Introduction
Septic shock is a major public health burden which
occurs in >230,000 patients in the US each year (1) and is the main cause of morbidity and
mortality in patients in non-cardiac intensive care units (2). During infection, the immune system
produces a broad inflammatory cascade, triggering a systemic
response which includes increased vascular permeability, myocardial
depression, impairment of the coagulation cascade and multiorgan
dysfunction during the late stage (3). Myocardial dysfunction is a predictor of
poor prognosis in patients with septic shock (4). Its mechanisms include the attenuation
of the adrenergic response at the cardiomyocyte level, alterations
in intracellular calcium trafficking and blunted calcium
sensitivity of contractile proteins, all of which are mediated by
cytokines (5). Therapeutic
management of septic shock includes addressing the existing
infection and fluid therapy (6). For
the treatment of sepsis-induced cardiac dysfunction, potentially
useful therapies include novel inotropes, which may reduce the
heart rate to cardiac oxygen expenditure and improving diastolic
filling, and β-blockers which may reduce local and systemic
inflammation (5) .
Lipopolysaccharide (LPS) is a major constituent of
the bacterial outer membrane, and serves a crucial function in the
initiation of the pathophysiological cascades (7). A reduction in LPS has been associated
with improved outcomes in patients with heart disease (8). Cultured H9c2 cardiomyocytes have been
shown to exhibit a marked inflammatory response to LPS stimulation
(9). Therefore, the construction of
a sepsis model using H9c2 cardiomyocytes may facilitate the
development of novel therapeutic agents for the direct treatment of
chronic heart failure.
Puerarin is the major bioactive ingredient isolated
from the root of the Pueraria iobata (Willd.) (10). Puerarin has been widely studied due
to its wide spectrum of pharmacological properties, which include
cardioprotective (11),
neuroprotective (12), vasodilatory,
antioxidative (13),
anti-inflammatory (14), anti-cancer
(15) and anti-diabetic (16) effects. In our previous study, it was
found that puerarin may offer a potentially effective and
relatively safe approach to mitigate pressure overload-induced
cardiac hypertrophy and the associated apoptosis (17). The aim of the present study was to
investigate the potential of puerarin to protect against bacterial
infection of the heart, by evaluating its effect on LPS-stimulated
H9c2 cells.
Materials and methods
Reagents
Puerarin (≥98% purity, as determined by
high-performance liquid chromatography) and LPS were purchased from
Sigma-Aldrich (St. Louis, MO, USA). Dulbecco's modified Eagle's
medium (DMEM)/nutrient mixture F12, fetal bovine serum (FBS),
trypsin, penicillin and streptomycin were purchased from Gibco-BRL
(Grand Island, NY, USA). TRIzol® for total RNA extraction was
obtained from Invitrogen (Thermo Fisher Scientific, Inc., Waltham,
MA, USA). Transcriptor First Strand cDNA Synthesis kit and
LightCycler® 480 SYBR Green I Master mix were purchased from Roche
Diagnostics (Basel, Switzerland). Alexa Fluor® 488 goat anti-mouse
immunoglobulin G (IgG) and SlowFade Gold antifade reagent with
4′,6-diamidino-2-phenylindole (DAPI) were purchased from Invitrogen
(Thermo Fisher Scientific, Inc.). An ApopTag® Plus Fluorescein
In Situ Apoptosis Detection kit was from EMD Millipore
(Billerica, CA, USA). A Bicinchoninic acid (BCA) protein assay kit
was obtained from Pierce (Thermo Fisher Scientific, Inc.). Primary
antibodies were from Cell Signaling Technology, Inc. (Danvers, MA,
USA). IRDye 800 CW conjugated secondary antibodies were obtained
from LI-COR Biosciences (Lincoln, NE, USA).
