Introduction
Brain damage following cardiac arrest (CA) is
typically the result of ischemic or hypoxic injury in vulnerable
areas of the brain, such as the hippocampus, cortex and thalamus,
and triggers a series of pathophysiological processes following
CA/cardiopulmonary resuscitation (CPR). The necrosis and apoptosis
of numerous nerve cells leads to various neuronal dysfunctions,
including anterograde amnesia, learning difficulties, emotional and
social behavioral changes, depression, and potentially coma,
persistent vegetative state and mortality. Thus, brain damage has
been considered to be a major sign of post-CA syndrome, which often
increases the mortality rate in addition to the effects caused by
the primary diseases that induce CA (1). Effective attenuation of brain injury is
one of the key aims of CPR.
As the third gaseous signaling molecule in
vivo, hydrogen sulfide (H2S) has a relatively small
molecular mass, which allows it to traverse the cell membrane
freely without requiring a receptor. Previous studies have
indicated that H2S is involved in the pathophysiological
process of ischemia-reperfusion injury and shock, and exerts
protective effects on neurons (2–7).
H2S has been demonstrated to increase the expression of
γ-aminobutyric acid B receptor subunits 1 and 2, and subsequently
reduce excitotoxic injury (8,9).
H2S mediates signals between neuronal cells and
astrocytes by increasing the influx of Ca2+ in order to
maintain calcium homeostasis and regulate synaptic activity
(10). Furthermore, H2S
is known to exert protective effects on neurons via its
antioxidative functions (11).
However, the effects of H2S on brain injury after CA are
not clear and the results of previous studies appear contradictory.
For example, the administration of the H2S donor sodium
sulfide (Na2S) 1 min prior to CPR has been demonstrated
to significantly improve the survival rate and neurological
function of rats at 24 h (12) or 10
days after CPR (13). Furthermore,
Derwall et al observed that high-dose Na2S
significantly reduces microglial activation in striatal areas,
although this did not translate into improved neurological outcome
in a porcine model of prolonged CA (14). Knapp et al reported that
Na2S therapy is associated with a temporary beneficial
effect on neurological outcome (3 days after CPR) (15). Therefore, the present study aimed to
clarify the modulation of H2S levels in the serum of
rats and to evaluate the effects of treatment with the
H2S donor sodium hydrosulfide (NaHS) or hydroxylamine, a
cystathionine-β-synthase (CBS) inhibitor, on brain injury following
CA. These effects were evaluated by examining biomarkers of brain
injury, neurologic deficit scoring (NDS) and the neural behavior
after CPR. CBS is able to catalyze the synthesis of H2S
from L-cysteine in mammalian central nervous system tissues
(16) and it was hypothesized that
hydroxylamine application may reduce the levels of
H2S.
Materials and methods
Subjects and groups
All animal procedures were approved and conducted in
accordance with the Animal Ethics Committee of Sun Yat-Sen
University (Guangzhou, China) and the UK Home Office Animals
(Scientific Procedures) Act 1986. All procedures conformed to
Directive 2010/63/EU of the European Parliament. Animal experiments
were conducted in the Assisted Circulation Key Laboratory of
Ministry of Health at Sun Yat-Sen University. Animals were provided
by the Laboratory Animal Center of Sun Yat-Sen University.
A total of 60 healthy male Sprague-Dawley rats, aged
10–12 months old and weighing 450–550 g, were allocated at random
into the NaHS, hydroxylamine or routine CPR control groups (n=20
per group). The further 40 rats were used as spare rats to
supplement the rats if any died prior to the end point of
observation. A further 100 male rats of the same weight and brood
were prepared as blood donors.
Induction of the CA model
The rat model of CA was established as described in
our previous study (17). Briefly,
the rats were anesthetized via a bolus intraperitoneal injection of
10% chloral hydrate (3 ml/kg). The degree of anesthesia was
evaluated by measurement of muscular tone, corneal reflex and pain
reflex. Tracheal intubation was performed through the mouth and
cardiac monitoring was performed by electrocardiography using limb
leads (V3404; SurgiVet, Inc., Waukesha, WI, USA). A 24G close-vein
indwelling needle (BD Intima II™ Vein™; Suzhou Becton Dickinson
Medical Devices Co., Ltd., Suzhou, China) was inserted into the
right femoral vein to establish a transfusion passage. A 22G
close-vein indwelling needle was inserted into the right femoral
artery. A three-way cock valve was connected to the remaining
arterial needle. One end was connected to an injector with heparin,
and the other end was connected to the BL-420E Biological Data
Acquisition and Analysis system (Chengdu TME Technology Co., Ltd.,
Chengdu, China). After the chest skin of the rats was shaved, two
disposable acupuncture needles (30G HuanQiu; Suzhou Acupuncture
Supplies Co., Ltd., Suzhou, China) were transcutaneously inserted
into the epicardium between the fourth rib of the left sternal
border and the third rib of the right sternal border. The
stimulator electrode of the BL-420E system was then connected,
which was used to supply direct and constant electrical stimulation
of the epicardium with crude current, continuous single
stimulation, a delay of 100 msec, a wave width of 1 msec, a
frequency of 50 Hz, an initial intensity of 1–2 mA and a
stimulation duration of 3 min. CA was defined as follows (18): i) Systolic arterial pressure quickly
reduced to <25 mmHg after electrical stimulation; ii) the
arterial pulse wave from blood pressure monitoring disappeared
after electrical stimulation; and iii) the electrocardiographic
wave displayed on the cardiac monitor indicated ventricular
fibrillation, pulseless electrical activity or asystole following
the cessation of electrical stimulation.
CPR
CPR was performed with the Utstein style (18) following a 6-min period without any
intervention. External chest compression was performed using an
external chest compression machine for small animals, developed by
the Key Laboratory of Assisted Circulation of Sun Yat-Sen
University, at a rate of 200 compressions/min, with equal
compression-relaxation, and depth of compression to 1/3 of the
anteroposterior chest diameter. Intermittent positive pressure
ventilation was performed using a Model 683 Small Animal Ventilator
(Harvard Apparatus, Inc., Holliston, MA, USA) with the initial
parameters as follows: Set frequency, 70 times/min; tidal volume,
0.65 ml/100 g; and inspired oxygen concentration, 21%. Parameters
for the next respirator were adjusted according to blood gas
analysis. Adrenalin (2 µg/100 g) was immediately administered to
the rats at the initiation of CPR and was readministered at 3 min
intervals as required. The administration of liquid during CPR was
limited to <2 ml. Defibrillation was performed with
direct-current single-phase (defibrillation energy, 5 J) if the
electrocardiogram displayed ventricular fibrillation at 1 min after
CPR. If the defibrillation failed, CPR was repeated and
defibrillations were repeated at 1 min after CPR. Spontaneous
circulation was restored (18) if
supraventricular cardiac rhythm was restored; the average arterial
pressure was >60 mmHg and was maintained for ≥10 min. If the
spontaneous circulation of the rats was not restored after 10 min
with the above treatment, CPR was considered to have failed.
