Introduction
Ultraviolet (UV) radiation and oxidative stress may
lead to the formation of cataracts; thus, a number of appropriate
mechanisms and relevant theories have been proposed to explain the
formation of lens opacities (1,2). Eyes
have a certain capacity to resist damage; however, for cataract
patients, functional defects in the defense against UV and
oxidative stress may enable damage factors to cause epithelial
apoptosis in the crystalline lens more easily, which may be the
cytological basis underlying the formation of cataracts (3). Generally, UV exposure may lead to a
series of complex acute or chronic reactions and produce a surplus
reactive oxygen species, including hydrogen peroxide and peroxide
ions (4,5). Degradation, cross-linking and
polymerization of proteins in the crystalline lens may occur if the
reactive oxygen species are not eliminated by reduction and
accumulate substantially (6). In
addition, UV radiation may lead to DNA damage, including the
formation of base-free loci and pyrimidine dimers, which severely
affect DNA duplication and transcription. Subsequently, DNA damage
may be repaired to enable the cells to survive continuously, while
programed cell death or apoptosis may be utilized to eliminate the
defective individual cells; thus, maintaining the structural and
functional completeness of the crystalline lens (7,8).
However, the intrinsic physiological functions of the crystalline
lens are likely to be affected, resulting in a series of
pathological changes if apoptosis occurs to a number of cells, or
if the apoptotic cells can not be offset by proliferation (9,10). UV
radiation is an inevitable environmental factor in daily lives and
is closely associated with the onset of ophthalmic diseases. Thus,
research on UV-induced apoptosis in the crystalline lens should
provide more understanding into the pathogenesis of cataracts.
Materials and methods
Materials
A CO2 incubator was purchased from
Shanghai Aoxin Technology Instrument Co., Ltd. (Shanghai, China);
the chemical luminous gel-imaging system was purchased from Hach
Company (Loveland, CO, USA); and the cryogenic desk centrifuge was
purchased from Beijing Jingli Centrifuge Co., Ltd. (Beijing,
China).
Laboratory Bcl-2 knockout (K.O) mice and normal
control mice were provided by Shanghai SLAC Laboratory Animal Co.,
Ltd. (Shanghai, China). The present study was approved by the
Ethics Committee of The Second Hospital of Jilin University
(Changchun, China) and was performed according to the Care and Use
of Laboratory Animals Guidelines of China.
Grouping
In total, 12 normal control mice served as the
control group and 12 Bcl-2 K.O mice served as the experimental
group. The mice were aged ~12 weeks in the two groups. One eye of
each mouse was selected randomly for UV irradiation, while the
other eye was covered and used as the control. Thus, the mice were
further divided into four groups, which included the normal-nonUV,
normal-UV, Bcl-2 K.O-nonUV and Bcl-2 K.O-UV groups.
Irradiation of the crystalline lens by
UV
In the mice, one eye was exposed to the UV radiator
(SP3-250D; Ushio Electric Co., Tokyo, Japan), while the other eye
was covered with a lead sheet patch. The total UV radiation dosage
was 8 kJ/m2 and the radiation time was 15 min. UV
radiation was produced by the UV radiator. The radiation level was
determined using an energy meter (GTFP200A7J; Meba Electric Co.,
Ltd., CA, USA) at the cornea plane, and the UV spectrum was
recorded using a spectrometer (F4600; Hitachi, Ltd., Tokyo, Japan).
The UV spectra ranged between 280 and 320 nm.
Test sample collection and observation
of the crystalline lens macrostructure
Mice were anesthetized with diethyl ether and
sacrificed by cervical fracture following irradiation with UV for
24 h. The two eyeballs were enucleated (optic nerve with an
approximate length of 2 mm was retained). Samples of the mouse
crystalline lens were microscopically obtained for reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
caspase-3 activity determination. The samples were also weighed.
The crystalline lens was washed three times with
diethylpyrocarbonate-phosphate-buffered saline (PBS) for use in
subsequent experiments.
