Introduction
Diabetes is a chronic progressive disease and the
most common endocrine disorder worldwide. According to projections
by the World Health Organization, the worldwide prevalence of
diabetes will reach a ~334 million individuals worldwide by the
2025 (1). Another study predicted
that the prevalence of diabetes in China alone will reach ~100
million individuals by 2030 (2).
Furthermore, 25.8% of these patients in China received treatment
for diabetes, and 39.7% of those treated had adequate glycemic
control. Currently, medical interventions, including diet,
exercise, and anti-diabetic medications, are the primary approaches
for managing type 2 diabetes mellitus (T2DM). However, there is
increasing evidence that surgery may help achieve complete
remission, particularly in morbidly obese patients with diabetes
(3). The positive effects of
bariatric surgery on the remission of T2DM are well established
(4–6). A number of conventional and
experimental surgical operations have been demonstrated to markedly
ameliorate T2DM, among which Roux-en-Y gastric bypass (RYGB) is the
most common surgical procedure (7).
A number of observational studies have detected improvements in
glycemic and metabolic disorders, and diabetic complications have
shown to be partially reversible due to weight loss and diabetes
control. In addition, patients received beneficial reductions in
cardiovascular morbidity and overall mortality.
The purpose of this retrospective study was to
analyze the results of laparoscopic Roux-en-Y gastric bypass
(LRYGB) treating for diabetes in Chinese people in our department,
the outcome of metabolic syndrome is also discussed.
Materials and methods
Patients
The study was conducted with the approval of the
ethics committee and institutional review board of the Shanghai
Sixth People's Hospital (Shanghai, China). All patients provided
written informed consent after being made aware of the current
standards of treatment for T2DM and of risks and benefits
associated with the procedure. A total of 85 patients (39 men and
46 women) with T2DM underwent LRYGB between February 2011 and May
2013. The mean age was 47.33±12.91 years (range, 24–65 years). The
mean duration from the onset of T2DM was 7.79±4.84 years (range, 1
month to 22 years).
Inclusion and exclusion criteria
Inclusion criteria for this study were as follows:
Age, 18–65 years; BMI, >28 kg/m2; and poorly
controlled T2DM as indicated by glycosylated hemoglobin (HbA1C)
level of ≥7%. Diagnosis of T2DM was based on the criteria of the
American Diabetes Association (ADA) (8) and was considered valid if established
by an endocrinologist or diabetes specialist. The study exclusion
criteria were as follows: Patients <18 or >65 years old;
those planning a pregnancy within 2 years after entry into the
study; and patients with established diagnoses of type 1 diabetes,
latent autoimmune diabetes in adults, malignancy, debilitating
disease, unresolved psychiatric illness, or substance abuse.
Data collection
Data were collected prospectively and entered into a
database. We collected data on patient demographics, fasting blood
glucose (FBG), postprandial blood glucose (PBG), blood pressure
(BP), C-peptide, insulin levels, HbA1c and blood lipid levels,
including profile include cholesterol, triglyceride, high-density
and low-density lipoprotein. Blood glucose was measured using the
glucose oxidase method. Serum insulin and C-peptide levels were
quantified using radioimmunoassays with specific insulin and
C-peptide detection kits according to the manufacturers
instructions (Beijing North Institute of Biological Technology,
Beijing, China). HbA1c was measured using high-performance liquid
chromatography, with a former reference range of 4.0–6.0% (Menarini
Group, Florence, Italy). In addition, glycosylated serum protein
(GSP) was measured using an ELISA (Hitachi 7100; Hitachi, Ltd.,
Tokyo, Japan). Lipid profiles were measured using standard
commercial methods on a parallel, multichannel analyzer (Hitachi
7600-020; Hitachi, Ltd.). Follow-up visits were scheduled at 6, 12
and 18 months after surgery. In addition, postoperative data
regarding patient levels of folic acid, vitamin B12, serum iron,
parathyroid hormone (PTH) and 25-hydroxy vitamin D [25(OH)D] were
collected as indices of anemic and hypocalcemic status.
