces, and 250 µl lysis
buffer (ab204733; Abcam, Cambridge, UK), containing 1 mM
MgCl2, 10 mM Tris-HCl (pH 7.4), 1% Triton X-100, 1 %
sodium dodecyl sulfate (SDS) and 1% NP-40, was added for every 20
mg tissue. The sample was then centrifuged at 4°C, 12000 × g for 15
min, and the supernatant was isolated. The bicinchoninic acid
method was used to determine the concentration of total protein in
the sample. For this, 12% SDS-polyacrylamide gel (Thermo Fisher
Scientific Inc., Waltham, MA, USA) and spacer gel were prepared and
the amount of protein loaded for each lane was quantified at 25 µg.
The spacer gel was run at 75 V for 30 min, and the separation gel
was run at 120 V for 60 min; the transfer was performed at 200 mA
for 30 min, and blocking was undertaken using 5% skimmed milk
powder at 4°C overnight. The membrane was subsequently incubated
with primary mouse anti-CCL19 monoclonal antibody (ab193000) at a
dilution of 1:500 (0.2 µg/ml) for 2 h at room temperature. Mouse
anti-β-actin monoclonal antibody (1:1,000; ab123034) was used as a
loading control. A horseradish peroxide (HRP)-conjugated secondary
antibody (1:1,000; ab131368; all Abcam) was incubated with the
membrane for 1 h at 37°C. Enhanced chemiluminescence detection
(12630S; Cell Signalling Technology, Inc., Danvers, MA, USA) was
used to observe the blots. The densitometry of the bands was
quantified using ImageJ 2X software (National Institutes of Health,
Bethesda, MA, USA).
Paraffin section