Cell culture
H9c2 cardiomyocytes were obtained from the Cell Bank
of the Chinese Academy of Sciences (Shanghai, China). The H9c2
cells were grown in high-glucose DMEM supplemented with 10% (v/v)
FBS, 100 U/ml penicillin and 100 mg/ml streptomycin in a humidified
CO2 incubator (18 M; Sanyo Electric Co., Ltd., Osaka,
Japan) in 5% CO2 at 37°C. Cells at exponential growth
phase were dissociated with 0.25% trypsin, seeded in six-well
culture plates at a density of 1×106 cells/well, and
incubated for 24 h. Subsequently, cells were cultured with
serum-free DMEM for 12 h. Puerarin was dissolved in dimethyl
sulfoxide at a concentration of 40 mmol/l. LPS (1 µg/ml) in the
presence or absence of different concentrations of puerarin (1, 5,
10, 20 and 40 µM) was added to the medium and the cells were
incubated for the indicated time.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RT-qPCR was used to detect the mRNA expression
levels of inflammatory markers, including interleukin (IL)-1β and
tumor necrosis factor (TNF)-α. Total RNA was isolated from cultured
H9c2 cardiomyocytes using TRIzol® and their yields and purities
were spectrophotometrically estimated using A260/A280 and A230/260
ratios via a SmartSpec Plus Spectrophotometer (Bio-Rad Laboratories
Inc., Hercules, CA, USA). RNA (2 µg per sample) was
reverse-transcribed into cDNA using oligo(dT) primers and the
Transcriptor First Strand cDNA Synthesis kit. The PCR
amplifications were quantified using a LightCycler 480 SYBR Green I
Master mix. The IL-1β and TNF-α gene signals were normalized
against that of glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
The primer used were as follows: GAPDH, forward
5′-GACATGCCGCCTGGAGAAAC-3′ and reverse 5′-AGCCCAGGATGCCCTTTAGT-3′;
TNF-α, forward 5′-AGCATGATCCGAGATGTGGAA-3′, reverse
5′-TAGACAGAAGAGCGTGGTGGC-3′; IL-1β, forward
5′-GGGATGATGACGACCTGCTAG-3′ and reverse
5′-ACCACTTGTTGGCTTATGTTCTG-3′. Briefly, following initial
denaturation at 95°C for 5 min, 42 primer-extension cycles were
completed. Each cycle consisted of a 10 sec denaturation step at
95°C, a 20 sec annealing step at 60°C and a 20 sec incubation at
72°C for extension. A final extension step was carried out at 72 °C
for 10 min. IL-1β and TNF-α expression levels were normalized to
glyceraldehyde-3-phosphate dehydrogenase (GAPDH). PCR results were
quantified using the double standard curve, calculated as follows:
Calibrator normalized ratio = [(concentration of
target)/(concentration of reference)(sample)]/[(concentration of
target)/(concentration of reference) (calibrator)].
Western blot analysis
Cells were lysed in radioimmunoprecipitation assay
(RIPA) lysis buffer containing 720 µl RIPA buffer, 20 µl
phenylmethylsulfonyl fluoride (1 mM), 100 µl cOmplete™
(04693124001), 100 µl phosSTOP™ (04906837001; both Roche
Diagnostics, Indianapolis, IN, USA), 50 µl NaF (1 mM) and 10 µl
Na3VO4 (per ml). Protein concentration was
measured using the BCA protein assay kit using a Synergy HT
microplate reader (BioTek, Winooski, VT, USA). Samples of total
proteins (50 µg) were separated by 10% sodium dodecyl
sulphate-polyacrylamide gel electrophoresis (Wuhan Goodbio
Technology Co., Ltd., Wuhan, China), and transferred onto
Immobilon-FL transfer membranes (EMD Millipore) in a transfer
buffer containing 14.4 g glycine, 3.03 g Tris base, 800 ml
H2O and 200 ml methanol (per liter), at 200 mA for 1.5
h. Membranes were blocked with 5% non-fat milk at room temperature
for 2 h and incubated overnight at 4°C with mouse anti-human
nuclear factor of kappa light polypeptide gene enhanced in B-cells
inhibitor alpha (IκBα) (1:1,000; 4814) monoclonal antibody, the
following rabbit anti-human monoclonal antibodies: phosphorylated
(p)-IκBα (1:1,000; 2859); nuclear factor (NF)-κB p65 (1:1,000;
8242), p-NF-κB p65 (1:1,000; 3033); B-cell lymphoma (Bcl)-2
(1:1,000; 2870) or the following rabbit anti-human polyclonal
antibodies: Bcl-2-associated X protein (Bax) (1:1,000; 2772; all
Cell Signaling Technology, Inc.) and GAPDH (1:200; sc-25778; Santa
Cruz Biotechnology, Inc.).