Patient outcome criteria
The aims for glycemic and BP control were based on
the criteria established by the ADA (8). If patients were not receiving
anti-diabetic medications and had normal FPG (<100 mg/dl) and
HbA1c (<6%) levels, their condition was considered to be
resolved. Patients with an HbA1c of ≤7%, despite no use of
anti-diabetic medications, were considered to have achieved
glycemic control. If the FPG decreased by >25 mg/dl or the HbA1c
reduced by >1%, the patients' condition was considered to have
improved. Surgery was considered to have failed if glycemic indices
showed no significant improvement, worsened or if a patient
required additional anti-diabetic medication.
Surgical technique
The patient was placed in the reverse Trendelenburg
position (9), with the surgeon
positioned between the patient's legs. General anesthesia supplied
by mechanical ventilation (Oxylog 3000 plus; Dräger, Lübeck,
Germany). Five ENDOPATH XCEL® trocars (Ethicon; Johnson &
Johnson, New Brunswick, NJ, USA) were inserted under direct
laparoscopic vision (Stryker 1188 HD autoclavable camera; Stryker
Corporation, Kalamazoo, MI, USA). A ~30-ml gastric pouch was
created using 2-0 mechanical sutures (Ethicon; Johnson &
Johnson), preventing the transection of the left gastric artery.
Subsequently, the angle of Treitz was identified, and a 100-cm long
jejunal loop (biliopancreatic limb) was ascended anterior to the
colon and anastomosed to the gastric pouch with mechanical linear
suture (30 mm). A lateral jejuno-jejunal anastomosisadjacent to
gastro-jejunal anastomosis was made at 100 cm from the previous
nastomosis (alimentary limb), using an EC60A articulating stapler
and a 6TB45 articulating linear cutter (Ethicon; Johnson &
Johnson).
Statistical analysis
Data extracted for this analysis included
preoperative and postoperative body weight, BMI, blood glucose,
HbA1c, BP and blood lipid. A weight loss outcome was indicated by a
reduction in BMI. Blood lipid included cholesterol, triglycerides,
high and low density lipoprotein (HDL and LDL). Comparisons among
groups were performed by one-way analysis of variance followed by
Tukey's multiple comparison test. Comparisons of means were
performed using Student's t-test, and contingency tables of
categorical variables were analyzed using Fisher's exact test.
P<0.05 was considered to indicate a statistically significant
difference, and 95% confidence interval (95% CI) was reported as a
measure of precision. All statistical analyses were performed using
SPSS statistical software, version 20.0 (IBM SPSS, Armonk, NY,
USA).
Results
Preoperative patient demographics
The preoperative mean BMI was 31.60±4.10
kg/m2 (range, 28.53–48.10 kg/m2) and the mean
percentage of body fat was 36.35±9.12% (range, 18–56%). The mean
HbA1c was 8.32±2.13% (range, 7–15.9%).
Surgical success and
complications
LRYGB was successfully completed in all patients.
There were no cases of patient mortality; however, 5 patients
(5.9%) developed complications. One patient had intra-abdominal
infection, another patient had stenosis of gastroenteric stoma,
while the other 3 patients had intestinal obstruction which caused
by intra-abdominal hernia. All the complications occurred in the
first 30 patients, which was within the learning curve (9).
Postoperative follow-up
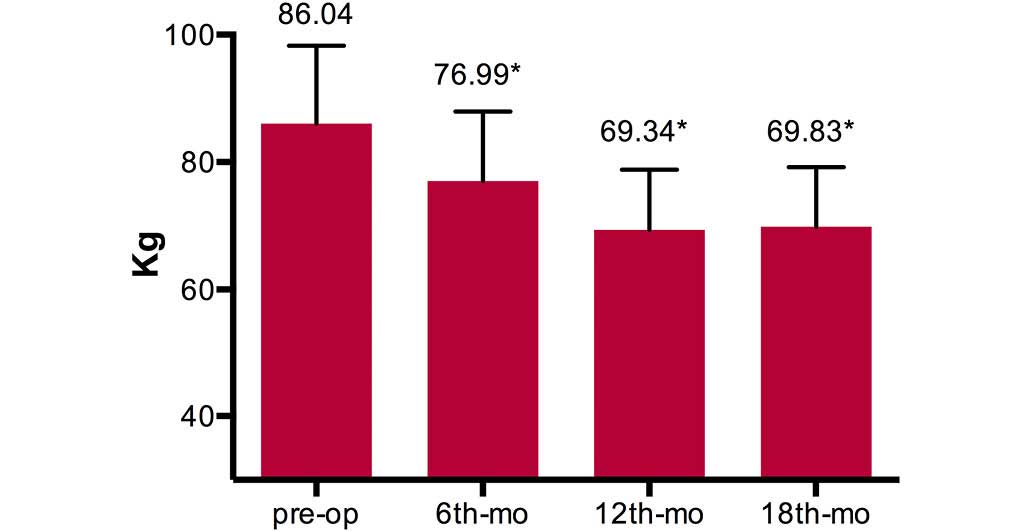
At the 18-month follow-up, a significant reduction
in mean patient body weight was observed in comparison to the mean
preoperative weight (P<0.01) (Fig.