immunofluorescence
Paraffin sections were dewaxed to permit water
penetration, and underwent high temperature microwave repair for 10
min. Blocking was conducted by incubation with normal sheep serum
(Gibco; Thermo Fisher Scientific, Inc.) for 30 min. The following
primary mouse monoclonal antibodies were incubated with the
sections for 1 h at room temperature: Rat anti-cluster of
differentiation (CD68) (1:100, 1 µg/ml; ab31630) and rabbit
anti-CCL19 (1:100, 1 µg/ml; ab126742; both Abcam). A wash with 0.01
M phosphate-buffered saline (PBS) was conducted. Fluorescein
isothiocyanate (FITC)-labeled goat anti-rabbit immunoglobulin (Ig)M
(1:100; LS-C86590-2000) and Luo Danming (Rhodamine)-labeled goat
anti-rat IgG secondary antibodies (1:100; LS-C61649-1000; both
LifeSpan BioSciences, Inc., Seattle, WA, USA) were added to the
sections and incubated for 1 h at room temperature. After a further
wash with 0.01 M PBS, the samples were counterstained with
4,6-diamidino-2-phenyl indole and images were captured using an
upright Olympus BX61 fluorescence microscope (Olympus Corporation,
Tokyo, Japan).
Statistical analysis
Experimental results were expressed as median values
and SPSS software (version 17.0; SPSS, Inc., Chicago, IL, USA) was
used to conduct statistical analyses. Independent samples were
compared with the t-test and the Spearman correlation coefficient
was used to analyze the association between CCL19 and eosinophils
in the blood. In addition, non-parametric Kruskal-Wallis tests were
