Introduction
Patent ductus arteriosus (PDA) is a common
congenital heart condition, the incidence of which is
1/2,500–1/5,000 (1). The ductus
arteriosus evolves from the sixth left aortic arch in the process
of aortic arch development; it is a normal route of blood
circulation in the fetus. In normal development, 82–96% of the
ductus arteriosus undergoes functional closure within 48 h after
birth, and anatomical closure with fibrosis is usually completed in
weeks 2–3 after birth (2). Various
genetic and/or environmental factors and premature birth may cause
arterial tissue elastic fibers to increase, reduce smooth muscle
tissue and cause endocardial cushion dysplasia. These factors may
lead to the delayed or lack of closure of the PDA. The ductus
arteriosus is generally situated at the aortic isthmus and at the
left pulmonary artery side of the main pulmonary artery
bifurcation. However, in patients with a right aortic arch, it may
be located between the aorta distal to the brachiocephalic artery
root and the right pulmonary artery. Bilateral ductus arteriosus is
very rare (3). This malformation is
assumed to occur during the transformation of the branchial-type
arterial system to the mammalian-type arterial system in the
development of the aorta and its branches (4).
Case report
A 2.5-year-old girl weighing 8.5 kg had a cardiac
murmur for >2 years. The pre-hospital echocardiographic