1). The mean BMI decreased from 31.60±4.10 kg/m2
preoperatively to 28.23±3.60, 24.69±2.45 and 24.88±2.99
kg/m2 at the 6-, 12- and 18-month follow-up
examinations, respectively (Fig.
2).
Metabolic syndrome indices
The changes in waistline (cm), hipline (cm) and
waist-hip ratio in comparison to the mean preoperative weight were
significant (P<0.01), which indicated a marked improvement in
central obesity. The blood cholesterol levels were significantly
decreased at the 12- and 18-month follow-up examinations
(P<0.01) and triglycerides levels were decreased significantly
at 6 (P=0.029), 12 (P=0.002) and 18 months (P=0.032)
post-operation. HDL levels did not exhibit an evident change
following surgery, while the LDL levels were significantly
decreased at 12 (P=0.000) and 18 months (P=0.002) following surgery
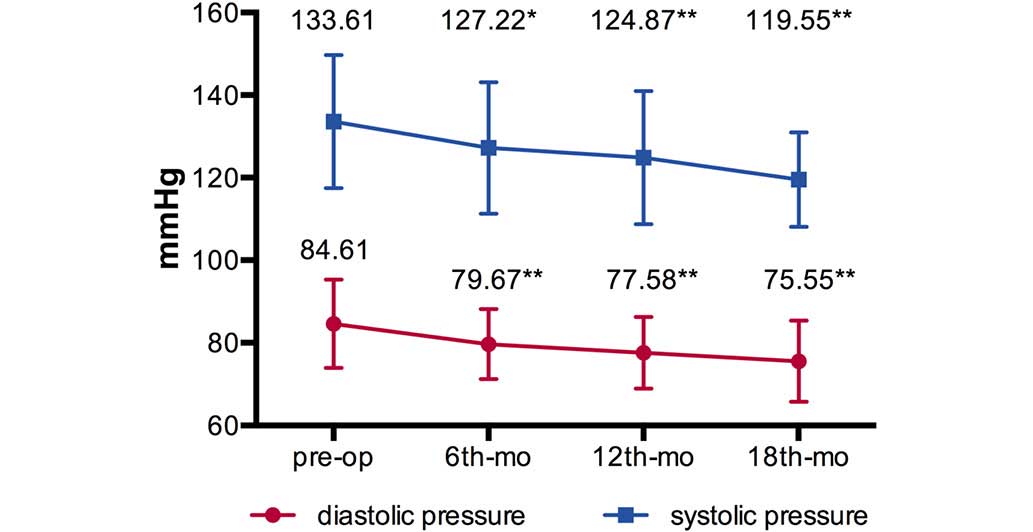
(Table I). The mean BPs
(systolic/diastolic pressure, mmHg) decreased from
133.61±16.12/84.61±10.66 mmHg preoperatively to [127.22±15.91 mmHg
(P=0.043)/79.67±8.46 mmHg (P=0.010)] at 6-months [124.87±16.12 mmHg
(P=0.008)/77.58±8.68 mmHg (P=0.001)] at 12 months and [119.55±11.43
mmHg (P=0.001)/75.55±9.83 mmHg (P=0.004)] at 18 months
post-operation (Fig. 3).
 | Table I.Metabolic syndrome improvement
following laparoscopic Roux-en-Y gastric bypass. |
Table I.