Membranes were then incubated with IRDye 800
CW-conjugated goat anti-rabbit immunoglobulin (Ig)G (1:10,000;
926–32211) and IRDye 800 CW-conjugated goat anti-mouse IgG
(1:10,000; 926–32210) secondary antibodies (both LI-COR
Biosciences) at 37°C in Odyssey® blocking buffer for 1 h. The blots
were subsequently scanned and analyzed using a two-color infrared
imaging system (Odyssey; LI-COR Biosciences).
Terminal deoxynucleotidyl
transferase-mediated dUTP nick end labeling (TUNEL)
The detection of apoptosis was performed by TUNEL
staining using the commercially available ApopTag® Plus Fluorescein
In Situ Apoptosis Detection kit, according to the
manufacturer's protocol. Briefly, cells on coverslips were fixed in
1% paraformaldehyde in phosphate-buffered saline (PBS) and stained
with TUNEL reagents, and the nuclei were stained using DAPI. The
relative number of apoptotic cells was calculated as the ratio of
the number of apoptotic nuclei to the total number of nuclei.
Immunocytochemistry for NF-κB p65
localization
The effect of puerarin on the nuclear translocation
of p65 protein was evaluated using immunocytochemical analysis.
Cells were grown on chamber slides at a concentration of
1×105 cells/ml and were either not treated or treated
with 40 µM puerarin. Cells were fixed in a solution of
methanol:acetic acid (95:5) for 20 min at −20°C and subsequently
permeabilised with 0.3% Triton X-100 (Amresco, LLC, Solon, OH, USA)
at 4°C for 5 min. Following blocking with 8% goat serum (Abcam,
Cambridge, UK) for 60 min, the slides were incubated with rabbit
polyclonal anti-p65 antibody (1:100). Following 2 h of incubation
in a humectation chamber at 37°C, the slides were washed with PBS
and incubated at 37°C for 40 min with a secondary goat anti-rabbit
antibody conjugated to Alexa Fluor 488 (1:200; A11008). The
relative number of cells with NF-κB p65 nuclear localization was
evaluated using a fluorescent microscope (BX51; Olympus
Corporation, Tokyo, Japan) and images were captured using an
Eclipse E800 microscope (Nikon Nederland, Amsterdam, The
Netherlands).
Statistical analysis
Data are presented as the mean ± standard error of
the mean. Between-group differences were determined using two-way
analysis of variance and Tukey's post-hoc analysis. Comparisons
between two groups were performed using the unpaired Student's
t-test. P<0.05 was considered to indicate a statistically
significantly difference.