Tissue fixation and preparation of the
paraffin sections
Glacial acetic acid solution, formaldehyde solution,
normal saline solution and 75% ethanol solution were mixed in the
ratio 1:2:7:10 to form a fixation solution. The entire eyeball was
immersed in the fixation mixture and fixed for 0.5 h at room
temperature. Once the eyeball surface had become hard, the eyeballs
were injected with the fixation solution from the posterior chamber
and fixed for 2 h. Samples were incised from the upper and lower
sections using a sharp blade or ophthalmological scissors, and
subsequently fixed for 2 h at room temperature. The samples were
then immersed in 10% neutral formaldehyde and fixed for a further
20 h. Following a series of dehydrations using gradient
concentrations of ethanol, the samples were blocked with
dimethylbenzene solution, embedded in paraffin at 60°C and cut into
4 µm sections.
Terminal deoxynucleotidyl transferase
dUTP nick-end labeling (TUNEL) assay
Following drop-wise addition of 50 µ1 alkali Triton
X-100 (0.1%; prepared by 0.1% sodium citrate), the samples were
incubated for 8 min at room temperature, and washed twice with PBS
for 5 min. Next, 50 µl H2O2 (3%) was added in
a drop-wise manner and the samples were left for 10 min at room
temperature, followed by two washes with PBS for 5 min. Following
drop-wise addition of 50 µl TUNEL reaction solution, the samples
were incubated for 2 h at 37°C in the dark, and washed twice with
PBS for 5 min. The staining results were observed microscopically
following subsequent procedures, as described in the relevant
literature (11).
Determination of caspase-3
activity
An appropriate volume of radioimmunoprecipitation
assay lysis buffer was added according to the mass and volume of
each specimen. The samples were placed on ice, pipetted into a
single-cell suspension, and subsequently placed on ice for a
further 5 min. The samples were centrifuged for 10 min at 10,000 ×
g at 4°C. Protein extract was obtained by separating the
supernatant and a caspase-3 detection kit was used to determine the
activity of caspase-3, as described in a previous study (12).
Statistical analysis
SPSS l2.0 statistical analysis software (SPSS, Inc.,
Chicago, IL, USA) was used for the statistical analyses, and the
data are expressed as the mean ± standard deviation. An independent
t-test was conducted for the comparison of mean values between
samples, where P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression of Bcl-2 in the crystalline
lens of the normal mice
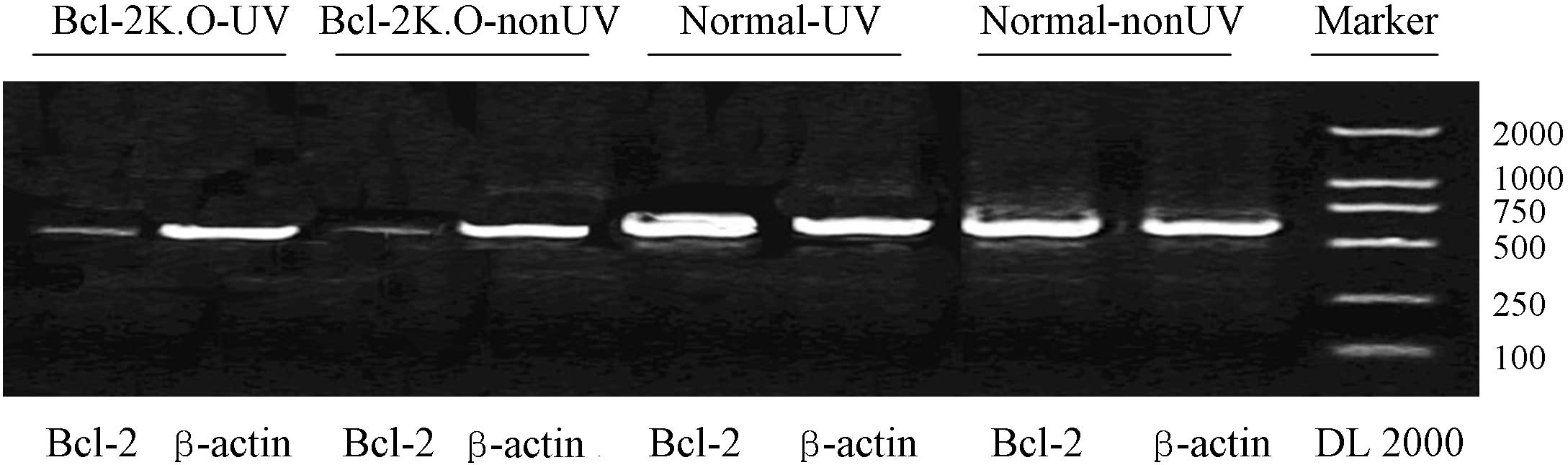
Total RNA was extracted from the crystalline lens of
mice by using the Total RNA Extraction kit (Tiangen Biotech Co.,
Ltd., Beijing, China). Total RNA (0.2 µg) was subjected to RT-qPCR
and the amplification products were subjected to agarose gel
electrophoresis. The results demonstrated that the mRNA expression
level of Bcl-2 was similar to that of the control gene, β-actin, in
the normal-UV and normal-nonUV groups. Thus, a considerable level
of Bcl-2 transcription was observed in the crystalline lens of the
adult mice (Fig. 1).