Treatments following
resuscitation
Rats that exhibited successful restoration of
spontaneous circulation (ROSC) were monitored using an
electrocardiogram and for hemodynamics for 4 h. During this period,
rats with weak autonomous respirations were mechanically
ventilated. The respiratory condition of each rat was evaluated
every 15 min to determine whether further mechanical ventilation
was required. Mechanical ventilation was stopped at 4 h, after all
tubes were removed and wounds were sutured. Each rat was fed in a
separate cage. All animal experiments were conducted on a
thermostatic table (Suzhou Liying Experimental Co., Ltd., Suzhou,
China), which maintained the rat body temperature at 37.5±0.2°C. A
heat lamp (Suzhou Liying Experimental Co., Ltd.) was additionally
used for rats with a body temperature of <36.5°C. Immediately
following ROSC, NaHS (14 µmol/kg/day, diluted in 1.5 ml normal
saline; Sigma-Aldrich Trading Co. Ltd, Shanghai, China) was
injected into the rats via the femoral vein and then every
subsequent 8 h intraperitoneally (three equal 0.5-ml doses/day) in
the NaHS group. Hydroxylamine solution (40 µmol/kg/day, diluted in
1.5 ml normal saline; Sigma-Aldrich Trading Co. Ltd) was
administered to the rats in the hydroxylamine group in the same
manner as that used for NaHS. The control group received routine
CPR, and an equal quantity of saline as a control in the same
manner as that used the first two groups. The end point of
observation was 7 days. Animals with failed CA induction, or
animals that died prior to the end point of observation, were
excluded from the final data analysis.
Neurological deficit scoring
(NDS)
NDS was performed prior to CA and at 4, 12, 24, 72
and 168 h after CPR, as previously described (19).
Sample collection
After NDS at each specified experimental time point
(prior to CA and 4, 12, 24, 72 and 168 h after), 2 ml blood was
obtained from the femoral vein. A 1-ml volume of this blood sample
was sealed in an EP tube containing coagulant and deproteinization
reagent for H2S measurement. The remaining 1 ml blood
was collected for the examination of neuron-specific enolase (NSE)
and S100β. Samples were centrifuged at 2,504 × g for 15 min at 4°C,
within 30 min of collection. The sealed EP tube with serum was
numbered and stored at −80°C. An equal volume of blood from donor
mice was transfused immediately into the experimental mice
following blood collection, in order to avoid unstable blood
circulation and its influence on the experiment results. Serum
levels of H2S were measured using the deproteinization
method (20) and the levels of NSE
and S100β were measured using ELISA kits (R&D Systems, Inc.,
Minneapolis, MN, USA).
Morris water maze, beam walking and
prehensile traction tests
As described in previous studies (21,22), the
Morris water maze test consisted of a hidden platform test, probe
test and visible platform test. Animals were subjected to the
Morris water maze test individually 1 day prior to CA and 3, 5 and
7 days after CPR. The hidden platform test was performed in
duplicate, once in the morning and once in the afternoon, with the
average number as the result for each day of testing. The water
maze apparatus (Zhenghua Biotech Co., Ltd., Hebei, China) consisted
of a circular glass pool of 150 cm diameter and a movable glass
platform 12 cm in diameter and 35 cm in height. A video camera was
positioned on a platform that was 210 cm above the bottom of the
pool. The wall of the maze was painted black and the water was dyed
black using ink. The pool was filled with water to the 36-cm mark
and maintained at 24±1°C. The pool was divided arbitrarily into
four equally sized quadrants. The platform was submerged in the
middle of zone I such that its surface was 1 cm below the water
surface and 20 cm away from the wall of the maze. The hidden
platform test measured rat behavioral change by quantifying escape
latency and swimming distance. The probe test was conducted 7 days
after CPR, subsequent to the hidden platform test. After removing
the platform, the animals were left to swim for 120 sec. During
this period, the number of instances the animal passed each
quadrant, as well as the swimming was recorded by a video tracking
system. In the visible platform test, which was performed after the
probe test, escape latency and swimming distance were recorded.
The beam walking test (BWT) and the prehensile
traction test were conducted 1 day prior to CA and 3, 5 and 7 days
after CPR, following established methods (23,24).