Metabolic syndrome improvement
following laparoscopic Roux-en-Y gastric bypass.
| Parameter | Pre-operative | 6 months | 12 months | 18 months |
|---|
| Waistline (cm) | 104.26±11.51 |
95.37±9.48a |
86.22±7.72a |
86.40±8.00a |
| Hipline (cm) | 107.42±9.64 |
100.80±7.85a |
95.34±7.16a |
95.40±6.08a |
| Waist-hip ratio | 0.970±0.523 |
0.946±0.057a |
0.905±0.051a |
0.91±0.055a |
| Heartrate (bpm) | 78.32±6.39 | 77.96±10.54 |
74.92±7.97a |
71.70±7.43a |
| Cholesterol
(mmol/l) | 4.94±1.10 | 4.68±1.08 |
4.01±0.73a |
3.97±0.57a |
| Triglycerides
(mmol/l) | 2.40±2.82 |
1.51±0.60b |
1.14±0.47a |
1.01±0.41b |
| HDL (mmol/l) | 1.03±0.23 | 0.98±0.18 |
1.15±0.27b | 1.13±0.27 |
| LDL (mmol/l) | 2.88±0.89 | 2.90±0.89 |
2.23±0.58a |
2.20±0.53a |
T2DM indices
Furthermore, the changes in fasting blood glucose,
postprandial blood glucose, HbA1c and GSP in comparison to the mean
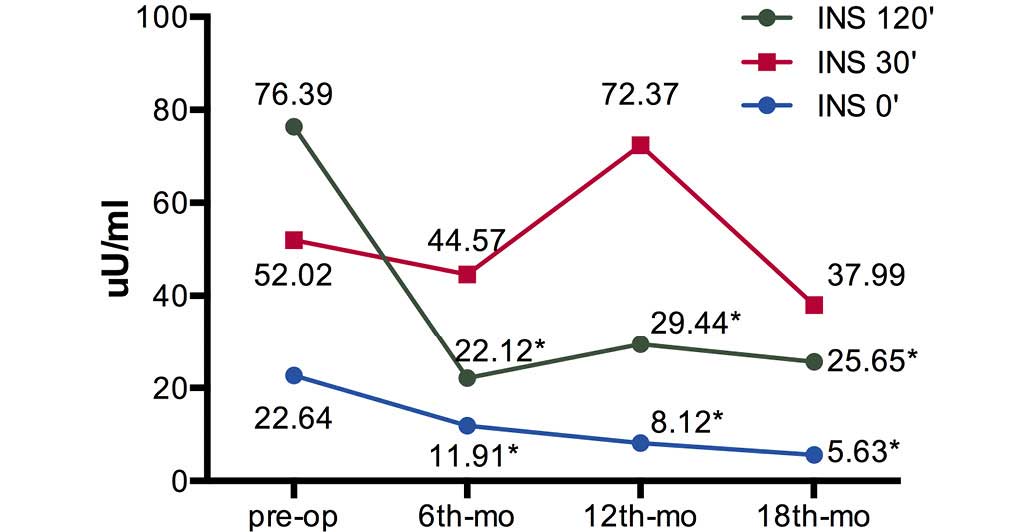
preoperative levels were significant (P<0.01) (Table II). The fasting insulin levels (Ins
0′) and 120 min postprandial insulin levels (Ins 120′) were
significantly decreased at 6, 12 and 18 months after surgery
(P<0.01), while the 30 min postprandial insulin levels (Ins 30′)
were not significantly reduced (Fig.
4). The fasting C-peptide levels were decreased progressively
without significant difference. The 30 (CP 30′) and 120 min
postprandial (CP 120′) C-peptide levels were increased at 6 months
(P=0.380 for CP 30′ and P=0.755 for CP 120′), 12 months (P=0.202
for CP 30′ and P=0.036 for CP 120′) and 18 months (P=0.001 for CP
30′ and P=0.024 for CP 120′) after surgery (Fig. 5).
 | Table II.Result of type 2 diabetes mellitus
following laparoscopic Roux-en-Y gastric bypass. |
Table II.