UV radiation does not affect Bcl-2
expression
A statistically significant difference was not
observed in the expression levels of Bcl-2 between the normal-UV
and normal-nonUV groups (Fig. 1),
indicating that the UV irradiation did not trigger Bcl-2 mRNA
transcription. In addition, UV radiation did not induce any changes
in the mRNA expression of Bcl-2 in the Bcl-2 K.O groups (Fig. 1).
No significant changes occur to the
macrostructure of the crystalline lens
UV radiation did not cause lens opacities in the
various specimens of the normal and Bcl-2 K.O mice. Furthermore, no
characteristic changes of cell apoptosis, including hydrofracturing
and vacuoles, were detected. Thus, the results indicated that the
UV irradiation dose used in the experiment was insufficient to
cause lens opacities prior to the sacrifice of the mice.
UV radiation induces apoptosis in the
crystalline lens
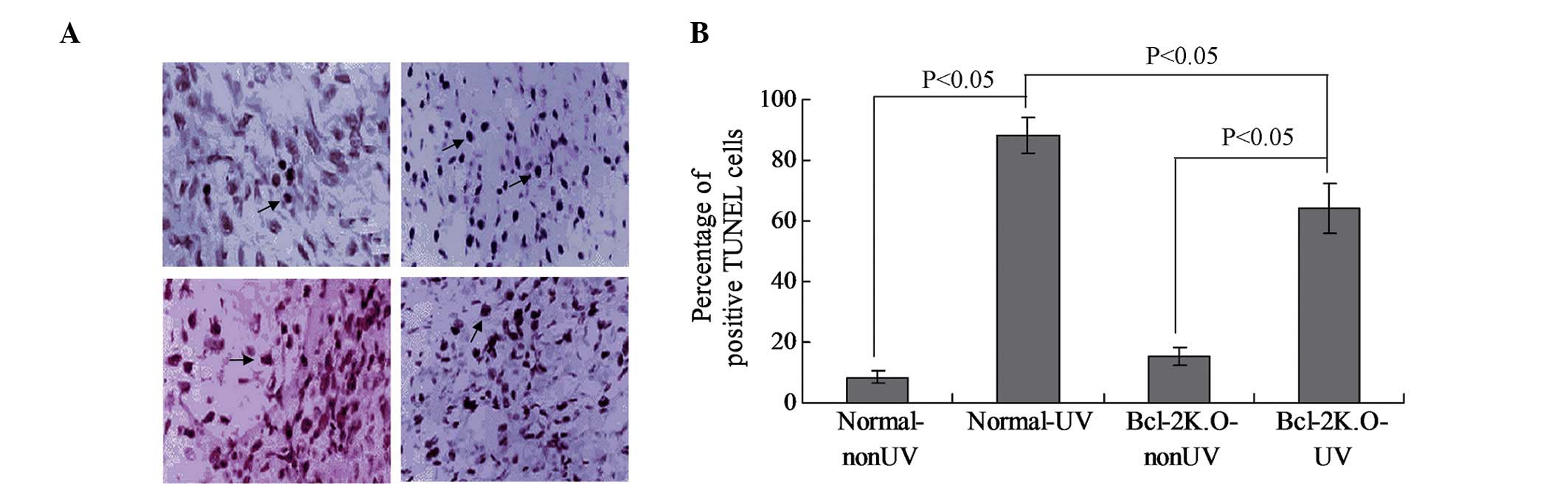
A TUNEL assay kit was used to investigate the rate
of apoptosis in the tissue samples of the mouse crystalline lens of
the various groups. The results of the present study indicated that
large doses of UV radiation were able to induce a significant level
of apoptosis in the epithelial cells of the crystalline lens
(Fig. 2). The apoptotic rate was
very low in the normal-nonUV group; however, a significant level of
apoptosis was observed in the normal-UV and Bcl-2 K.O-UV groups
(Fig. 2A; P<0.05). Furthermore,
the apoptosis rate in the Bcl-2 K.O-nonUV group was not significant
(Fig. 2B). Notably, the apoptotic
rate in the normal-UV group was significantly higher, as compared
with that of the Bcl-2 K.O-UV group (Fig. 2B; P<0.05).