used to analyze the expression levels of CCL19. P<0.05 was
considered to indicate a statistically significant result.
Results
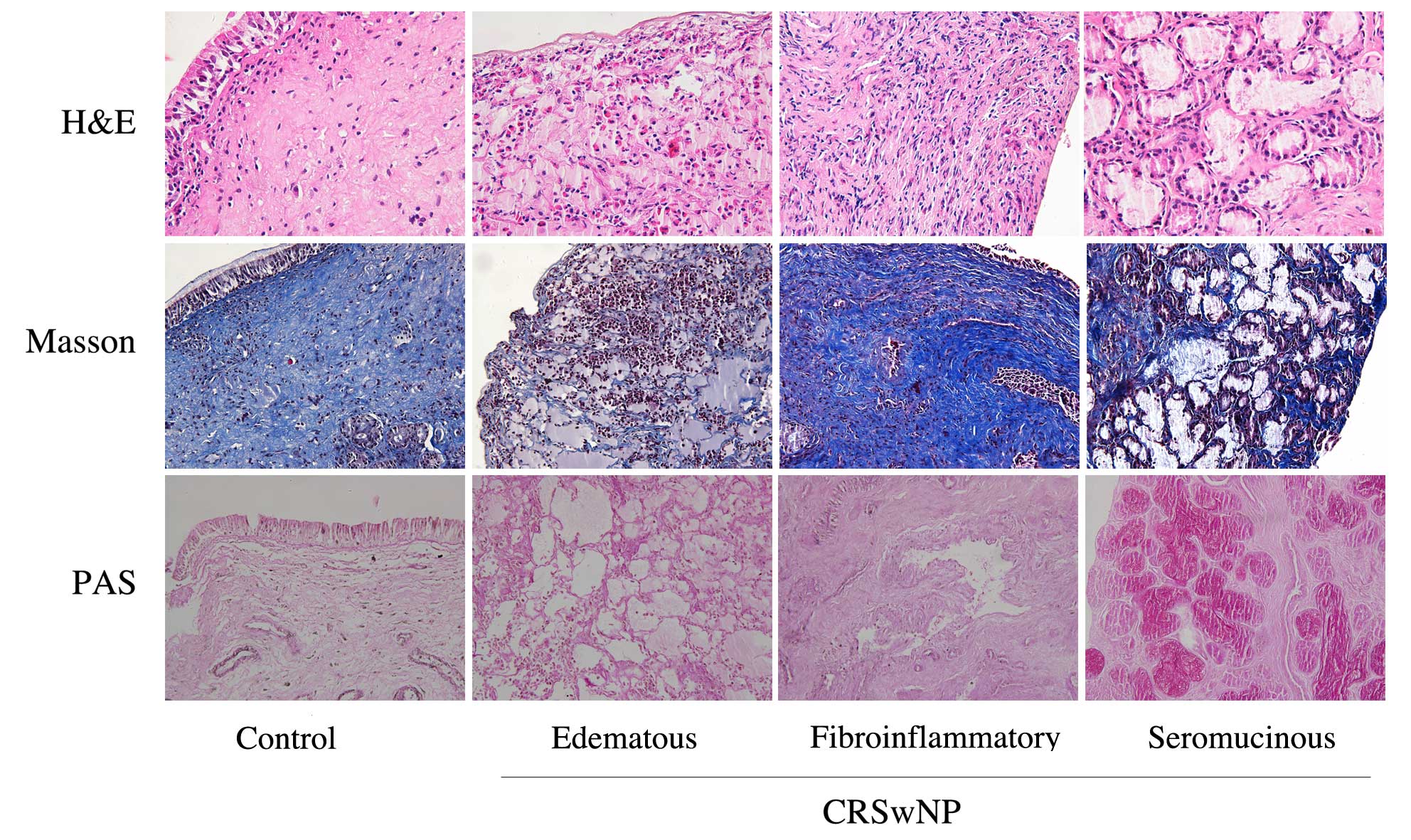
Inflammation and tissue typing of
chronic rhinosinusitis
In accordance with previously used eosinophilic
CRSwNP standards, which are that CRSsNP and CRSwNP may be
classified as eosinophilic when the percentage of eosinophils is
>10% of the mean of controls (2),
the CRSwNP cases were further divided into 31 cases of eosinophilic
type, and 40 cases of non-eosinophilic type. With a combination of
Masson and PAS staining, the CRSwNP cases were divided into three
types in accordance with their histopathological features: The
edematous type, in which there are many eosinophilic granulocytes;
the fibroinflammatory type, in which collagen fibers show
significant proliferation with varying degrees of inflammatory cell
infiltration, and the seromucinous type, in which mucous glands
have clear evidence of hypertrophy. In this study, there were 40
cases of the edematous type, 15 cases of the fibroinflammatory
type, and 16 cases of the seromucinous type. Representative images
of the three different types are shown in Fig. 1.
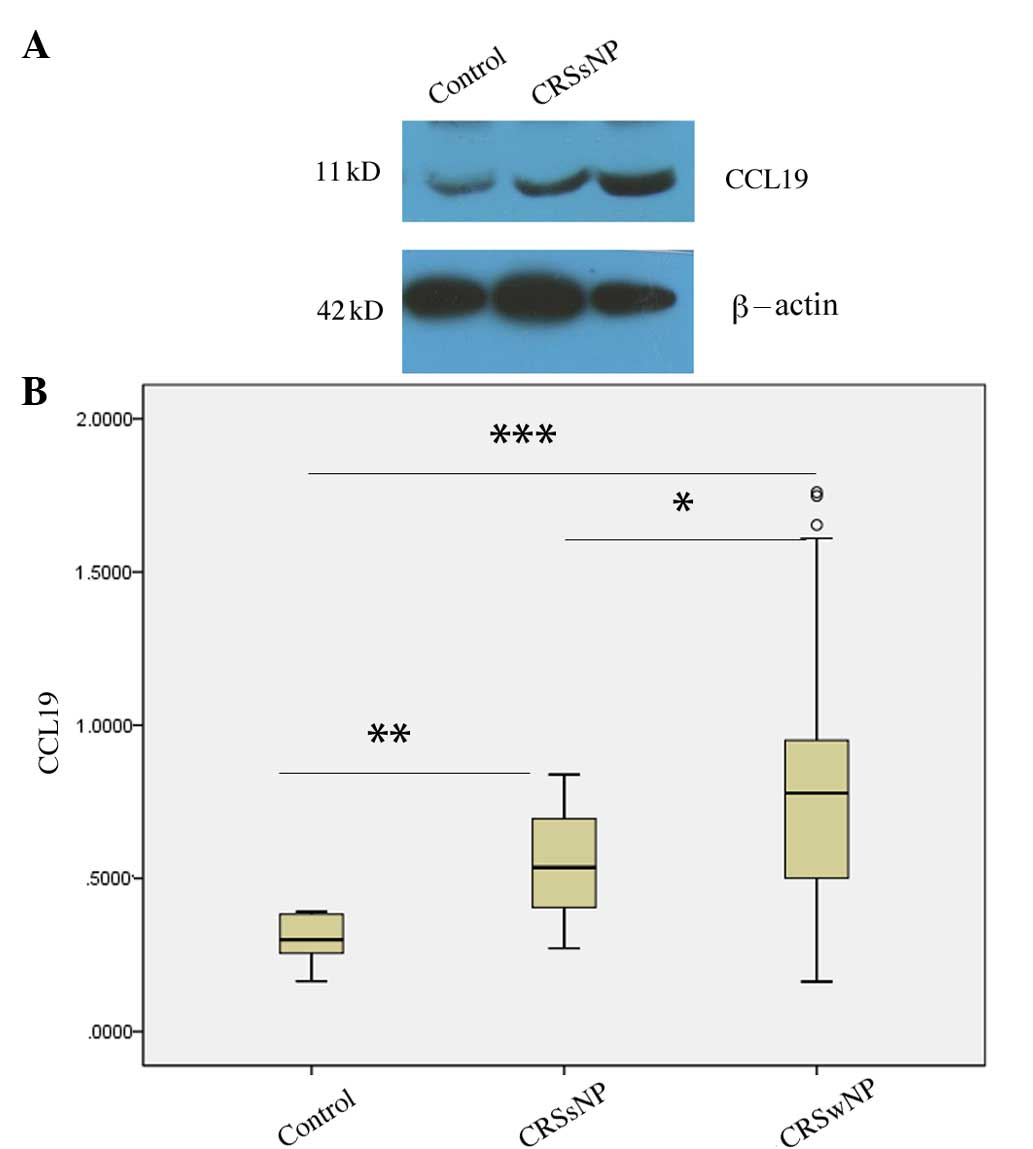
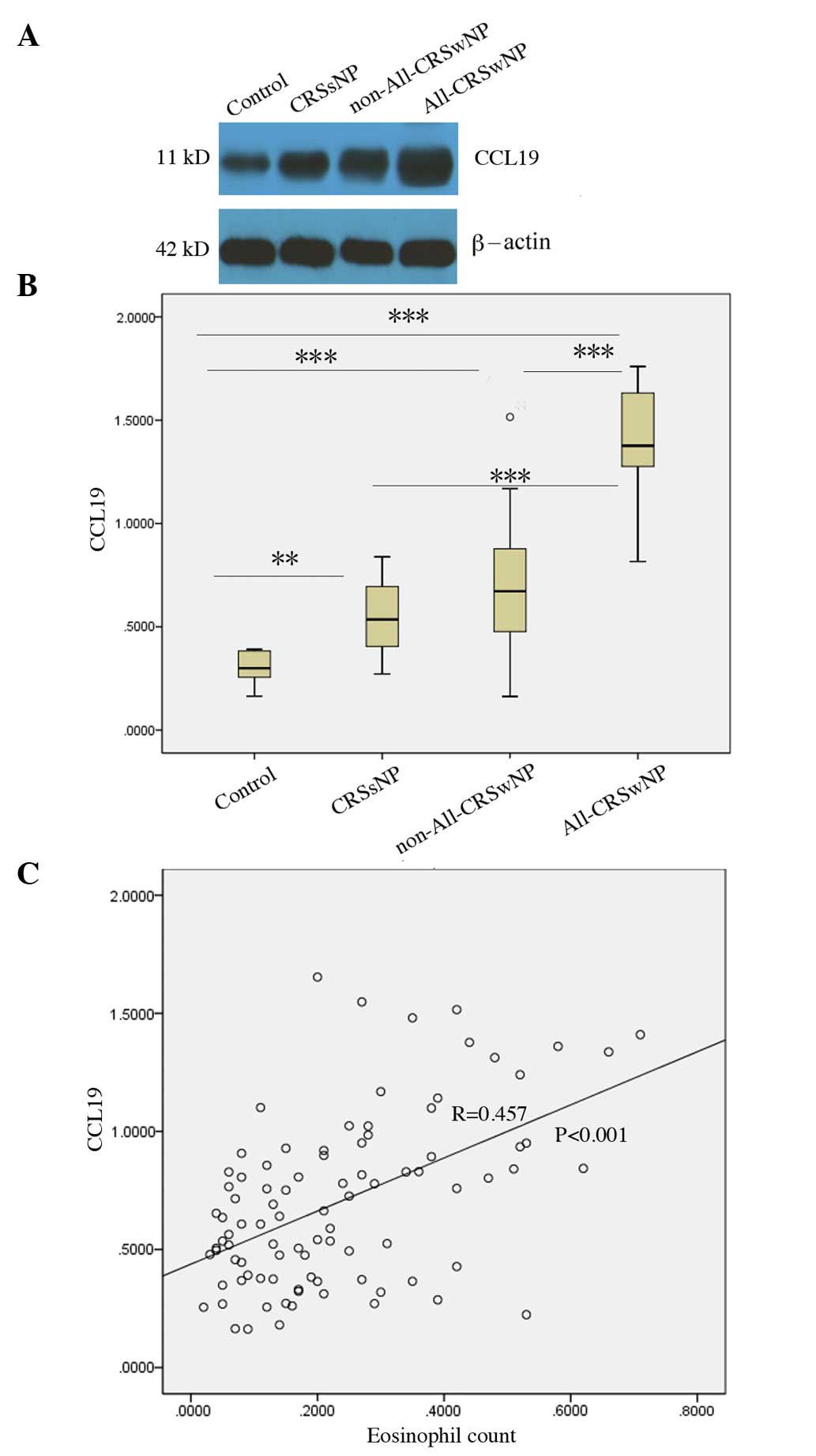
Differential expression of CCL19
protein in CRSsNP and CRSwNP
According to the presence or absence of nasal polys,
the patients with CRS were divided into CRSsNP and CRSwNP groups.