Result of type 2 diabetes mellitus
following laparoscopic Roux-en-Y gastric bypass.
| Parameter | Pre-operative | 6 months | 12 months | 18 months |
|---|
| FBG (mmol/l) | 8.64±2.95 |
7.13±2.29a |
5.78±1.29a |
5.77±1.13a |
| PBG (mmol/l) | 13.55±4.85 |
8.32±3.18a |
7.73±3.22a |
7.70±3.13a |
| HbA1c (%) | 8.32±2.13 |
6.97±1.22a |
6.10±0.85a |
6.23±1.16a |
| GSP (%) | 20.34±6.65 |
16.02±3.38a |
16.41±3.01a |
16.50±4.23a |
Nutritive status indices
Serum folic acid, vitamin B12 and serum iron levels
were detected as indicators of anemia. Furthermore, the levels of
PTH and 25(OH)D indicated hypocalcemic status. All patients
received follow-up examinations regularly in order to assess their
nutritive status, and no malnutrition or severe anemia were
observed as a result of the treatments (Table III).
 | Table III.Assessment of nutritive status
indices following laparoscopic Roux-en-Y gastric bypass. |
Table III.
Assessment of nutritive status
indices following laparoscopic Roux-en-Y gastric bypass.
| Parameter | Pre-operative | 6 months | 12 months | 18 months |
|---|
| Folic acid
(ng/l) | 8.57±3.07 |
15.06±16.00a |
15.11±5.19a |
14.94±5.40a |
| Vitamin B12
(ng/l) | 580.30±293.91 |
763.72±373.64a | 490.03±380.27 | 443.90±342.16 |
| Serum iron
(µmol/l) | 16.95±5.60 |
14.27±4.70a | 15.29±5.88 | 16.49±5.41 |
| PTH (pg/ml) | 37.97±14.63 |
52.06±18.33a |
41.30±15.71a |
46.37±15.14a |
| 25(OH)D
(ng/ml) | 15.48±6.40 | 13.62±6.48 | 16.14±6.95 | 17.02±8.13 |
Discussion
Diabetes encompasses a group of chronic progressive
metabolic diseases that are characterized by hyperglycemia
resulting from defects in insulin secretion or activity (10). Diabetes is grouped into four clinical
classes, and T2DM is the most common form of diabetes
worldwide.
In the medical management of T2DM, the aim of
treatment is to achieve glycemic control in order to reduce
complications; which differs from the potential novel end point of
euglycemia that metabolic surgery offers (11).
A previous meta-analysis that summarized the
diabetic outcomes of 3,188 patients reported a resolution rate of
80.3% using LRYGB, 95.1% after biliopancreatic diversion (BPD), and
56.7% after laparoscopic adjustable gastric banding in morbidly
obese individuals (4). Metabolic
surgery has been approved as an effective and potentially useful
treatment for patients with T2DM and obesity (4,12).
Although the precise mechanisms underlying the amelioration of
diabetes following surgery are poorly understood, innovative
procedures based on the current understanding of mechanisms, such
as duodenojejunal bypass and ileal interposition have been
investigated (13–16). Furthermore, bariatric surgery has
been shown to result in a significant reduction in excess weight,
an effective control of comorbidities and a significant reduction
in long-term mortality (17–21).
Laparoscopic RYGBP was performed in the present
study as the procedure has previously been shown to be a safe and
effective, with low mortality rate (0.16–0.40%) (3) and a known morbidity rate (7.4%)
(22). Although the exact mechanism
remained unclear, prior studies have indicated that weight loss,
malabsorptive surgery and change in gut hormone contributed the
diabetes control (23,24). We recognize that the extent of
excluded intestine is a point still under discussion. A previous
study published by our group in 2010 showed the results in glycemic
control of patients with T2DM that underwent total or subtotal
gastrectomy and Roux-en-Y reconstruction with a 30–50-cm
biliopancreatic limb and 70-cm alimentary limb, performed for an
indication other than obesity (predominantly cancer), with a
remission rate of 65% at two years of follow-up (25).
PTH may be an early detection index for a disorder
of calcium-phosphate metabolism in patients with obesity and T2DM
following Roux-en-Y gastric bypass (26). Sufficient supplement for relevant
trace elements and regular follow-up are crucial postoperatively
(27).