Results
Effect of puerarin on LPS-induced
proinflammatory cytokine production
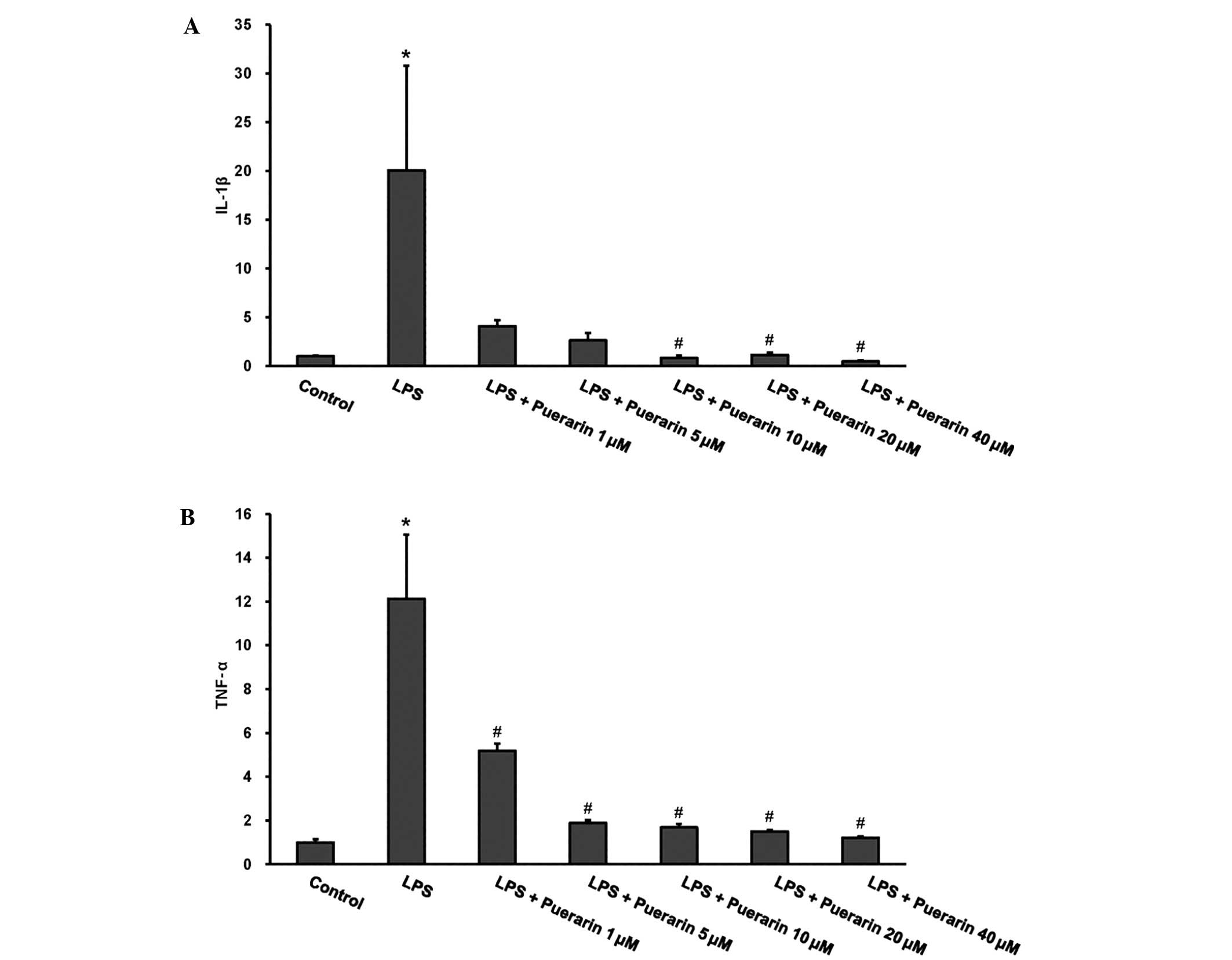
As demonstrated in Fig.
1, stimulation with LPS for 24 h induced a significant increase
in the mRNA levels of IL-1β and TNF-α in H9c2 cardiomyocytes
(P<0.05 vs. the control). Various concentrations of puerarin
(1–40 µM) were used to detect its effect on the induction of IL-1β
and TNF-α expression in response to LPS. Puerarin treatment
markedly attenuated the LPS-induced increase in proinflammatory
cytokine production in a concentration-dependent manner (P<0.05
vs. the LPS group).