Preparation of samples for apoptosis
analyses
Following the endpoint of the neural behavioral
tests, the animals in each group were divided at random into two
subgroups for the analysis of apoptosis by either
immunohistological examination by TUNEL assay, or by western blot
analysis. The brains of rats that were to undergo
immunohistochemical (IHC) examination were fixed via transcardial
perfusion of the rats with 4% paraformaldehyde for 20–30 min prior
to decapitation and removal of the brain. Brain tissue was then
fixed in 4% paraformaldehyde. Sections of brain tissue 3–4 mm in
size were excised between the optic chiasm and optic nerve,
embedded in paraffin and sectioned for IHC examination. Hippocampal
volume was measured by stereological analysis after hematoxylin and
eosin staining (25). Rats that were
to undergo western blot analysis were sacrificed by cervical
dislocation. The brains were removed immediately, and the
hippocampal region was isolated and rapidly frozen using liquid
nitrogen. Samples were stored at −80°C prior to detection of
cysteine-containing aspartate-specific protease-3 (caspase-3) and
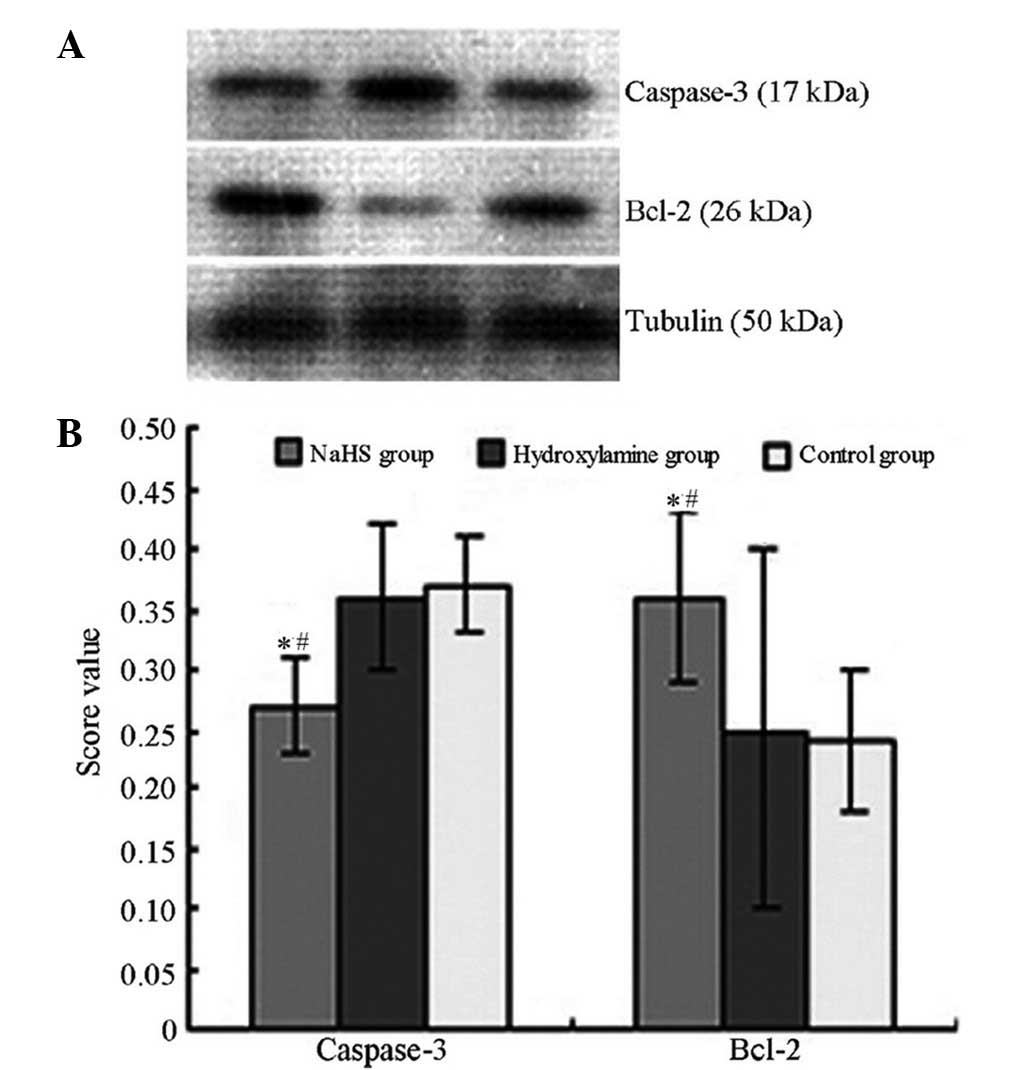
B-cell leukemia/lymphoma 2 (Bcl-2) protein expression levels.
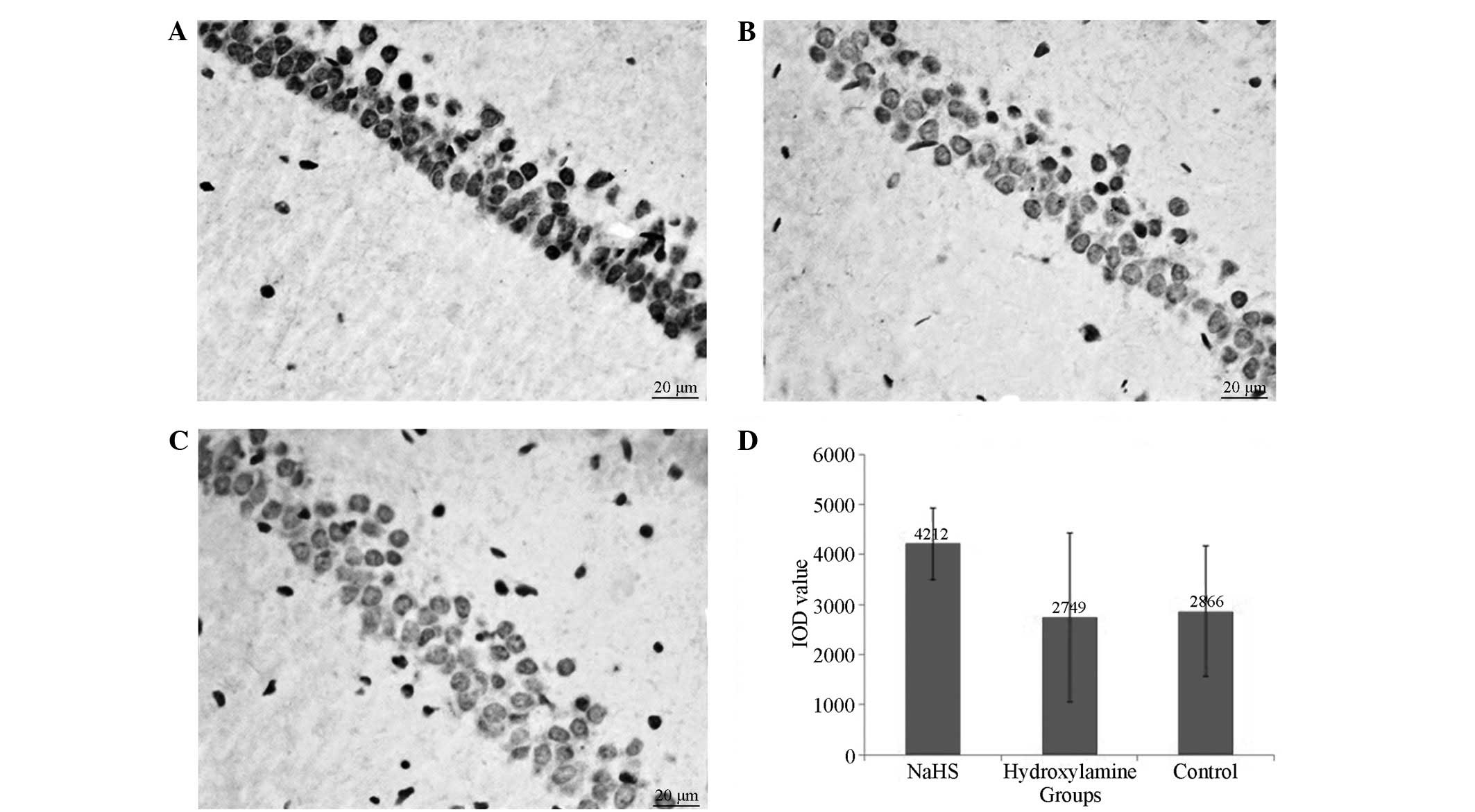
TUNEL assay for apoptosis
analysis
Apoptosis assays were performed using a TUNEL assay
(TUNEL Cell Apoptosis Detection kit; Nanjing KeyGen Biotech, Co.,
Ltd., Nanjing, China). Cells exhibiting characteristics of
apoptosis, combined with brown nuclear staining, were considered to
be positive for apoptosis. Expression of caspase-3 and Bcl-2 in the
hippocampal CA1 region was examined using streptavidin biotin
peroxidase complex immunohistochemical staining kits (Wuhan Boster
Bio-Engineering Ltd. Co., Wuhan, China). Cells displaying dark
brown staining were considered to be positive.