Bcl-2 protein can elevate the
expression level of intracellular caspase-3
Caspase-3 is an important member in the family of
cysteine-aspartic acid proteolytic enzymes. The enzyme is able to
catalyze reactions of itself and other substrates, including
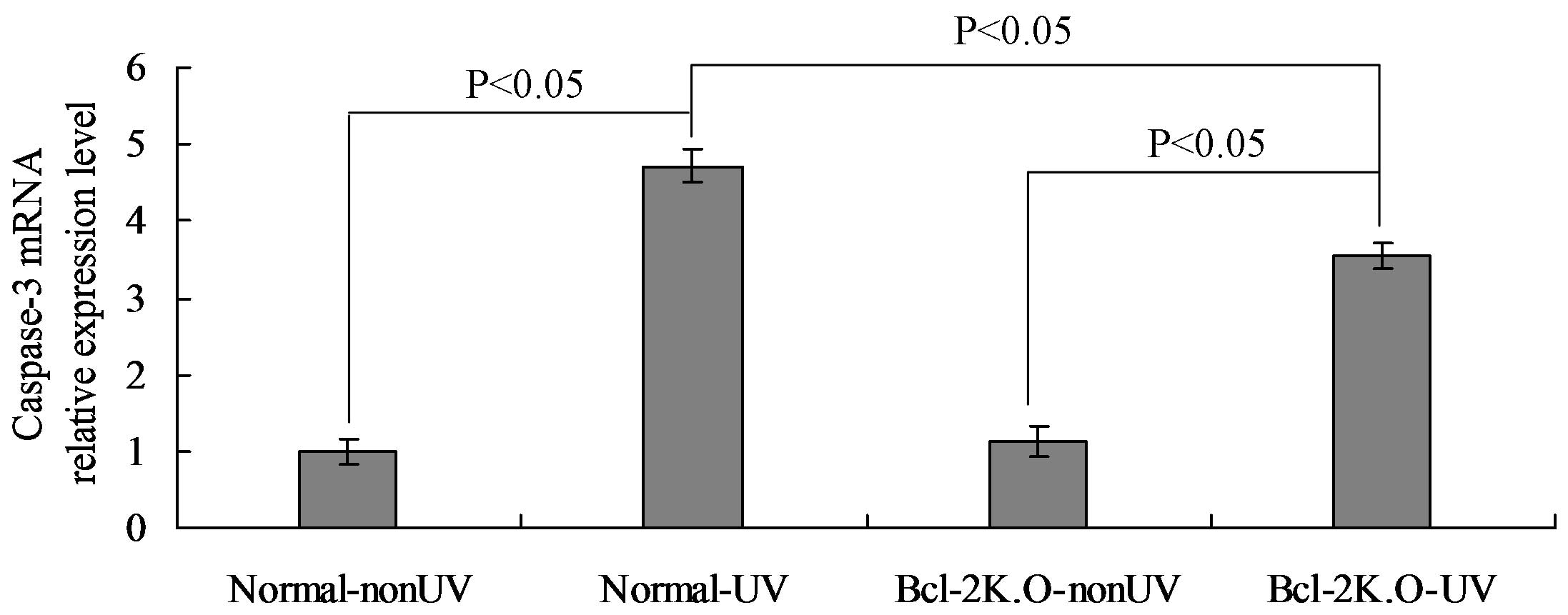
caspase-7. The mRNA expression of caspase-3 was quantitatively
determined using RT-qPCR analysis. The results indicated that the
mRNA expression of caspase-3 in the normal-UV group was
significantly higher compared with the normal-nonUV group
(P<0.05), while the mRNA expression in the Bcl-2 K.O-UV group
was also significantly higher compared with the Bcl-2 K.O-nonUV
group (P<0.05). In addition, the mRNA expression of caspase-3 in
the normal-UV group was significantly higher compared with that in
the Bcl-2 K.O-UV group (P<0.05), as demonstrated in Fig. 3.