Previous studies on bariatric-metabolic surgery have
produced novel perspectives for the treatment of T2DM (5,6,12). However, this therapeutic approach
requires adjustment to further increase its effectiveness. In
addition, the establishment of well-defined recommendations and
guidelines for the clinical use and the definition of specific
criteria for consideration of T2DM remission and control are
required. Therefore, further multicenter studies are required to
investigate the benefits of the surgery in different populations,
by analyzing the procedures used and investigating the mechanisms
involved, in order to propose a model for the development of a safe
and effective surgical procedure for T2DM remission in patients
with a BMI of <28 kg/m2.
In conclusion, the results of the present study
suggest that LRYGB may be introduced relatively safely on a larger
scale in small hospitals; with acceptable complication and
mortality rates, good short-term weight loss and a valuable
ameliorative effect on T2DM. However, the effects of the learning
curve could not be entirely avoided. In order to ensure the surgery
outcome, an appropriate surgical team and standardized surgery is
necessary, which required adequate experience in order to overcome
the learning curve associated with performing surgery.
Acknowledgements
This study was supported by grants from the Chinese
Society of Endocrinology, Key Program of the Shanghai Municipality
for Basic Research (no. 11JC1409600), the 973 program (no.
2011-CB504001) and the National Major Scientific and Technological
Special Project for “Significant New Drugs Development” (no.
2011-ZX09307-001-02).
References
|
1
|
Wild S, Roglic G, Green A, Sicree R and
King H: Global prevalence of diabetes: Estimates for the year 2000
and projections for 2030. Diabetes Care. 27:1047–1053. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang W, Lu J, Weng J, Jia W, Ji L, Xiao J,
Shan Z, Liu J, Tian H, Ji Q, et al: Prevalence of diabetes among
men and women in China. N Engl J Med. 362:1090–1101. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Buchwald H, Avidor Y, Braunwald E, Jensen
MD, Pories W, Fahrbach K and Schoelles K: Bariatric surgery: A
systematic review and meta-analysis. JAMA. 292:1724–1737. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Buchwald H, Estok R, Fahrbach K, Banel D,
Jensen MD, Pories WJ, Bantle JP and Sledge I: Weight and type 2
diabetes after bariatric surgery: Systematic review and
meta-analysis. Am J Med. 122:248–256. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schauer PR, Kashyap SR, Wolski K,
Brethauer SA, Kirwan JP, Pothier CE, Thomas S, Abood B, Nissen SE
and Bhatt DL: Bariatric surgery versus intensive medical therapy in
obese patients with diabetes. N Engl J Med. 366:1567–1576. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mingrone G, Panunzi S, De Gaetano A,
Guidone C, Iaconelli A, Leccesi L, Nanni G, Pomp A, Castagneto M,
Ghirlanda G and Rubino F: Bariatric surgery versus conventional
medical therapy for type 2 diabetes. N Engl J Med. 366:1577–1585.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dixon JB, Zimmet P, Alberti KG and Rubino
F: International Diabetes Federation Taskforce on Epidemiology and
Prevention: Bariatric surgery: An IDF statement for obese Type 2
diabetes. Diabet Med. 28:628–642. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
American Diabetes Association: Standards
of medical care in diabetes - 2006. Diabetes Care. 29(Suppl 1):
S4–S42. 2006.PubMed/NCBI
|
|
9
|
Huang CK, Lee YC, Hung CM, Chen YS and Tai
CM: Laparoscopic Roux-en-Y gastric bypass for morbidly obese
Chinese patients: Learning curve, advocacy and complications. Obes
Surg. 18:776–781. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
American Diabetes Association: Diagnosis
and classification of diabetes mellitus. Diabetes Care. 33(Suppl
1): S62–S69. 2010.PubMed/NCBI
|
|
11
|
Rubino F, Schauer PR, Kaplan LM and
Cummings DE: Metabolic surgery to treat type 2 diabetes: Clinical
outcomes and mechanism of action. Annu Rev Med. 61:393–411. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schauer PR, Bhatt DL, Kirwan JP, Wolski K,
Brethauer SA, Navaneethan SD, Aminian A, Pothier CE, Kim ES, Nissen
SE and Kashyap SR: STAMPEDE Investigators: Bariatric surgery versus
intensive medical therapy for diabetes - 3-year outcomes. N Engl J
Med. 370:2002–2013. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rubino F, Forgione A, Cummings DE, Vix M,
Gnuli D, Mingrone G, Castagneto M and Marescaux J: The mechanism of
diabetes control after gastrointestinal bypass surgery reveals role
of the proximal small intestine in the pathophysiology of type 2
diabetes. Ann Surg. 244:741–749. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cohen RV, Schiavon CA, Pinheiro JS, Correa
JL and Rubino F: Duodenal-jejunal bypass for the treatment of type
2 diabetes in patients with body mass index of 22–34
kg/m2: A report of 2 cases. Surg Obes Relat Dis.