Puerarin attenuated LPS-induced
apoptosis
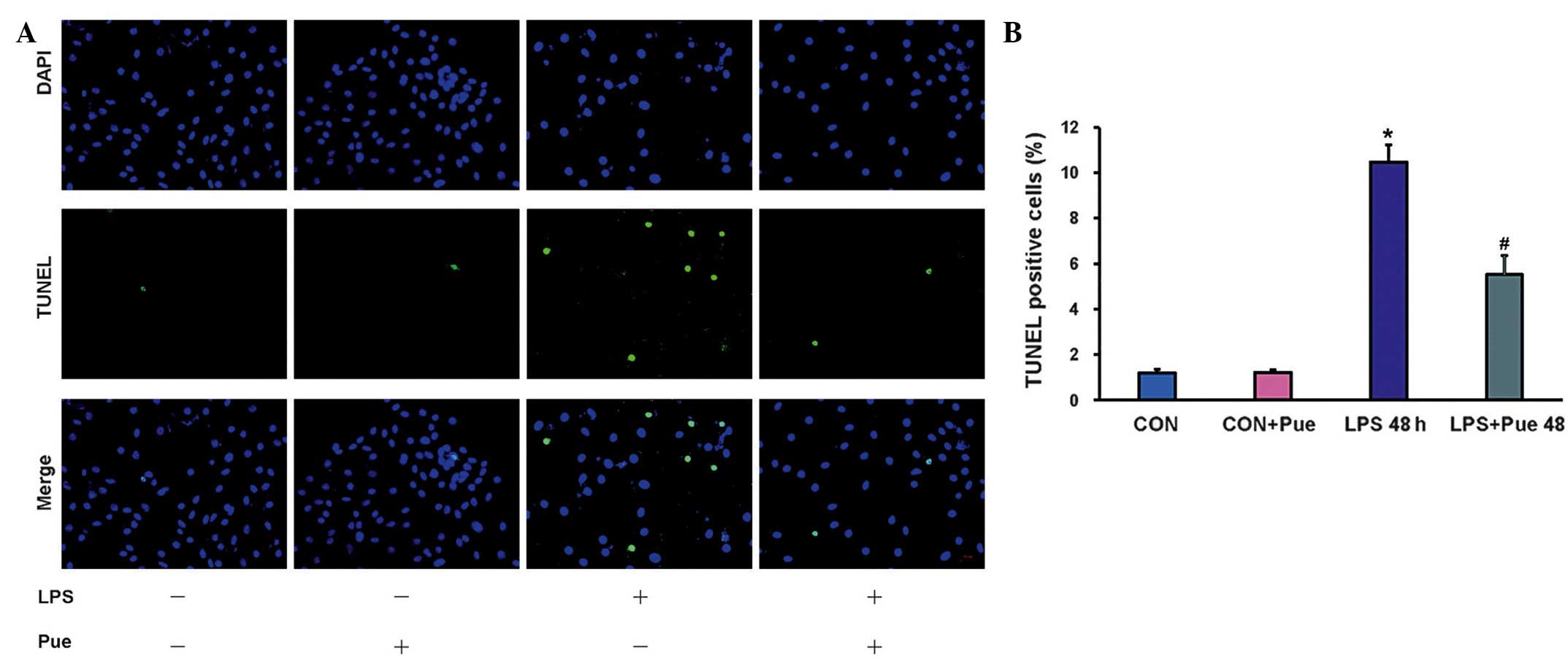
Only 1.2±0.2% TUNEL-positive nuclei were detected in
the control cells following the experiment. A significantly
increased percentage of TUNEL-positive nuclei were observed in
cells incubated with LPS (10.5±0.8%; P<0.01 vs. the control
group); however, puerarin treatment significantly reduced the
percentage of TUNEL-positive cells (5.5±0.8%; P<0.01 vs. the
LPS-only group) (Fig. 2).
H9c2 cardiomyocytes were treated with 40 µM puerarin
while exposed to 1 µg/ml LPS for 12, 24 and 48 h, respectively, and
the Bax and Bcl-2 protein expression levels were subsequently
evaluated (Fig. 3). Western blot
analysis demonstrated that stimulation with LPS significantly
increased the protein expression levels of Bax, while significantly
decreasing those of Bcl-2 (both P<0.05 vs. the control).