Western blot for apoptosis
analysis
The expression levels of caspase-3 and Bcl-2 in the
hippocampal CA1 region were additionally examined using western
blot analysis, according to a previously reported method (26). The primary antibodies included
polyclonal rabbit anti-active caspase-3 antibody (1:1,000; #9664;
Cell Signaling Technology, Inc., Danvers, MA, USA) and polyclonal
rabbit anti-Bcl-2 antibody (1:50; ab7973; Abcam, Inc., Cambridge,
MA, USA). The secondary antibody was goat-anti-rabbit IgG (1:5,000;
SC-2004; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) for 2
h. Semi-quantitative image analysis was performed using Image Pro
Plus 6.0 software (Media Cybernetics, Inc., Rockville, MD, USA).
Five optical microscope fields (DVM6; Leica Microsystems GmbH,
Berlin, Germany) were selected at random from the same region of
each tissue section and the apoptotic index (AI) was calculated as
a percentage of total apoptotic cells. For caspase-3 and Bcl-2
expression, integrated optical density (IOD) was determined for
each field. Average IOD from the five fields was calculated as the
mean IOD for the region. Western blot analysis results were
analyzed following scanning of the blots. Quantity One image
processing software (Bio-Rad Laboratories, Inc., Hercules, CA, USA)
was used to semi-quantitatively calculate the luminance ratio of
each protein band.
Statistical analysis
Statistical analyses were performed using SPSS
software, version 13.0 (SPSS, Inc., Chicago, IL, USA). Categorical
data were organized into contingency tables. Quantitative data are
expressed as the mean ± standard deviation. Pair-wise comparisons
between groups were performed using the Student-Newman-Keuls
method. P<0.05 was considered to indicate a statistically
significant difference.
Results
CA model
In the NaHS group, the establishment of the CA model
was attempted in 31 Sprague-Dawley rats with a success rate of
93.5% (29/31) and an ROSC rate of 93.1% (27/29). Seven rats died
after ROSC prior to the end point of observation (7/27, 25.9%). In
the hydroxylamine group, establishment of the CA model was
attempted in 35 rats with a success rate of 91.4% (32/35) and an
ROSC rate of 93.8% (30/32). A total of 10 rats died after ROSC
prior to the end point of observation, resulting in a mortality
rate of 33.3% (10/30). In the control group, CA was successfully
induced in a total of 32 rats (97.0%, 32/33). The ROSC rate was
90.6% (29/32) and 9 rats died after ROSC but prior to the end point
of observation, resulting in a mortality rate of 31.0% (9/29). No
significant differences were observed in the rates of successful CA
model induction, ROSC and mortality following ROSC among the three
groups (P>0.05).
Comparison of serum H2S,
NSE and S100β concentrations and NDS after CPR
As presented in Fig.
1, the differences in serum H2S, NSE and S100β
concentrations and NDS before and after CPR were statistically
significant (F=223.496, 111.667, 30.194 and 7.318; P<0.001,
<0.001, <0.001 and 0.001, respectively). In the control
group, the H2S concentration gradually increased
following CA, peaking at 4 h after CPR, then reduced over time. The
H2S concentration in the NaHS group increased after CPR
while the H2S concentration in the hydroxylamine group
reduced after CPR. Significant differences were detected in
H2S levels among the three groups at each time point
(P<0.05). Serum concentrations of NSE and S100β in all animals
gradually increased and peaked respectively at 24 and 4 h,
respectively, after CPR prior to declining. The peak values of the
two parameters were lowest in the NaHS group and highest in the
hydroxylamine group. NSE and S100β concentrations in the NaHS group
were significantly reduced compared with those in the hydroxylamine
group at 4, 12, 24 and 72 h after CA/CPR (P<0.05), but not at
168 h. Following CA/CPR, NDS values reduced significantly, with the
lowest values observed at 4 h, followed by a trend of gradual
increase. The range of NDS decline was the lowest in the NaHS group
and was largest in the hydroxylamine group following CPR. NDS
values from the NaHS group were significantly higher compared with
those in the hydroxylamine group at 4, 12 and 24 h after CA/CPR
(P<0.05).
BWT and the prehensile traction
test
As presented in Fig.
2 the scores of the beam walking and prehensile traction tests
suggest significant differences among the three groups (F=5.503 and
3.246; P=0.007 and 0.046, respectively). After CA/CPR, the scores
in all groups declined and reached the lowest value at 3 days.
Among the three groups, scores in the hydroxylamine group exhibited
the most marked decline at 3 days; however, the scores subsequently
recovered. At all time points after CPR conduction, the NaHS group
exhibited the highest score, whereas the hydroxylamine group
presented significantly reduced scores (P<0.05).
Morris water maze test
As displayed in Fig.
3, in the hidden platform test, there were significant
differences in escape latency, swimming distance and speed among
the three groups (F=12.289, 3.712 and 17.441; P=0.000, 0.031 and
<0.001, respectively). At all time points after CPR, the NaHS
group exhibited the shortest escape latency and swimming distance,
and highest swimming speed, whereas the hydroxylamine group
displayed the longest escape latency and swimming distance, and
lowest swimming speed. As shown in Table
I, in the probe test, the swimming distance and number of
passings of the platform differed significantly among the groups
(P<0.05). The NaHS group exhibited the longest swimming distance
and the highest number of passings of the platform, whereas the
hydroxylamine group had the shortest swimming distance and the
least number of passings of the platform. In the visible platform
test, no significant differences were observed in escape latency
among the three groups (F=1.131, P=0.330).
 | Table I.Comparison of results from the Morris
water maze probe test and visible platform test after
cardiopulmonary resuscitation (mean ± standard deviation). |
Table I.