The CCL19 protein levels in the CRSsNP (P=0.004) and CRSwNP
(P<0.001) groups were increased compared with those in the
normal controls, and the CCL19 protein levels in the CRSwNP group
were significantly higher than those observed in the CRSsNP group
(P=0.037; Fig. 2).
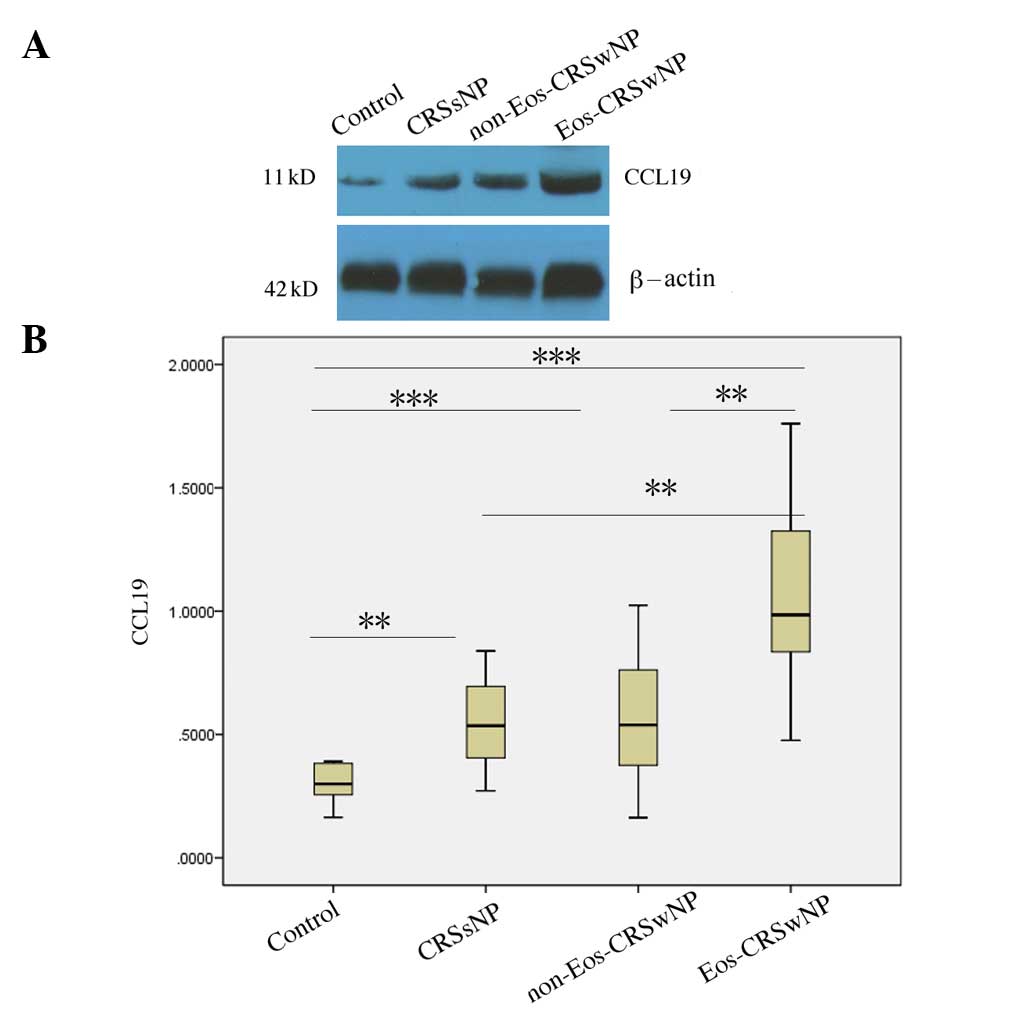
Differential expression of CCL19
protein in eosinophilic and non-eosinophilic CRSwNP
Since eosinophilic and non-eosinophilic CRSwNP have
different immunologic characteristics, CRSwNP was divided into
eosinophilic and non-eosinophilic types. It was found that the
CCL19 protein levels in the eosinophilic CRSwNP group were higher
than those in the normal controls (P<0.001), the CRSsNP group
(P<0.01) and the non-eosinophilic CRSwNP group (P<0.01). In
addition, the CCL19 protein levels in non-eosinophilic CRSwNP were
higher than those observed in the normal controls (P<0.001), but
were not significantly different from those in CRSsNP (P=0.819;
Fig. 3).