Bcl-2 protein can elevate the activity
of caspase-3 protease
Further analysis of caspase-3 activity indicated
that UV radiation significantly increased the activity of the
proteolytic enzyme. In addition, caspase-3 activity levels were
significantly higher in the normal-UV group when compared with the
Bcl-2 K.O-UV group (P<0.05), under the same dose of UV radiation
(Fig. 4). Therefore, following the
determination of mRNA expression and the proteinase activity of
caspase-3, the results indicated that the expression of the Bcl-2
protein aggravates UV-induced apoptosis in the crystalline
lens.
Discussion
Previous studies have demonstrated that Bcl-2 mRNA
transcription is present in the mouse crystalline lens during
embryonic development, and the expression of Bcl-2 occurs following
the appearance of ganglion cells in the retina (13,14). The
expression of Bcl-2 has also been demonstrated in primary lens
fibers. Expression has not been detected in the epithelial cells at
the center of the anterior envelope of the crystalline lens;
however, transcription has been shown to initiate when these cells
move to the equator (15,16). The specificity of tissue localization
indicates that Bcl-2 is transcribed and expressed in the fibrocytes
of the epithelial and crystalline lens that are present in the
equator area. With a relatively active growth, Bcl-2 may
participate in a number of physiological processes associated with
development; for example, Bcl-2 may promote the expression of
important structural proteins in cells, or have a role in the
structural formation and functional maturation of the crystalline
lens (17,18). In the present study, the
transcriptional expression of Bcl-2 mRNA was detected in the
crystalline lens of adult mice, indicating that Bcl-2 may
participate in certain physiological processes of mature
crystalline lens tissues.
The results of the present study demonstrated that
Bcl-2 is capable of elevating the level of UV-induced apoptosis in
the crystalline lens; thus, the protein may play a certain
promoting role during apoptosis. In addition, the same result has
been confirmed at a cellular level in previous studies (19,20). The
promoting role may be due to the action of the Bcl-2 protein on a
certain factor in the known apoptosis pathway. Bcl-2 may also
affect the mechanism underlying apoptosis signaling transduction,
which has yet to be fully elucidated (21). The internal and external pathways of
apoptosis can activate the precursor of caspase-3, pro-caspase-3,
to reveal the activity of the proteolytic enzyme; therefore,
damaging the cellular structure via apoptosis and subsequently
forming a series of morphological changes in significance (22,23).
Caspase-3 is an important indicator of apoptosis,
and the activity of the enzyme presents the apoptosis capacity in a
numeric form in order to conduct quantitative research (24,25). In
the present study, the significant increase in caspase-3 activity
confirmed the positive role of Bcl-2 in apoptosis. In addition, the
experimental results demonstrated that UV radiation does not cause
any change in the expression levels of Bcl-2, indicating that Bcl-2
is the regulating object in DNA damage. Therefore, the effect of
the changes in the intrinsic protein level of Bcl-2 on apoptosis
can be excluded when comparing the difference in the apoptosis rate
in the crystalline lens between the group of K.O mice and the group
of normal control mice that had been subjected to the same UV
radiation conditions.
UV light is a component in solar radiation.
Individuals may be more or less exposed to UV radiation every day.
In particular, for eyes receiving external light for imaging, a
small-dose of UV radiation accompanies each healthy individual
during their lifetime. Thus, how to offset the damage of UV
radiation to human eyes is the premise for maintaining normal
visual functions. Bcl-2 may increase the sensitivity of cells in
the crystalline lens to UV radiation damage, allowing the abnormal
cells to be identified as early as possible and eliminated by
apoptosis to ensure that the functions of the crystalline lens are
not interfered by these abnormal cells. In this respect, Bcl-2 has
a positive significance in maintaining cellular ‘metabolism’.