Comparison of results from the Morris
water maze probe test and visible platform test after
cardiopulmonary resuscitation (mean ± standard deviation).
|
| Probe test
| Visible platform
test
|
|---|
| Groups | Distance (cm) | Platform passing
(n) | Escape latency
(sec) | Swimming speed
(cm/sec) |
|---|
| NaHS |
5,329.6±540.0a,b |
7.6±2.0a,b | 5.8±2.3 |
44.7±4.3a,b |
| Hydroxylamine | 4,070.4±500.9 | 3.4±1.9 | 6.4±2.5 | 36.4±3.9 |
| Control | 4,835.8±700.7 | 5.1±2.2 | 5.3±2.4 | 40.9±5.1 |
Apoptosis of neurons in the
hippocampal region
All rats that underwent CA presented with numerous
TUNEL-positive pyramidal neurons in the CA1 region of the
hippocampus at 7 days after CPR. Significantly reduced numbers of
TUNEL positive neurons were observed in the NaHS group compared
with the hydroxylamine group (Fig.
4). Concomitantly, significantly reduced caspase-3 expression
levels (Figs. 5 and 6) and increased Bcl-2 (Figs. 6 and 7) expression levels were observed in the
NaHS group compared with the hydroxylamine and control groups. No
significant differences were detected in the expression levels of
caspase-3 and Bcl-2 between the hydroxylamine and control
groups.
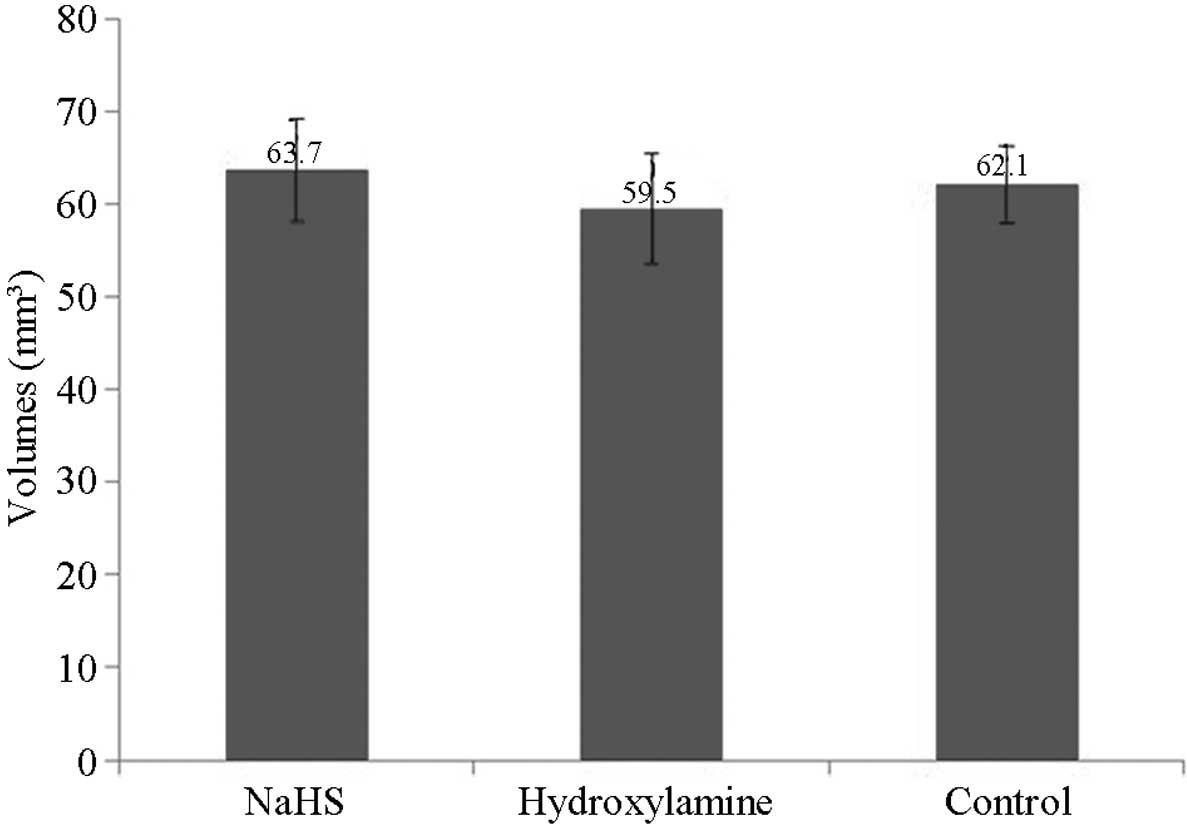
Stereological analysis of the
hippocampus
Stereological analyses detected no significant
differences in the volume of the hippocampus among the groups at 7
days after CPR (P>0.05; Fig.
8).
Discussion
The results of the present study indicate that the
serum H2S concentration in rats of the NaHS group after
CPR was significantly elevated, whereas the serum H2S
concentration in rats treated with hydroxylamine was reduced,
suggesting that exogenous treatments are able to alter
H2S levels in vivo. Furthermore, NaHS treatment
attenuates neuronal injury and improves neural functional
performance, whereas hydroxylamine exaggerates neuronal injury and
exacerbates learning and memory problems. NaHS treatment markedly
increases Bcl-2 expression levels and reduces caspase-3 expression.
The present results suggest that H2S may possess
potential therapeutic value for brain injury following CA.
NSE is a specific enzyme located primarily in brain
neurons and neuroendocrine cells, and is involved in glycolysis.
Following CA/CPR, NSE may be released from ischemically injured
neurons and traverse the blood brain barrier into the circulation;
therefore, serum NSE levels reflect the severity of brain damage
after CA and indicate disease outcome (27,28). The
calcium binding protein S100β is an axonal growth factor that is
predominantly produced and secreted by glial cells, and is widely
expressed in nervous tissue. Serum S100β levels are used as
biomarkers for disease progression after CA/CPR and neurological
prognosis (29) due to their
regularity of alteration and marked correlation with the severity
of the injury (30). In the present
study, the serum concentrations of NSE and S100β gradually
increased and peaked at 24 or 4 h, respectively, after CA/CPR. In
addition, serum NSE and S100β concentrations reduced or increased
according to the H2S levels in serum, suggesting that
H2S therapy may be able to reduce rat brain damage
following CA to certain extent. Compared with NSE and S100β, NDS is
able to more directly indicate the extent of cerebral function
damage after CPR, and to determine the effectiveness of treatment
(19,31,32).