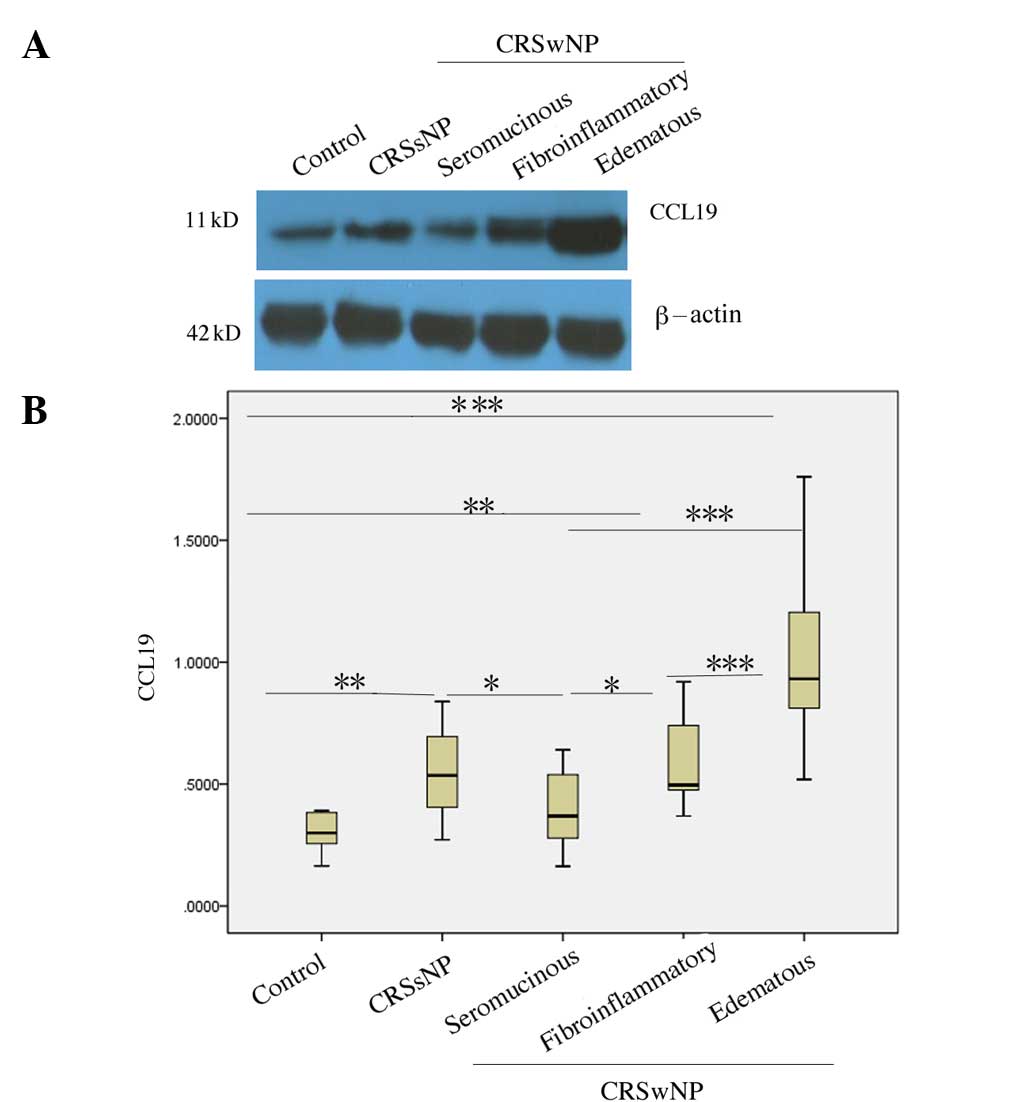
Expression characteristics of CCL19
protein in different histological types of CRSwNP
According to its main tissue component and the
nature of the infiltrative inflammatory cells, CRSwNP was divided
into edematous, fibroinflammatory and seromucinous types. The CCL19
protein levels in the edematous type of CRSwNP were higher than
those observed in the normal controls, CRSsNP, and the
fibroinflammatory and seromucinous types of CRSwNP (all,
P<0.001). The CCL19 protein levels in the fibroinflammatory type
of CRSwNP were higher than those in normal controls (P=0.004) and
the seromucinous type (P=0.016), but were not significantly
different from those in CRSsNP (P=0.775). The CCL19 protein levels
in the seromucinous type were lower than those of CRSsNP tissues
(P=0.048), but not significantly different from those in normal
controls (P=0.140; Fig. 4).
CCL19 protein levels correlate with
blood eosinophilia and allergies
Since the CCL19 protein levels were significantly
elevated in the edematous and eosinophilic types of CRSwNP, the
possibility that CCL19 protein levels might correlate with
peripheral blood eosinophilia and a history of allergy was
investigated. Spearman correlation analysis showed that CCL19
protein levels were positively correlated with the number of
eosinophils in the blood (R=0.457, P<0.001), and the CCL19
protein levels in CRSwNP patients with allergic rhinitis or asthma
were also significantly higher than in the patients with CRSsNP
(P<0.001) and CRSwNP without allergy (P<0.001; Fig. 5).