However, excessive UV radiation or the repeated accumulation of
small doses of UV radiation may lead to extensive apoptosis and
further induce the occurrence of cataracts (26,27).
Therefore, the role of Bcl-2 in apoptosis appears to be a
contradictory with negative and positive aspects.
References
|
1
|
Galichanin K, Svedlund J and Söderberg P:
Kinetics of GADD45α, TP53 and CASP3 gene expression in the rat lens
in vivo in response to exposure to double threshold dose of UV-B
radiation. Exp Eye Res. 97:19–23. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xin G, Du J, Wang YT and Liang TT: Effect
of oxidative stress on heme oxygenase-1 expression in patients with
gestational diabetes mellitus. Exp Ther Med. 7:478–482.
2014.PubMed/NCBI
|
|
3
|
Hengerer FH, Conrad-Hengerer I, Buchner SE
and Dick HB: Evaluation of the Calhoun Vision UV Light Adjustable
Lens implanted following cataract removal. J Refract Surg.
26:716–721. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mesa R and Bassnett S: UV-B-induced DNA
damage and repair in the mouse lens. Invest Ophthalmol Vis Sci.
54:6789–6797. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Deng ZC, Zhao P, Cao C, Sun SF, Zhao F, Lu
CY and Ma HY: C-reactive protein as a prognostic marker in chronic
obstructive pulmonary disease. Exp Ther Med. 7:443–446.
2014.PubMed/NCBI
|
|
6
|
Kubo E, Hasanova N, Tanaka Y, Fatma N,
Takamura Y, Singh DP and Akagi Y: Protein expression profiling of
lens epithelial cells from Prdx6-depleted mice and their
vulnerability to UV radiation exposure. Am J Physiol Cell Physiol.
298:C342–C354. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kalay S, Oztekin O, Tezel G, Aldemir H,
Sahin E, Köksoy S, Akçakuş M and Oygur N: Role of immunoglobulin in
neuronal apoptosis in a neonatal rat model of hypoxic ischemic
brain injury. Exp Ther Med. 7:734–738. 2014.PubMed/NCBI
|
|
8
|
Varma SD, Hegde KR and Kovtun S:
UV-B-induced damage to the lens in vitro: Prevention by caffeine. J
Ocul Pharmacol Ther. 24:439–444. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Heo J, Lee BR and Koh JW: Protective
effects of epigallocatechin gallate after UV irradiation of
cultured human lens epithelial cells. Korean J Ophthalmol.
22:183–186. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Avetisov SE, Polunin GS, Sheremet NL,
Makarov IA, Fedorov AA, Karpova OE, Muranov KO, Dizhevskaia AK,
Soustov LV, Chelnokov EV, et al: Chaperon-like anticataract agents,
the antiaggregants of lens crystallin. Communication 4. Study of
the effect of a mixture of di- and tetrapeptides on a prolonged rat
model of UV-induced cataract. Vestn Oftalmol. 124:12–16. 2008.(In
Russian). PubMed/NCBI
|
|
11
|
Kernt M, Neubauer AS, Liegl R, Eibl KH,
Alge CS, Lackerbauer CA, Ulbig MW and Kampik A: Cytoprotective
effects of a blue light-filtering intraocular lens on human retinal
pigment epithelium by reducing phototoxic effects on vascular
endothelial growth factor-alpha, Bax, and Bcl-2 expression. J
Cataract Refract Surg. 35:354–362. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Galichanin K, Löfgren S, Bergmanson J and
Söderberg P: Evolution of damage in the lens after in vivo close to
threshold exposure to UV-B radiation: Cytomorphological study of
apoptosis. Exp Eye Res. 91:369–377. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gao J, Zhao L, Wang Y, Teng Q, Liang L and
Zhang J: Effect of limb ischemic preconditioning on myocardial
apoptosis-related proteins in ischemia-reperfusion injury. Exp Ther
Med. 5:1305–1309. 2013.PubMed/NCBI
|
|
14
|
Avetisov SE, Polunin GS, Sheremet NL,
Muranov KO, Makarov IA, Fedorov AA, Karpova OE and Ostrovskiĭ MA:
Search for chaperon-like anticataract agents, the antiaggregants of
lens crystallin. Communication 3. Possibilities of a follow-up of
caractogenesis processes on a prolonged rat model of UV-induced
cataract. Vestn Oftalmol. 124:8–12. 2008.(In Russian). PubMed/NCBI
|
|
15
|
Wu XH, Lu Y, Fang YW and Jiang YX: The
polyamidoamine-mediated inhibition of bcl-2 by small hairpin RNA to
induce apoptosis in human lens epithelial cells. Mol Vis. 18:74–80.