Therefore, NDS was also evaluated in the present study. The NDS
score reduced significantly in rats following CA/CPR. However, the
NDS score of NaHS-treated rats was higher that that of rats treated
with hydroxylamine at 24 h after CPR, indicating a protective
effect against brain damage after CA.
Neural behavior is a crucial function of the body,
which controls and coordinates the body's normal activities, in
addition to being influenced and regulated by the state of other
organ systems. Following CPR, the body may not display clear signs
and symptoms of the neurological state, but patients may present
with abnormal memory, sensory, motor and cognitive functions.
Therefore, neurobehavioral examination is frequently a key index
for evaluating brain functional status and prognosis after CPR
(33–35). The Morris water maze experiment is a
classical neurobehavioral test, which includes a hidden platform
test, space exploration test and visible platform test. The Morris
water maze is designed to allow animals to learn to search for a
hidden platform under water, and by analyzing the time and path
taken to search for the platform, animal memory function may be
assessed. The Morris water maze allows the effective detection of
rat spatial learning and memory abilities, which involves the
medial temporal lobe limbic region including the hippocampus
(36). In the present study, the
spatial learning and memory ability of the rats reduced following
CA, but subsequently recovered to a certain extent. In the hidden
platform test, the escape latency of the NaHS group was lower than
that of the control group at every time point after CPR; however,
in the hydroxylamine group the escape latency and swimming distance
increased after CPR. In the space exploration experiments, the NaHS
group displayed a significant increase in the number of passings of
the platform, while the hydroxylamine group exhibited a significant
reduction. This indicated that improving body levels of
H2S led to improved rat spatial learning and memory
ability after CPR, and reducing H2S levels may result in
delayed recovery of spatial learning and memorizing abilities.
The BWT is used to detect whether the established
operant conditioning reflex is impaired after injury in rats. The
BWT is frequently used to assess whether there are impairments in
the functions of dynamic balance, motor coordination, learning and
memorizing abilities (37,38). The prehensile traction test is an
index for evaluating rat forepaw gripping power, muscle strength,
balance and motor function (24,39,40). In
the present study, the scores for the beam walking and prehensile
traction testing reduced significantly in the rats after CA, and
increased gradually over the 7 days after CPR. A comparison of the
results in these three groups showed that the performances of the
NaHS-treated rats were the most improved, while the hydroxylamine
group displayed the worst performance. This indicated that
increasing H2S levels improved the balance, coordination
and muscle strength of rats after CPR, and reducing H2S
levels delayed the recovery of motor ability.
The results of the neural behavior and function
analysis are consistent with previous studies, in which Knapp et
al reported that the administration of Na2S reduces
sensorimotor deficits 72 h after CA/CPR in rats (15) and Kida et al found that
Na2S improves neurological function at 96 h after CA and
CPR (13). In contrast to previous
studies involving rodents, Derwall et al observed in a
porcine model that high-dose Na2S (1 mg/kg) bolus
followed by infusion at 1 mg/kg/h for 2 h did not
improve neurological outcomes (14).
This may be attributable to the hypothesis that a lower
concentration of H2S exerts a protective effect on cells
while higher levels of H2S exposure lead to cytotoxicity
(41). In the present study, 14
µmol/kg/day NaHS was intraperitoneally administered, and it has
previously been confirmed that the protective effect against
neuronal injury conferred by NaHS is dose-dependent (42). In addition to its function as an
inhibitor of CBS, hydroxylamine can be metabolized to NO (43), which may be neurotoxic and
contributes to neuronal damage (44).
In the present study, levels of neuronal apoptosis
in the hippocampus were assessed at 7 days after CPR, and it was
observed that in response to the elevated levels of serum
H2S in the NaHS group, the apoptotic index of pyramidal
neurons in the hippocampal CA1 region and the expression level of
the pro-apoptotic protein caspase-3 appeared to reduce, while the
expression of the anti-apoptotic protein Bcl-2 increased. By
contrast, in response to the reduced levels of serum H2S
in the hydroxylamine group, the apoptosis index of pyramidal
neurons and caspase-3 expression levels in the CA1 region
increased, whereas Bcl-2 expression levels decreased. This result
suggests that increased endogenous H2S levels may
inhibit neuronal apoptosis, whereas inhibiting endogenous
H2S production may promote apoptosis. However, this is
contradictory to the study by Knapp et al, in which the
administration of Na2S failed to significantly reduce
the number of TUNEL-positive cells and caspase activity in the CA1
region of the hippocampus 7 days after CPR (15). This may be attributable to the
difference in potentials of the H2S donors that were
used and the short infusion time (0.5 mg/kg, 1 min prior to the
initiation of CPR, followed by a continuous infusion of
Na2S for 6 h, 1 mg/kg/h) in the previous study.
In conclusion, the in vivo modulation of
H2S levels may influence the occurrence and development
of brain damage in rats following CA. After ROSC, elevating the
H2S levels via a therapeutic intervention may improve
neural function damage in rats that have undergone CPR.
The present study has certain limitations. i) It was
observed that certain doses of NaHS appeared to reduce the
biochemical markers of nerve injury, improve nervous function
defect score, improve neurobehavioral symptoms and reduce neuronal
apoptosis in hippocampal tissue. However, these indices in the
hydroxylamine group exhibited adverse changes to various degrees.
No significant differences were observed between the hydroxylamine
and saline control groups when multiple level comparisons were
performed. ii) Theoretically, as cellular apoptosis increases,
hippocampal volume is expected to exhibit a certain degree of
atrophy; however, no differences in hippocampal volume were
observed among the groups in the current study. iii) There were no
obvious increases in mortality rate following ROSC, which is the
primary index for evaluating the effectiveness of CPR. As for the
reason for this, as only a single dose level was used in this
experiment, it is hypothesized that there may be an optimal
effective dose range of H2S. iv) Body temperature, heart
rate, respiratory rate and blood pressure were measured during the
experiment. However, all animals were placed on a thermostatic
table 4 h after ROSC and the body temperature was maintained at
37.5±0.2°C. From 5 h after ROSC to the end point of observation, a
heat lamp was additionally used for the rats with poor status and a
body temperature <36.5°C. Heart rate and the respiratory rate
are directly associated with body temperature. Furthermore,
adrenalin affects heart rate and blood pressure in the early stages
following ROSC. Blood pressure measurements may differ
significantly between these two methods. In addition, mechanical
ventilation may effect the respiratory rate. Thus, although the
physiological indices were maintained as consistently as possible
during the experiment, the above factors were not excluded. Thus,
the systemic detection and comparison of certain physiological
indices were not performed. The differences between these indices
may have affected the analysis of the experimental results. v) The
scale of the present study was relatively small, which may have
resulted in experimental bias.