diagnosis was congenital heart disease with PDA (funnel-type with
left-to-right shunt). The X-ray suggested increased bilateral
pulmonary blood; cloud-like high-density shadows were observed in
the field of the right lower lung. The cardio-thoracic ratio was
0.57.
Following preoperative preparation, the intent was
to perform surgical ligation of the PDA under general anesthesia. A
conventional thoracotomy was made at the fourth left intercostal
space. The left pulmonary artery was observed to be parallel to the
esophagus. The descending aorta was below the esophagus. Freeing
the descending aorta was difficult because of obstruction by the
esophagus and left pulmonary artery. Considering the poor
accessibility, the search for the PDA was abandoned. A drainage
tube was placed in the left chest, and the chest was finally
closed.
Enhanced computed tomography (CT) of the heart was
conducted 2 days after the surgery. The CT images clearly showed
the double PDA (Figs. 1 and 2). A 6.6-mm-wide shadow indicated the PDA
(PDA-1) in the descending aorta connecting to the right pulmonary
artery. The aortic arch was the origin of the left common carotid
artery, the right common carotid artery, and the right
subclavicular artery. The left subclavian artery originated from
the left vertebral artery. The artery was connected to the main
pulmonary artery by a duct (PDA-2) 4.5 mm in diameter. The child