3:195–197. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
DePaula AL, Macedo AL, Mota BR and
Schraibman V: Laparoscopic ileal interposition associated to a
diverted sleeve gastrectomy is an effective operation for the
treatment of type 2 diabetes mellitus patients with BMI 21–29. Surg
Endosc. 23:1313–1320. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
DePaula AL, Macedo AL, Schraibman V, Mota
BR and Vencio S: Hormonal evaluation following laparoscopic
treatment of type 2 diabetes mellitus patients with BMI 20–34. Surg
Endosc. 23:1724–1732. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sjöström L, Narbro K, Sjöström CD, Karason
K, Larsson B, Wedel H, Lystig T, Sullivan M, Bouchard C, Carlsson
B, et al: Effects of bariatric surgery on mortality in Swedish
obese subjects. N Eng J Med. 357:741–752. 2007. View Article : Google Scholar
|
|
18
|
Sjöström L, Gummesson A, Sjöström CD,
Narbro K, Peltonen M, Wedel H, Bengtsson C, Bouchard C, Carlsson B,
Dahlgren S, et al: Effects of bariatric surgery on cancer incidence
in obese patients in Sweden (Swedish Obese Subjects Study): A
prospective controlled intervention trial. Lancet Oncol.
10:653–662. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Adams TD, Gress RE, Smith SC, Halverson
RC, Simper SC, Rosamond WD, Lamonte MJ, Stroup AM and Hunt SC:
Long-term mortality after gastric bypass surgery. N Engl J Med.
357:753–761. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Christou NV, Sampalis JS, Liberman N, Look
D, Auger S, McLean AP and MacLean LD: Surgery decreases long-term
mortality, morbidity and health care use in morbidly obese
patients. Ann Surg. 240:416–423. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Csendes A, Burdiles P, Papapietro K and
Burgos AM: Review of the results of medical and surgical treatment
of morbid obesity. Rev Med Chil. 137:559–566. 2009.(In Spanish).
PubMed/NCBI
|
|
22
|
Nguyen NT, Hinojosa M, Fayad C, Varela E
and Wilson SE: Use and outcomes of laparoscopic versus open gastric
bypass at academic medical centers. J Am Coll Surg. 205:248–55.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Allen RE, Hughes TD, Ng JL, Ortiz RD,
Ghantous MA, Bouhali O, Froguel P and Arredouani A: Mechanisms
behind the immediate effects of Roux-en-Y gastric bypass surgery on
type 2 diabetes. Theor Biol Med Model. 10:452013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Samat A, Malin SK, Huang H, Schauer PR,
Kirwan JP and Kashyap SR: Ghrelin suppression is associated with
weight loss and insulin action following gastric bypass surgery at
12 months in obese adults with type 2 diabetes. Diabetes Obes
Metab. 15:963–966. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lanzarini E, Csendes A, Lembach H, Molina
J, Gutiérrez L and Silva J: Evolution of type 2 diabetes mellitus
in non morbid obese gastrectomized patients with Roux en Y
reconstruction: Retrospective study. World J Surg. 34:2098–2102.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Karefylakis C, Näslund I, Edholm D,
Sundbom M, Karlsson FA and Rask E: Vitamin D status 10 years after
primary gastric bypass: Gravely high prevalence of hypovitaminosis
D and raised PTH levels. Obes Surg. 24:343–348. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vidal P, Ramón JM, Goday A, Parri A, Crous
X, Trillo L, Pera M and Grande L: Lack of adherence to follow-up
visits after bariatric surgery: Reasons and outcome. Obes Surg.
24:179–183. 2014. View Article : Google Scholar : PubMed/NCBI
|