Treatment with puerarin markedly reduced Bax expression to a level
comparable to that of the control cells, and significantly
increased Bcl-2 expression (both P<0.05 vs. the LPS-treated
cells), demonstrating that the protective antiapoptotic effect of
puerarin against LPS-induced damage may be associated with the
regulation of Bax and Bcl-2 expression levels by puerarin .
Puerarin blocked the LPS-induced
activation of p-p65 pathways
NF-κB activation is known to involve the regulation
of LPS-induced inflammatory factor expression in cardiomyocytes
(18). To further elucidate the
mechanism underlying the anti-inflammatory and anti-apoptotic
effects of puerarin on LPS-treated H9c2 cells, western blot
analysis was used to detect the activation of NF-κB. Cardiomyocytes
were treated with LPS in the presence or absence of puerarin (40
µM) for 12, 24 and 48 h respectively. NF-κB translocation was
determined by western blotting at 12, 24 and 48 h and by
immunocytochemical analysis at 48 h after treatment. Puerarin
appeared to block the phosphorylation and degradation of IκB in
H9c2 cells in response to LPS, and subsequently decreased the
nuclear translocation and phosphorylated levels of NF-κB p65
(P<0.05 vs. the LPS-treated cells) (Figs. 3 and 4).
Discussion
The results of the present study demonstrated that
puerarin significantly attenuated the inflammatory responses of
H9c2 cardiomyocytes by repressing the expression levels of the
proinflammatory cytokines, IL-1β and TNF-α. Furthermore, puerarin
exhibited a protective effect against the LPS-induced apoptosis of
H9c2 cardiomyocytes by reversing LPS-induced downregulation of Bax
and upregulation of Bcl-2. Therefore, the protective effect of
puerarin in LPS-stimulated cardiomyocytes may be mediated by the
inhibition of the NF-κB signaling cascade.
The incidence rate of cardiac dysfunction among
patients with septic shock is 80% (19). Septic shock may induce hypotension,
depression of myocardial systolic performance and the alteration of
the diastolic function (20). In
addition, myocardial dysfunction as a result of septic shock
contributes to the high mortality rate associated with sepsis
(21). The inhibition of cardiac
inflammatory processes in sepsis may exert a beneficial effect on
cardiac dysfunction (22). It has
been demonstrated that the LPS-induced inflammatory response in
cardiomyocytes is characterized by the induction of inflammatory
mediators, such as IL-1β and TNF-α (23). In the present in vitro study,
the LPS-induced expression of inflammatory factors was
downregulated by puerarin treatment in a concentration-dependent
manner, suggesting that puerarin may have the ability to protect
cardiomyocytes against the inflammation resulting from sepsis.
In a previous study of adult male C57 mice that
received 4 mg/kg LPS, increased myocardial caspase-3 activity and
the number of apoptotic cells were detected (24), suggesting that apoptosis and
inflammation coexist in sepsis. The balance between the
upregulation and downregulation of pro-apoptotic proteins, such as
Bax, and anti-apoptotic proteins, such as Bcl-2, determines whether
the cells will undergo apoptosis (25,26). The
present results demonstrated that puerarin was able to counteract
LPS-induced apoptosis by inhibiting the expression of Bax and
increasing the expression of Bcl-2.
The role of NF-κB activation in septic
pathophysiology and the signal transduction pathways leading to
NF-κB activation during sepsis/septic shock have been extensively
investigated (27,28). Following cell stimulation, the IκB
kinase complex phosphorylates IκB proteins on specific serine
residues, subsequently leading to their polyubiquitylation and
proteasomal degradation (29). As a
result, NF-κB dimers accumulate in the nucleus and activate the
transcription of numerous genes in various cells. Following LPS
stimulation, NF-κB dimers accumulate in the nucleus of B cells and
activate the transcription of IL-1 and TNF-α (30,31). In
order to identify the mechanism underlying the cytoprotective
effect of puerarin, the activation of NF-κB was evaluated. The
present results indicated that treatment with puerarin resulted in
a marked reduction in p-NF-κB levels, and an increase in IκB
levels.