Acknowledgements
The present study was supported by grants from
Medical innovation project of Fujian Province, China (no.
2011-CXB-31) and the social development research project of Xiamen,
China (no. 3502Z20114006, 3502Z20124042 and 3502Z20134003). The
funding source had no role in the study design, the collection and
interpretation of the data, writing of the report, or decision to
submit the paper for publication. The authors would like to thank
the staff of the Emergency Department of the First Affiliated
Hospital of Xiamen University and the Key Laboratory on Assisted
Circulation of Ministry of Health of Sun Yat-Sen University for
their excellent technical assistance and constructive criticism.
The authors also thank Professor Wen Jie and Dr Lin Gui-ping for
their valuable help.
References
|
1
|
Lemiale V, Dumas F, Mongardon N,
Giovanetti O, Charpentier J, Chiche JD, Carli P, Mira JP, Nolan J
and Cariou A: Intensive care unit mortality after cardiac arrest,
The relative contribution of shock and brain injury in a large
cohort. Intensive Care Med. 39:1972–1980. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McCook O, Radermacher P, Volani C, Asfar
P, Ignatius A, Kemmler J, Möller P, Szabó C, Whiteman M, Wood ME,
et al: H2S during circulatory shock: Some unresolved questions.
Nitric Oxide. 41:48–61. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Luo Y, Yang X, Zhao S, Wei C, Yin Y, Liu
T, Jiang S, Xie J, Wan X, Mao M, et al: Hydrogen sulfide prevents
OGD/R-induced apoptosis via improving mitochondrial dysfunction and
suppressing an ROS-mediated caspase-3 pathway in cortical neurons.
Neurochem Int. 63:826–831. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang Y, Jia J, Ao G, Hu L, Liu H, Xiao Y,
Du H, Alkayed NJ, Liu CF and Cheng J: Hydrogen sulfide protects
blood-brain barrier integrity following cerebral ischemia. J
Neurochem. 129:827–838. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang M, Shan H, Chang P, Wang T, Dong W,
Chen X and Tao L: Hydrogen sulfide offers neuroprotection on
traumatic brain injury in parallel with reduced apoptosis and
autophagy in mice. PLoS One. 9:e872412014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jiang X, Huang Y, Lin W, Gao D, Fei Z,
Chen X and Tao L: Protective effects of hydrogen sulfide in a rat
model of traumatic brain injury via activation of mitochondrial
adenosine triphosphate-sensitive potassium channels and reduction
of oxidative stress. J Surg Res. 184:e27–e35. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang M, Shan H, Wang T, Liu W, Wang Y,
Wang L, Zhang L, Chang P, Dong W, Chen X, et al: Dynamic change of
hydrogen sulfide after traumatic brain injury and its effect in
mice. Neurochem Res. 38:714–725. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Han Y, Qin J, Bu DF, Chang XZ, Yang ZX and
Du JB: Gamma-aminobutyric acid B receptor regulates the expression
of hydrogen sulfide/cystathionine-beta-synthase system in recurrent
febrile seizures. Zhongguo Dang Dai Er Ke Za Zhi. 8:141–143.
2006.(In Chinese). PubMed/NCBI
|
|
9
|
Han Y, Qin J, Chang X, Yang Z, Bu D and Du
J: Modulating effect of hydrogen sulfide on gamma-aminobutyric acid
B receptor in recurrent febrile seizures in rats. Neurosci Res.
53:216–219. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Markova J, Hudecova S, Soltysova A, Sirova
M, Csaderova L, Lencesova L, Ondrias K and Krizanova O:
Sodium/calcium exchanger is upregulated by sulfide signaling, forms
complex with the β1 and β3 but not β2 adrenergic receptors, and
induces apoptosis. Pflugers Arch. 466:1329–1342. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kimura Y and Kimura H: Hydrogen sulfide
protects neurons from oxidative stress. FASEB J. 18:1165–1167.
2004.PubMed/NCBI
|
|
12
|
Minamishima S, Bougaki M, Sips PY, Yu JD,
Minamishima YA, Elrod JW, Lefer DJ, Bloch KD and Ichinose F:
Hydrogen sulfide improves survival after cardiac arrest and
cardiopulmonary resuscitation via a nitric oxide synthase
3-dependent mechanism in mice. Circulation. 120:888–896. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kida K, Minamishima S, Wang H, Ren J,
Yigitkanli K, Nozari A, Mandeville JB, Liu PK, Liu CH and Ichinose
F: Sodium sulfide prevents water diffusion abnormality in the brain
and improves long term outcome after cardiac arrest in mice.
Resuscitation. 83:1292–1297. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Derwall M, Westerkamp M, Löwer C,
Deike-Glindemann J, Schnorrenberger NK, Coburn M, Nolte KW, Gaisa
N, Weis J, Siepmann K, et al: Hydrogen sulfide does not increase
resuscitability in a porcine model of prolonged cardiac arrest.
Shock. 34:190–195. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Knapp J, Heinzmann A, Schneider A, Padosch
SA, Böttiger BW, Teschendorf P and Popp E: Hypothermia and
neuroprotection by sulfide after cardiac arrest and cardiopulmonary
resuscitation. Resuscitation. 82:1076–1080. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Qu K, Lee SW, Bian JS, Low CM and Wong PT:
Hydrogen sulfide, Neurochemistry and neurobiology. Neurochem Int.
52:155–165. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lin JY, Liao XX, Li H, Wei HY, Liu R, Hu
CL, Huang GQ, Dai G and Li X: Model of cardiac arrest in rats by
transcutaneous electrical epicardium stimulation. Resuscitation.