had situs solitus right-sided aortic arch with right-sided
descending aorta, isolated left subclavian artery, and double
PDA.
Interventional treatment was conducted in an attempt
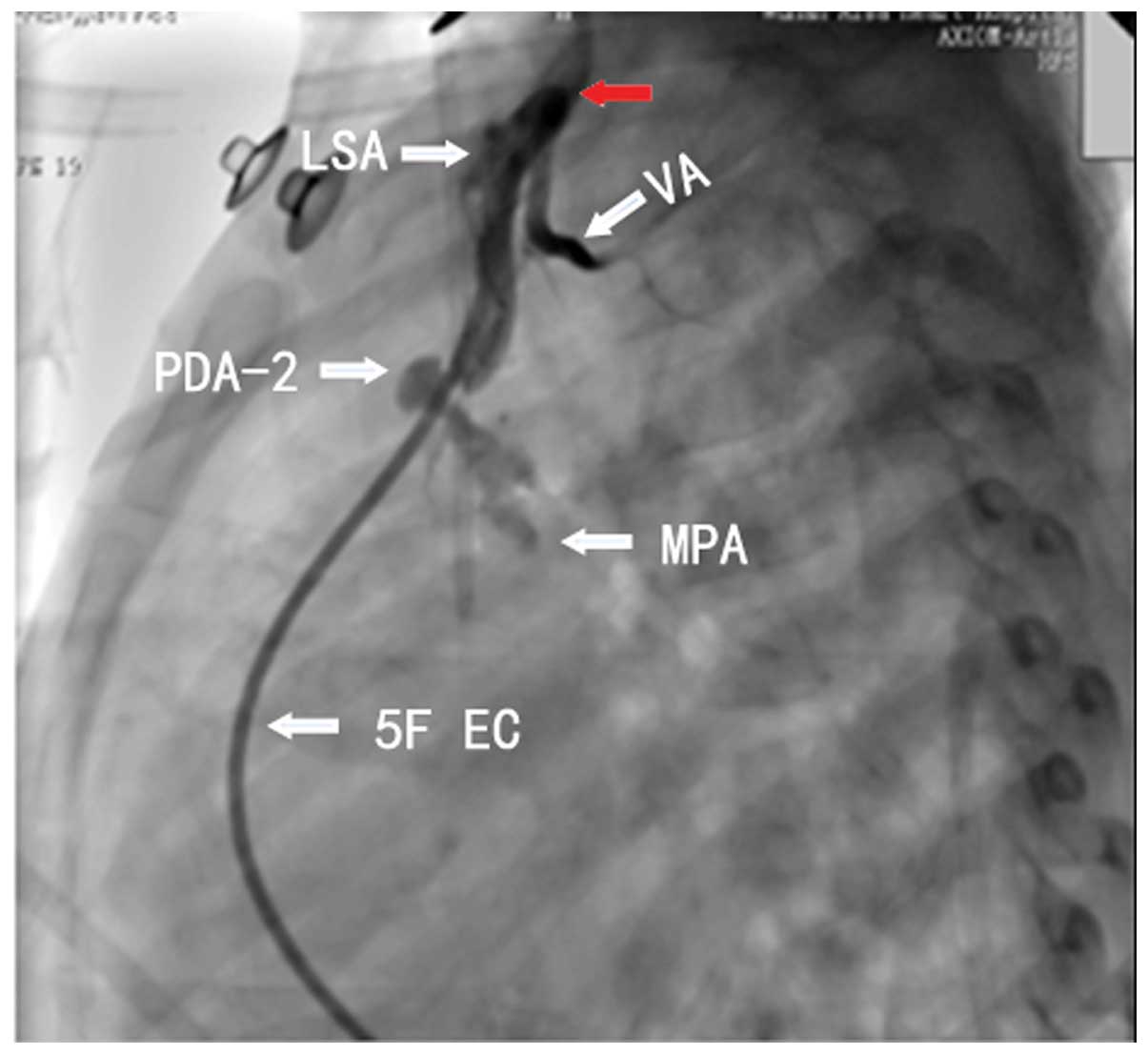
to block the double PDA. Intraoperative angiography showed the
bilateral ductus arteriosus (Figs. 3
and 4). The angiography showed that
the narrowest region of the PDA-2 had a diameter of ~2 mm.
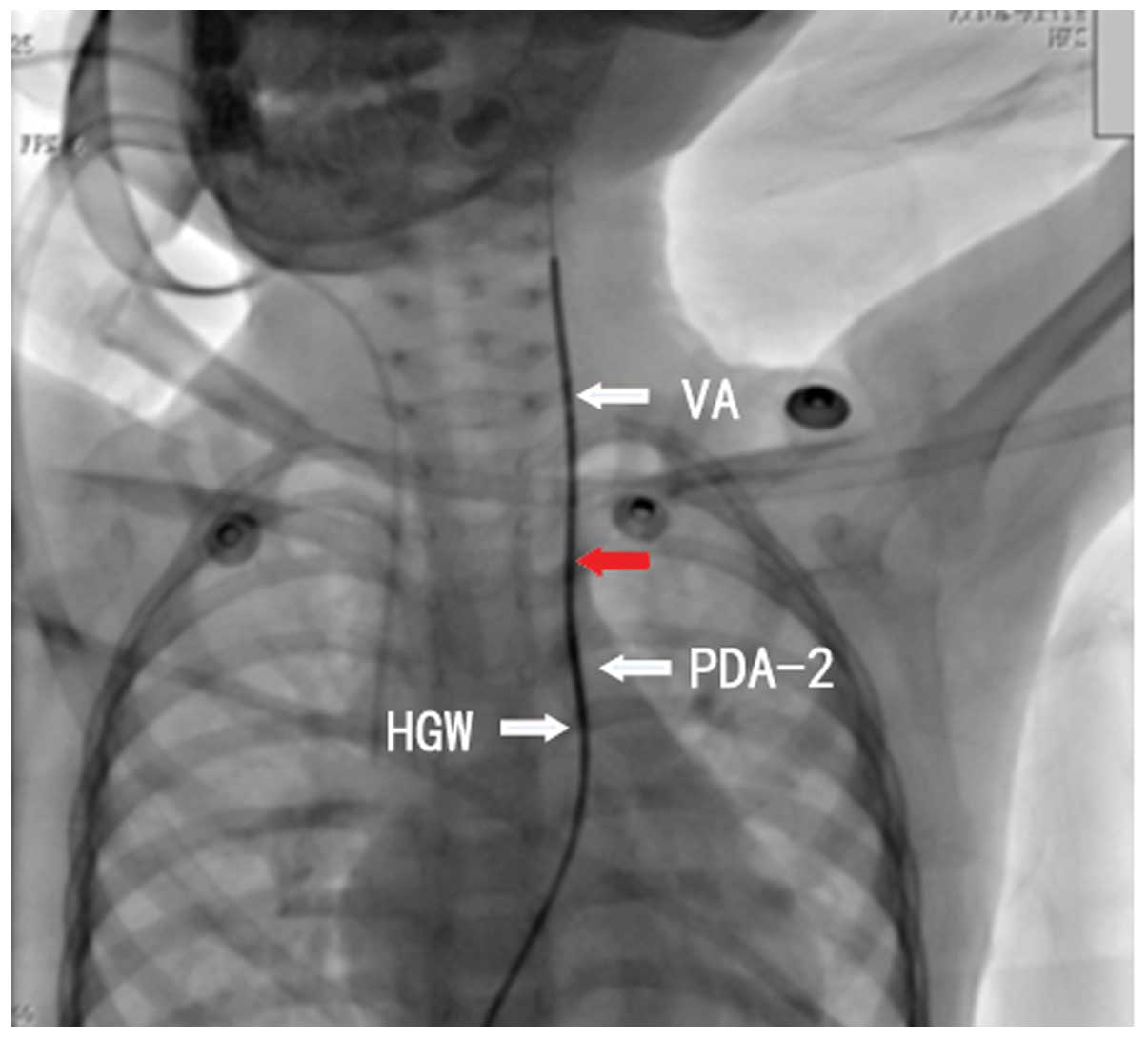
Establishing a path from PDA-2 to the vertebral artery with the
260-cm hardened guidewire was challenging (Fig. 5). Thus, an attempt was made to
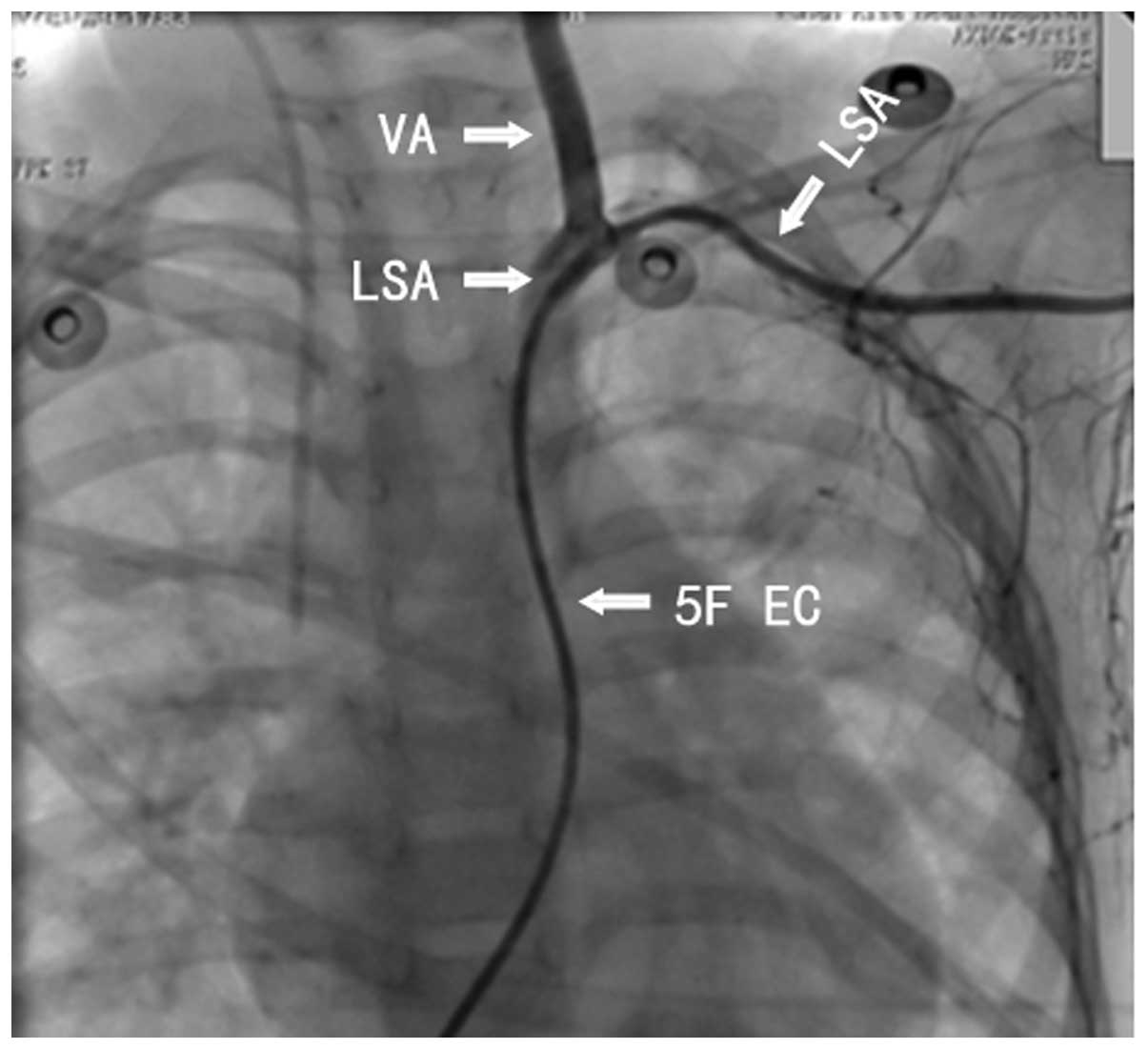
establish a path from PDA-2 to the subclavian artery (Fig. 6). However, passing through the
twisted PDA-2 was also challenging. Therefore, the closure was
abandoned and the surgery was terminated.
Following the interventional surgery, the child was
transferred to the surgical operation room for double PDA ligation
plus left subclavian artery reconstruction under median
thoracotomy. The surgery was successful and after the surgery, the
sick child recovered stably.
Discussion
To the best of our knowledge there are no relevant
reports about double patent ductus arteriosus. A similar
malformation is anomalous origin of the left subclavian artery from
the pulmonary artery, which was originally identified at autopsy
(5). These abnormalities are divided
into two typies. One is aberrant left subclavian artery, the other
is the variation of the origin area of subclavian artery. Among
them, aberrant left subclavian artery is accounted for 76.2% and
the incidence rate is 0.8%. Among these, aberrant left subclavian
artery was rarer than aberrant right subclavian artery; from a
reported 16 cases of aberrant subclavian artery there was only one
case where the left subclavian artery was affected (6). That case reported the concomitant
congenital heart disease tetralogy of Fallot, which was very
similar to the present case, but differed in that the left
subclavian artery arose from the proximal descending aorta and
detoured to the rear of the esophagus. It has been reported that
cardiovascular malformations may be associated with 22q11.2
deletion (7). Anatomical variations
of these vessels have the following clinical implications: i)
Compression by the vascular ring may cause a sense of obstruction
of the esophagus; ii) the anatomical variations may be associated
with congenital heart disease; iii) the variations may be
associated with steal syndrome, causing transient cerebral ischemia
(8); iv) they may extend the time of
examination of neck vessels and the cerebrovascular system by
digital subtraction angiography, and increase the incidence of
complications; v) the arterial variation should be considered when
conducting thoracic surgery to avoid damaging large blood vessels.