In conclusion, the present results indicated that
puerarin may protect against LPS-induced inflammation and apoptosis
in H9c2 cardiomyocytes via the inhibition of the NF-κB pathway.
Furthermore, puerarin may be used as an adjuvant treatment in order
to reduce cardiomycocyte inflammation and apoptosis in patients
suffering from sepsis. Future studies are required in order to
fully elucidate the underlying mechanisms.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81300070, 81300104
and 81270303), the Specialized Research Fund for the Doctoral
Program of Higher Education of China (grant no. 20130141120042) and
the Fundamental Research Funds for the Central Universities of
China (grant no. 2012302020212).
References
|
1
|
Murray CJ, Atkinson C, Bhalla K, Birbeck
G, Burstein R, Chou D, Dellavalle R, Danaei G, Ezzati M, Fahimi A,
et al: The state of US health, 1990-2010: Burden of diseases,
injuries, and risk factors. JAMA. 310:591–608. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Martin GS, Mannino DM, Eaton S and Moss M:
The epidemiology of sepsis in the United States from 1979 through
2000. N Engl J Med. 348:1546–1554. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tupchong K, Koyfman A and Foran M: Sepsis,
severe sepsis, and septic shock, A review of the literature.
African J Emerg Med. 5:127–135. 2015. View Article : Google Scholar
|
|
4
|
Romero-Bermejo FJ, Ruiz-Bailen M,
Gil-Cebrian J and Huertos-Ranchal MJ: Sepsis-induced
cardiomyopathy. Curr Cardiol Rev. 7:163–183. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rudiger A and Singer M: The heart in
sepsis: From basic mechanisms to clinical management. Curr Vasc
Pharmacol. 11:187–195. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Seymour CW and Rosengart MR: Septic Shock:
Advances in Diagnosis and Treatment. JAMA. 314:708–717. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Plociennikowska A, Hromada-Judycka A,
Borzecka K and Kwiatkowska K: Co-operation of TLR4 and raft
proteins in LPS-induced pro-inflammatory signaling. Cell Mol Life
Sci. 72:557–581. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Charalambous BM, Stephens RC, Feavers IM
and Montgomery HE: Role of bacterial endotoxin in chronic heart
failure, The gut of the matter. Shock. 28:15–23. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Frazier WJ, Xue J, Luce WA and Liu Y: MAPK
signaling drives inflammation in LPS-stimulated cardiomyocytes, The
route of crosstalk to G-protein-coupled receptors. PLoS One.
7:e500712012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou YX, Zhang H and Peng C: Puerarin: A
review of pharmacological effects. Phytother Res. 28:961–975. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen R, Xue J and Xie M: Puerarin prevents
isoprenaline-induced myocardial fibrosis in mice by reduction of
myocardial TGF-β1 expression. J Nutr Biochem. 23:1080–1085. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhu G, Wang X, Wu S, Li X and Li Q:
Neuroprotective effects of puerarin on
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine induced Parkinson's
disease model in mice. Phytother Res. 28:179–186. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang JW, Wang HD, Cong ZX, Zhou XM, Xu JG,
Jia Y and Ding Y: Puerarin ameliorates oxidative stress in a rodent
model of traumatic brain injury. J Surg Res. 186:328–337. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Singh AK, Jiang Y, Gupta S, Younus M and
Ramzan M: Anti-inflammatory potency of nano-formulated puerarin and
curcumin in rats subjected to the lipopolysaccharide-induced
inflammation. J Med Food. 16:899–911. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kapoor S: Anti-neoplastic effects of
puerarin in systemic malignancies besides colon carcinomas. Int J
Pharm. 443:3062013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu K, Liang T, Duan X, Xu L, Zhang K and
Li R: Anti-diabetic effects of puerarin, isolated from Pueraria
lobata (Willd.), on streptozotocin-diabetogenic mice through
promoting insulin expression and ameliorating metabolic function.