81:1197–1204. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Idris AH, Becker LB, Ornato JP, Hedges JR,
Bircher NG, Chandra NC, Cummins RO, Dick W, Ebmeyer U, Halperin HR,
et al: Writing Group: Utstein-style guidelines for uniform
reporting of laboratory CPR research. A statement for healthcare
professionals from a task force of the American Heart Association,
the American College of Emergency Physicians, the American College
of Cardiology, the European Resuscitation Council, the Heart and
Stroke Foundation of Canada, the Institute of Critical Care
Medicine, the Safar Center for Resuscitation Research, and the
Society for Academic Emergency Medicine. Circulation. 94:2324–2336.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Neumar RW, Bircher NG, Sim KM, Xiao F,
Zadach KS, Radovsky A, Katz L, Ebmeyer E and Safar P: Epinephrine
and sodium bicarbonate during CPR following asphyxial cardiac
arrest in rats. Resuscitation. 29:249–263. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lin JY, Wei HY, Li H, Li X, Liu R, Hu CL,
Huang GQ, Dai G and Liao XX: Change of hydrogen sulfide content in
serum of rats after cardiopulmonary resuscitation. Xi Bao Yu Fen Zi
Mian Yi Xue Za Zhi. 26:363–365. 2010.(In Chinese). PubMed/NCBI
|
|
21
|
Morris R: Developments of a water-maze
procedure for studying spatial learning in the rat. J Neurosci
Methods. 11:47–60. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vorhees CV and Williams MT: Morris water
maze: Procedures for assessing spatial and related forms of
learning and memory. Nat Protoc. 1:848–858. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Feeney DM, Gonzalez A and Law WA:
Amphetamine, haloperidol, and experience interact to affect rate of
recovery after motor cortex injury. Science. 217:855–857. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Plaisier F, Bastide M, Ouk T, Pétrault O,
Laprais M, Stolc S and Bordet R: Stobadine-induced hastening of
sensorimotor recovery after focal ischemia/reperfusion is
associated with cerebrovascular protection. Brain Res.
1208:240–249. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gundersen HJ, Bendtsen TF, Korbo L,
Marcussen N, Møller A, Nielsen K, Nyengaard JR, Pakkenberg B,
Sørensen FB, Vesterby A, et al: Some new, simple and efficient
stereological methods and their use in pathological research and
diagnosis. APMIS. 96:379–394. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li J, Han B, Ma X and Qi S: The effects of
propofol on hippocampal caspase-3 and Bcl-2 expression following
forebrain ischemia-reperfusion in rats. Brain Res. 1356:11–23.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fink EL, Berger RP, Clark RS, Watson RS,
Angus DC, Richichi R, Panigrahy A, Callaway CW, Bell MJ and
Kochanek PM: Serum biomarkers of brain injury to classify outcome
after pediatric cardiac arrest. Crit Care Med. 42:664–674. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sulaj M, Saniova B, Drobna E and
Schudichova J: Serum neuron specific enolase and malondialdehyde in
patients after out-of-hospital cardiac arrest. Cell Mol Neurobiol.
29:807–810. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Stammet P, Wagner DR, Gilson G and Devaux
Y: Modeling serum level of s100β and bispectral index to predict
outcome after cardiac arrest. J Am Coll Cardiol. 62:851–858. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kleindienst A, Hesse F, Bullock MR and
Buchfelder M: The neurotrophic protein S100B: Value as a marker of
brain damage and possible therapeutic implications. Prog Brain Res.
161:317–325. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Drabek T, Stezoski J, Garman RH, Wu X,
Tisherman SA, Stezoski SW, Fisk JA, Jenkins L and Kochanek PM:
Emergency preservation and delayed resuscitation allows normal
recovery after exsanguination cardiac arrest in rats, A feasibility
trial. Crit Care Med. 35:532–537. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Popp E, Padosch SA, Vogel P, Schäbitz WR,
Schwab S and Böttiger BW: Effects of intracerebroventricular
application of brain-derived neurotrophic factor on cerebral
recovery after cardiac arrest in rats. Crit Care Med. 32(Suppl):
S359–S365. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cronberg T, Lilja G, Rundgren M, Friberg H
and Widner H: Long-term neurological outcome after cardiac arrest
and therapeutic hypothermia. Resuscitation. 80:1119–1123. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Meert KL, Donaldson A, Nadkarni V, Tieves
KS, Schleien CL, Brilli RJ, Clark RS, Shaffner DH, Levy F, Statler
K, et al: Pediatric Emergency Care Applied Research Network:
Multicenter cohort study of in-hospital pediatric cardiac arrest.
Pediatr Crit Care Med. 10:544–553. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Schreckinger M, Geocadin RG, Savonenko A,
Yamashita S, Melnikova T, Thakor NV and Hanley DF: Long-lasting
cognitive injury in rats with apparent full gross neurological
recovery after short-term cardiac arrest. Resuscitation.
75:105–113. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Garthe A and Kempermann G: An old test for
new neurons: Refining the Morris water maze to study the functional
relevance of adult hippocampal neurogenesis. Front Neurosci.
7:632013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tahamtan M, Allahtavakoli M, Abbasnejad M,
Roohbakhsh A, Taghipour Z, Taghavi M, Khodadadi H and Shamsizadeh
A: Exercise preconditioning improves behavioral functions following
transient cerebral ischemia induced by 4-vessel occlusion (4-VO) in
rats. Arch Iran Med. 16:697–704. 2013.PubMed/NCBI
|
|
38
|
Tamakoshi K, Ishida A, Takamatsu Y,
Hamakawa M, Nakashima H, Shimada H and Ishida K: Motor skills
training promotes motor functional recovery and induces
synaptogenesis in the motor cortex and striatum after intracerebral
hemorrhage in rats. Behav Brain Res. 260:34–43. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang H, Sun R, Liu XY, Shi XM, Wang WF,
Yu LG and Guo XL: A tetramethylpyrazine piperazine derivate CXC137
prevents cell injury in SH-SY5Y cells and improves memory
dysfunction of rats with vascular Dementia. Neurochem Res.
39:276–286. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Thal SC, Mebmer K, Schmid-Elsaesser R and
Zausinger S: Neurological impairment in rats after subarachnoid
hemorrhage - a comparison of functional tests. J Neurol Sci.
268:150–159. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Szabó C: Hydrogen sulphide and its
therapeutic potential. Nat Rev Drug Discov. 6:917–935. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang LM, Jiang CX and Liu DW: Hydrogen
sulfide attenuates neuronal injury induced by vascular dementia via
inhibiting apoptosis in rats. Neurochem Res. 34:1984–1992. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
DeMaster EG, Raij L, Archer SL and Weir
EK: Hydroxylamine is a vasorelaxant and a possible intermediate in
the oxidative conversion of L-arginine to nitric oxide. Biochem
Biophys Res Commun. 163:527–533. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chao CC, Hu S, Molitor TW, Shaskan EG and
Peterson PK: Activated microglia mediate neuronal cell injury via a
nitric oxide mechanism. J Immunol. 149:2736–2741. 1992.PubMed/NCBI
|