Macrophages in chronic rhinosinusitis
highly express CCL19
Although the CCL19 protein levels in eosinophilic
CRSwNP were high and correlated with the degree of blood
eosinophilia, to the best of our knowledge, there are no reports
concerning the expression of CCL19 by eosinophils in the
literature. Eosinophils, macrophages and other cells are
increasingly implicated in the pathophysiology of CRS. In addition,
mature macrophages, dendritic cells and T cells can also express
CCL19 (27). Using double-labeling
immunofluorescence, it was found that
CCL19+CD68+ cells accounted for 72% of all
CCL19+ cells. Therefore, the principal cells expressing
CCL19 in CRS were CD68+ macrophages (Fig. 6).
Discussion
CCL19 has a dual role, promoting the immune response
or having anti-inflammatory and immunosuppressive effects. It has
been reported that CCL19-knockout mice have more severe allergic
features and an enhanced Th2 response compared with wild-type mice,
and their allergic reactions were significantly inhibited when a
plasmid encoding CCL19 DNA was used as gene therapy, with possible
mechanisms including the promotion of IL-10 production, restriction
of the function of dendritic cells, or the induction of apoptosis
of mature dendritic cells (17,18). CRS
is a chronic inflammatory disease of the mucosa of the nasal cavity
and paranasal sinuses. CRSsNP is based on a Th1 response
characterized by elevated levels of granulocytes and interferon
(IFN)-γ; by contrast, CRSwNP is dominated by Th2, mastocytes and
eosinophilic infiltration (2,28). The
present study found that the CCL19 protein levels in CRSsNP and
CRSwNP were significantly upregulated, particularly in eosinophilic
and edematous CRSwNP. Since CCL19 has anti-inflammatory and
immunosuppressive effects, its moderate increase in expression in
inflammation may be associated with the restriction of eosinophil
infiltration and tissue edema in CRSwNP (22,23).
The present study found that CCL19 was expressed at
the highest levels in the edematous and eosinophilic types of
CRSwNP, and that its expression was higher in CRSwNP with a history
of allergic rhinitis and asthma. Also, the expression of CCL19 was
positively associated with the number of peripheral blood
eosinophils, despite the fact that there are no reports concerning
the expression of CCL19 by eosinophils in the literature. In
addition to the Th2 response and eosinophilia, the role of
macrophages in CRSwNP is coming under increasing scrutiny.
Macrophages in different microenvironments can differentiate into
different types. Under the influence of stimulation by IFN-γ and
lipopolysaccharide, they can differentiate into classical M1
macrophages to resist microbial infection, mainly secreting IFN-γ.
By contrast, in the context of Th2 cytokines such as IL-4 and
IL-13, they selectively differentiate into M2 macrophages, mainly
secreting Th2 cytokines, and promoting allergic inflammation
(13). Our previous study found that
total counts of CD68+ macrophages in CRSsNP and CRSwNP
were significantly upregulated compared with their levels in normal
controls (2). The present study
found using CD68+ and CCL19+ double
immunofluorescence that 72% of CCL19 was expressed by
CD68+ cells in the submucosa. The expression of CCL19 in
eosinophilic and edematous CRSwNP was significantly upregulated
compared with that in non-eosinophilic CRSwNP. Thus, the
upregulation of CCL19 in CRSwNP may be related to the increase in
CD68+ macrophages, even if the kind of macrophage is not
distinguished.
The main symptoms of patients with CRSwNP differ,
but typically include nasal obstruction, olfactory dysfunction
and/or rhinorrhea. These differences may be associated not only
with the site and stage of CRSwNP, but also with its tissue types
(7,8). Hellquist (7) and Couto et al (8) have undertaken detailed research into
the tissue types of CRSwNP and their classification, and divided
CRSwNP into four types, namely edematous, seromucinous and
fibroinflammatory types, and atypical hyperplasia. The edematous
type is the most common type with higher levels of eosinophils in
the nasal polyp, and a high relapse rate. The atypical hyperplasia
type belongs to the category of benign hyperplasia, and is
relatively rare (7,8,27). In
the present study, the CRSwNP cases included only edematous,
seromucinous and fibroinflammatory types, with no typical
hyperplasia samples. These findings were further confirmed by the
observation of three different types of histopathological staining.
The present study showed that the CCL19 protein levels in the
edematous and fibroinflammatory CRSwNP were significantly
upregulated compared with the controls. This suggests that CRSwNP
with the upregulation of CCL19 has the main characteristics of
inflammatory cell infiltration, tissue edema or fibrosis, which may
correlate with a history of allergies. These factors may be
considered to indicate that the use of glucocorticoid therapy
postoperatively should be intensified.
In addition to macrophages, dendritic cells and
other cells can also express CCL19 (24). Our previous study reported that
dendritic cells were increased in the samples from patients with
CRSsNP and CRSwNP compared with the controls, but the number of
dendritic cells in CRSwNP was much less than that of macrophages
(2). Double-labeling
immunofluorescence demonstrated that CCL19 was mainly expressed by
macrophages in CRSwNP. The previous study also found that the
expression levels of IL-10 in CRSsNP and CRSwNP were significantly
higher than those in the controls, with the highest levels in
eosinophilic CRSwNP (2). In the
present study, we found that the CCL19 protein levels were the
highest in eosinophilic CRSwNP, allowing us to speculate that the
upregulated CCL19 in CRSwNP may promote the expression of
immune-suppressive factor IL-10, thus limiting the inflammatory
cascade, which is consistent with a previous study (22). Ocampo et al (29) demonstrated that CCL19 mRNA expression
was elevated in CRSwNP. Consistent with their study, the present
study confirmed that CCL19 protein expression was also upregulated
in CRSwNP and CRSsNP, and correlated with different histologic
features of CRSwNP.