Food Chem Toxicol. 60:341–347. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yuan Y, Zong J, Zhou H, Bian ZY, Deng W,
Dai J, Gan HW, Yang Z, Li H and Tang QZ: Puerarin attenuates
pressure overload-induced cardiac hypertrophy. J Cardiol. 63:73–81.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu X, Jia B, Wang F, Lv X, Peng X, Wang Y,
Li H, Wang Y, Lu D and Wang H: α1 Adrenoceptor activation by
norepinephrine inhibits LPS-induced cardiomyocyte TNF-α production
via modulating ERK1/2 and NF-kappaB pathway. J Cell Mol Med.
18:263–273. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Beraud AS, Guillamet CV, Hammes JL, Meng
L, Nicolls MR and Hsu JL: Efficacy of transthoracic
echocardiography for diagnosing heart failure in septic shock. Am J
Med Sci. 347:295–298. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lemarie J, Blet A, Bouazza Y,
Boisrame-Helms J, Meziani F and Levy B: Dexamethasone and
recombinant human activated protein C improve myocardial function
and efficiency during experimental septic shock. Shock. 41:522–527.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ha T, Xia Y, Liu X, Lu C, Liu L, Kelley J,
Kalbfleisch J, Kao RL, Williams DL and Li C: Glucan phosphate
attenuates myocardial HMGB1 translocation in severe sepsis through
inhibiting NF-κB activation. Am J Physiol Heart Circ Physiol.
301:H848–H855. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ceylan-Isik AF, Zhao P, Zhang B, Xiao X,
Su G and Ren J: Cardiac overexpression of metallothionein rescues
cardiac contractile dysfunction and endoplasmic reticulum stress
but not autophagy in sepsis. J Mol Cell Cardiol. 48:367–378. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fallach R, Shainberg A, Avlas O, Fainblut
M, Chepurko Y, Porat E and Hochhauser E: Cardiomyocyte Toll-like
receptor 4 is involved in heart dysfunction following septic shock
or myocardial ischemia. J Mol Cell Cardiol. 48:1236–1244. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li X, Luo R, Jiang R, Meng X, Wu X, Zhang
S and Hua W: The role of the Hsp90/Akt pathway in myocardial
calpain-induced caspase-3 activation and apoptosis during sepsis.
BMC Cardiovasc Disord. 13:82013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Alladi PA, Roy T, Singh N and Wadhwa S:
Prenatal auditory enrichment with species-specific calls and sitar
music modulates expression of Bcl-2 and Bax to alter programmed
cell death in developing chick auditory nuclei. Int J Dev Neurosci.
23:363–373. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tichý A: Apoptotic machinery: T he Bcl-2
family proteins in the role of inspectors and superintendents. Acta
Medica (Hradec Kralove). 49:13–18. 2006.PubMed/NCBI
|
|
27
|
Chen G, Zhao J, Yin Y, Wang B, Liu Q, Li
P, Zhao L and Zhou H: C-type natriuretic peptide attenuates
LPS-induced endothelial activation, Involvement of p38, Akt and
NF-kappaB pathways. Amino Acids. 46:2653–2663. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Walley KR: Deeper understanding of
mechanisms contributing to sepsis-induced myocardial dysfunction.
Crit Care. 18:1372014. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Baeuerle PA and Baltimore D: I kappaB: A
specific inhibitor of the NF-kappaB transcription factor. Science.
242:540–546. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Grilli M, Chiu JJ and Lenardo MJ:
NF-kappaB and Rel, Participants in a multiform transcriptional
regulatory system. Int Rev Cytol. 143:1–62. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Muller JM, Ziegler-Heitbrock HW and
Baeuerle PA: Nuclear factor kappaB, a mediator of
lipopolysaccharide effects. Immunobiology. 187:233–256. 1993.
View Article : Google Scholar : PubMed/NCBI
|