Double PDA generally indicates the presence of other congenital
cardiac defects (9). The main
treatment methods for simple PDA include surgery and interventional
closure (10). However, studies and
treatment experience of double PDA are limited worldwide (11).
The reasons for failed interventional closure of the
present case were as follows. i) It was difficult to establish a
path to the vertebral artery because the shape of PDA-2 was
severely twisted. The soft tip of the hardened guidewire was
relatively long. If the hardened portion reached the appropriate
site to support the pathway, the soft tip would be forced to enter
the vertebrobasilar artery system. ii) When the left subclavian
artery was selected for intervention, the soft tip of the hardened
guidewire had already reached the farthest point of the left
subclavian artery without fully passing through the variant PDA.
Thus, the surgery could not be completed. It is hypothesized that
shortening the length of the soft head of the hardened guidewire
could have enabled the doctor to smoothly complete the
establishment of the path. This type of hardened guidewire would
require special production.
This unsuccessful interventional treatment was
considered very disappointing by the surgeon, who may never again
get the opportunity to treat this rare condition. It is
hypothesized that the surgery could have been successfully
completed if the process had been performed slowly and carefully.
Moreover, an operator with good manual dexterity should have
performed the insertion of the thread.
References
|
1
|
Al-Hamash SM, Wahab HA, Khalid ZH and
Nasser IV: Transcatheter closure of patent ductus arteriosus using
ADO device, Retrospective study of 149 patients. Heart Views.
13:1–6. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang ZW, Liu WY and Zhang BR: Patent
ductus arteriosus. Cardiac Surgery. People's Medical Publishing
House. 5862003.(In Chinese).
|
|
3
|
Amabile N, Ghez O, Aubert F, Ovaert C,
Fraisse A, Kreitmann B and Metras D: Complete correction of
interrupted right aortic arch with isolation of left subclavian
artery. Ann Thorac Surg. 80:733–735. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang ZW, Liu WY and Zhang BR: Embryonic
development of the heart. Cardiac Surgery. People's Medical
Publishing House. 18–20. 2003.(In Chinese).
|
|
5
|
Guo LN and Jiang Y: The abnorm of figure
of aortic arch and right subclavian artery: A case report. Zhong
Guo Lin Chuang Jie Pou Xue Za Zhi. 26:5932008.(In Chinese).
|
|
6
|
Zhu JQ, Tao XF, Hao NX and Zhang L: CT
angiography of variations of subclavian artery. Zhong Guo Yi Xue
Jisuan Ji Cheng Xiang Za Zhi. 19:132–135. 2013.(In Chinese).
|
|
7
|
Lee ML, Chen M, Tsao LY, Chiu HY, Chiu IS,
Yang AD and Tsai PL: Congenital stridor and wheezing as harbingers
of the del22q11.2 syndrome presenting cardiovascular malformations
of right aortic arch, aberrant left subclavian artery, Kommerell's
diverticulum, and left ligamentum arteriosum. Cardiovasc Pathol.
20:124–129. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cao YJ, Xiao GD, Zhang CY, Li W and Liu
CF: Successful treatment of the left subclavian artery steal
syndrome use endovascular stent. Zhong Hua Nao Xue Guan Bing Za
Zhi. 4:216–218. 2010.(In Chinese).
|
|
9
|
Ugurlucan M, Sayin OA, Dayioglu E and
Tireli E: Bilateral PDA in a patient with VSD and pulmonary
atresia. J Card Surg. 26:107–110. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kulkarni A, Richards J and Duffy D: Survey
of management of patent ductus arteriosus in neonatal units across
England. Arch Dis Child Fetal Neonatal Ed. 98:465–466. 2013.
View Article : Google Scholar
|
|
11
|
Zhu XY, Han XM, Zhang YW, Wang ZG, Quan Z,
Sheng XT, Jin Y and Deng DA: Catheterization analysis of 133
infants with congenital heart disease. Zhong Guo Shi Yong Er Ke Za
Zhi. 17:534–536. 2013.(In Chinese).
|