2012.PubMed/NCBI
|
|
16
|
Giblin FJ, Lin LR, Simpanya MF, Leverenz
VR and Fick CE: A Class I UV-blocking (senofilcon A) soft contact
lens prevents UVA-induced yellow fluorescence and NADH loss in the
rabbit lens nucleus in vivo. Exp Eye Res. 102:17–27. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Soustov LV, Chelnokov EV, Sapogova NV,
Bitiurin NM, Nemov VV, Karpova OE, Sheremet NL, Polunin GS,
Avetisov SE and Ostrovskiĭ MA: Like anticataract agents, the
antiaggregants of lens crystallin. Communication 2. Study of the
impact of chaperon-like (protective) activity of short-chain
peptides on the rate of UV-induced aggregation of betaL-crystallins
by eximer laser. Vestn Oftalmol. 124:6–8. 2008.(In Russian).
PubMed/NCBI
|
|
18
|
Jiang Q, Cao C, Zhou C, Song X, Healey S,
Kouttab N, Chu W, Xu A, Bi Z and Wan Y: Quercetin attenuates UV-
and H(2)O(2)-induced decrease of collagen type I in cultured human
lens epithelial cells. J Ocul Pharmacol Ther. 24:164–174. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Z and Wang Z: Effects of rapamycin on
expression of Bcl-2 and Bax in human lens epithelial cells and cell
cycle in rats. J Huazhong Univ Sci Technolog Med Sci. 31:555–559.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mafia K, Gupta R, Kirk M, Wilson L,
Srivastava OP and Barnes S: UV-A-induced structural and functional
changes in human lens deamidated alphaB-crystallin. Mol Vis.
14:234–248. 2008.PubMed/NCBI
|
|
21
|
Kernt M, Hirneiss C, Neubauer AS, Ulbig MW
and Kampik A: Coenzyme Q10 prevents human lens epithelial cells
from light-induced apoptotic cell death by reducing oxidative
stress and stabilizing BAX / Bcl-2 ratio. Acta Ophthalmol.
88:e78–e86. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pajer V, Tiboldi A, Bae N, Li K, Kang SU,
Hopp B, Kolozsvári L, Lubec G and Nógrádi A: The molecular
background of the differential UV absorbance of the human lens in
the 240–400 nm range. Photochem Photobiol. 89:856–863. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Basu S, Rajakaruna S, De Arcangelis A,
Zhang L, Georges-Labouesse E and Menko AS: α6 integrin
transactivates insulin-like growth factor receptor-1 (IGF-1R) to
regulate caspase-3-mediated lens epithelial cell differentiation
initiation. J Biol Chem. 289:3842–3855. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee HE, Choi ES, Shin JA, Lee SO, Park KS,
Cho NP and Cho SD: Fucoidan induces caspase-dependent apoptosis in
MC3 human mucoepidermoid carcinoma cells. Exp Ther Med. 7:228–232.
2014.PubMed/NCBI
|
|
25
|
Basu S, Rajakaruna S and Menko AS:
Insulin-like growth factor receptor-1 and nuclear factor κB are
crucial survival signals that regulate caspase-3-mediated lens
epithelial cell differentiation initiation. J Biol Chem.
287:8384–8397. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang TT and Xu GX: [Effect of cinobufagin
on the expressions of bcl-2 mRNA and bax mRNA and the proliferation
of lens epithelial cells]. Zhongguo Zhong Xi Yi Jie He Za Zhi.
29:915–917. 2009.(In Chinese). PubMed/NCBI
|
|
27
|
Lou JS, Chen XE, Zhang Y, Gao ZW, Chen TP,
Zhang GQ and Ji C: Photoprotective and immunoregulatory capacity of
ginsenoside Rg1 in chronic ultraviolet B-irradiated BALB/c mouse
skin. Exp Ther Med. 6:1022–1028. 2013.PubMed/NCBI
|