Determining the exact mechanism, however, will
require a high fidelity CRSwNP animal model with CCL19 gene
knockout, plus assessment of the therapeutic effect of recombinant
CCL19 in such a model. The present study is also limited by the
lack of analysis of expression of the CCL19 receptor CCR7 in CRSwNP
(22,23), and the role of specific receptors and
their ligands in the pathogenesis of CRSwNP remains unknown. Blood
eosinophil counts are simple and affordable to obtain, and it has
been reported that they have diagnostic significance for
eosinophilic CRSwNP (30).
Therefore, in the present study, the correlation between CCL19 and
eosinophils was analyzed, but the correlation with CD68 was
not.
In conclusion, this study shows that CCL19 is mainly
expressed by an expanded population of CD68+ macrophages
in CRSwNP, and positively correlates with eosinophil counts in the
blood and with a history of allergy. The upregulation of CCL19 may
play a protective role in limiting eosinophil infiltration and the
extent of edema to provide anti-inflammatory and immunomodulatory
effects.
Acknowledgements
This study was supported by the Joint Fund of 2012
from the Health Nonprofit Industry Research Project of National
Ministry of Health, China (grant no. 201202005), the National
Natural Science Foundation of China (grant nos. 81070766, 81001214
and 81372880) and the Natural Science Foundation of Hubei Province,
China (grant no. 2012FFB04312).
References
|
1
|
Shi JB, Fu QL, Zhang H, Cheng L, Wang YJ,
Zhu DD, Lv W, Liu SX, Li PZ, Ou CQ and Xu G: Epidemiology of
chronic rhinosinusitis: Results from a cross-sectional survey in
seven Chinese cities. Allergy. 70:533–539. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cao PP, Li HB, Wang BF, Wang SB, You XJ,
Cui YH, Wang DY, Desrosiers M and Liu Z: Distinct immunopathologic
characteristics of various types of chronic rhinosinusitis in adult
Chinese. J Allergy Clin Immunol. 124:478–484. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Akdis CA, Bachert C, Cingi C, Dykewicz MS,
Hellings PW, Naclerio RM, Schleimer RP and Ledford D: Endotypes and
phenotypes of chronic rhinosinusitis: A PRACTALL document of the
European Academy of Allergy and Clinical Immunology and the
American Academy of Allergy, Asthma & Immunology. J Allergy
Clin Immunol. 131:1479–1490. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Feazel LM, Robertson CE, Ramakrishnan VR
and Frank DN: Microbiome complexity and Staphylococcus
aureus in chronic rhinosinusitis. Laryngoscope. 122:467–472.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Polzehl D, Moeller P, Riechelmann H and
Perner S: Distinct features of chronic rhinosinusitis with and
without nasal polyps. Allergy. 61:1275–1279. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kanda A, Fleury S, Kobayashi Y, Tomoda K,
Julia V and Dombrowicz D: Th2-activated eosinophils release Th1
cytokines that modulate allergic inflammation. Allergology Int.
64(Suppl): S71–S73. 2015. View Article : Google Scholar
|
|
7
|
Hellquist HB: Nasal polyps update.
Histopathology. Allergy Asthma Proc. 17:237–242. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Couto LG, Fernades AM, Brandão DF, Santi
Neto D, Valera FC and Anselmo-Lima WT: Histological aspects of
rhinosinusal polyps. Braz J Otorhinolaryngol. 74:207–212. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shi LL, Xiong P, Zhang L, Cao PP, Liao B,
Lu X, Cui YH and Liu Z: Features of airway remodeling in different
types of Chinese chronic rhinosinusitis are associated with
inflammation patterns. Allergy. 68:101–109. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yoshikawa M, Wada K, Yoshimura T, Asaka D,
Okada N, Matsumoto K and Moriyama H: Increased CXCL10 expression in
nasal fibroblasts from patients with refractory chronic
rhinosinusitis and asthma. Allergol Int. 62:495–502. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
El-Shazly AE, Doloriert HC, Bisig B,
Lefebvre PP, Delvenne P and Jacobs N: Novel cooperation between
CX3CL1 and CCL26 inducing NK cell chemotaxis via CX3CR1: A possible
mechanism for NK cell infiltration of the allergic nasal tissue.
Clin Exp Allergy. 43:322–331. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu X, Mimms R, Lima R, Peters-Hall J, Rose
MC and Peña MT: Localization of inflammatory mediators in pediatric
sinus mucosa. Arch Otolaryngol Head Neck Surg. 138:389–397. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Peterson S, Poposki JA, Nagarkar DR,
Chustz RT, Peters AT, Suh LA, Carter R, Norton J, Harris KE,
Grammer LC, et al: Increased expression of CC chemokine ligand 18
in patients with chronic rhinosinusitis with nasal polyps. J
Allergy Clin Immunol. 129:119–127, e1-e9. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Petrek M, Kolek V, Szotkowská J and du
Bois RM: CC and C chemokine expression in pulmonary sarcoidosis.
Eur Respir J. 20:1206–1212. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kalwitz G, Andreas K, Endres M, Neumann K,
Notter M, Ringe J, Sittinger M and Kaps C: Chemokine profile of
human serum from whole blood: Migratory effects of CXCL-10 and
CXCL-11 on human mesenchymal stem cells. Connect Tissue Res.
51:113–122. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ozdemir C, Akdis M and Akdis CA: T-cell
response to allergens. Chem Immunol Allergy. 95:22–44. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Barnes PJ: Pathophysiology of allergic
inflammation. Immunol Rev. 242:31–50. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kellermann SA, Hudak S, Oldham ER, Liu YJ
and McEvoy LM: The CC chemokine receptor-7 ligands 6Ckine and
macrophage inflammatory protein-3 beta are potent chemoattractants
for in vitro- and in vivo-derived dendritic cells. J Immunol.
162:3859–3864. 1999.PubMed/NCBI
|
|
19
|
Kim CH, Pelus LM, White JR, Applebaum E,
Johanson K and Broxmeyer HE: CK beta-11/macrophage inflammatory
protein-3 beta/EBI1-ligand chemokine is an efficacious
chemoattractant for T and B cells. J Immunol. 160:2418–2424.
1998.PubMed/NCBI
|
|
20
|
Rangel-Moreno J, Moyron-Quiroz J, Kusser
K, Hartson L, Nakano H and Randall TD: Role of CXC chemokine ligand
13, CC chemokine ligand (CCL) 19, and CCL21 in the organization and
function of nasal-associated lymphoid tissue. J Immunol.
175:4904–4913. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ellingsen T, Hansen I, Thorsen J, Møller
BK, Tarp U, Lottenburger T, Andersen LS, Skjødt H, Pedersen JK,
Lauridsen UB, et al: Upregulated baseline plasma CCL19 and CCR7
cell-surface expression on monocytes in early rheumatoid arthritis
normalized during treatment and CCL19 correlated with radiographic
progression. Scandinavian J Rheumatol. 43:91–100. 2014. View Article : Google Scholar
|
|
22
|
Yamashita N, Tashimo H, Matsuo Y, Ishida
H, Yoshiura K, Sato K, Yamashita N, Kakiuchi T and Ohta K: Role of
CCL21 and CCL19 in allergic inflammation in the ovalbumin-specific
murine asthmatic model. J Allergy Clin Immunol. 117:1040–1046.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bosè F, Petti L, Diani M, Moscheni C,
Molteni S, Altomare A, Rossi RL, Talarico D, Fontana R, Russo V, et
al: Inhibition of CCR7/CCL19 axis in lesional skin is a critical
event for clinical remission induced by TNF blockade in patients
with psoriasis. Am J Pathol. 183:413–421. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Middel P, Raddatz D, Gunawan B, Haller F
and Radzun HJ: Increased number of mature dendritic cells in
Crohn's disease: Evidence for a chemokine mediated retention
mechanism. Gut. 55:220–227. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Takamura K, Fukuyama S, Nagatake T, Kim
DY, Kawamura A, Kawauchi H and Kiyono H: Regulatory role of
lymphoid chemokine CCL19 and CCL21 in the control of allergic
rhinitis. J Immunol. 179:5897–5906. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fokkens WJ, Lund VJ, Mullol J, Bachert C,
Alobid I, Baroody F, Cohen N, Cervin A, Douglas R, Gevaert P, et
al: European position paper on rhinosinusitis and nasal polyps
2012. Rhinol. 50(Suppl 23): 1–298. 2012.
|
|
27
|
Robbiani DF, Finch RA, Jäger D, Muller WA,
Sartorelli AC and Randolph GJ: The leukotriene C(4) transporter
MRP1 regulates CCL19 (MIP-3β, ELC)-dependent mobilization of
dendritic cells to lymph nodes. Cell. 103:757–768. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Van Zele T, Holtappels G, Gevaert P and
Bachert C: Differences in initial immunoprofiles between recurrent
and nonrecurrent chronic rhinosinusitis with nasal polyps. Am J
Rhinol Allergy. 28:192–198. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ocampo CJ, Kato A, Norton J, Kern RC,
Conley DB, Chandra R, Tan B, Peters AT, Grammer LC and Schleimer
RP: Elevated expression of mRNA for CCL2, CCL19, CCR7 and CXCR3 in
chronic rhinosinusitis with nasal polyposis. J Allergy Clin
Immunol. 129(Suppl): AB432012.
|
|
30
|
Hu Y, Cao PP, Liang GT, Cui YH and Liu Z:
Diagnostic significance of blood eosinophil count in eosinophilic
chronic rhinosinusitis with nasal polyps in Chinese adults.
Laryngoscope. 122:498–503. 2012. View Article : Google Scholar : PubMed/NCBI
|