Introduction
Although various treatments for pulmonary
tuberculosis (TB) are available, TB remains a major health problem
worldwide, with an estimated 8.6 million new cases and 1.3 million
mortalities in 2012 (1). The World
Health Organization estimates that one-third of the global
population has latent Mycobacterium tuberculosis (M.
tuberculosis) infection, among which 5–10% are likely to
develop active TB during their lifetime (1). A rapid and accurate diagnosis test for
active TB would enable the early treatment of this disease and
reduce transmission, thereby facilitating TB control (2). However, the diagnostic tests currently
available have significant deficiencies, such as a lack of
sensitivity and specificity (3). In
addition, a marker that precisely reflects the effectiveness of
antimicrobial therapy would be useful in assessing the response to
anti-TB treatments.
Endothelin (ET)-1 is a potent vasoconstrictor that
exerts various effects in the respiratory tract (4), including the stimulation of mucus
secretion, airway edema, smooth muscle mitogenesis and bronchial
hyperresponsiveness (5). In
addition, it is considered to have important pro-inflammatory
effects in the airways, where it acts as a chemoattractant and also
upregulates other inflammatory mediators such as interleukin
(IL)-6, IL-8 and granulocyte-macrophage colony-stimulating factor
(GM-CSF) (4). ET-1 is produced by
human airway epithelial and endothelial cells and macrophages
(4). Sputum levels of ET-1 have been
reported to increase in patients with chronic obstructive pulmonary
disease (COPD) during exacerbation (5). In addition, sputum ET-1 levels are also
elevated in patients with cystic fibrosis and COPD compared with
the levels in normal subjects (6).
Our pilot study suggested that elevated sputum ET-1
levels might indicate active disease in patients with pulmonary
M. tuberculosis infection. In the present study, the
association of the sputum ET-1 level with active pulmonary TB and
the effectiveness of anti-TB chemotherapy were explored.
Materials and methods
Subjects
From December 2012 to December 2013, 56 newly
diagnosed patients with active pulmonary TB, 56 age- and
gender-matched non-TB controls, and 43 subjects with latent TB were
recruited at the Second Xiangya Hospital of Central South
University (Changsha, China). Diagnosis of active pulmonary TB was
based on clinical symptoms, chest radiography, microscopy for acid
fast bacilli and sputum M. tuberculosis culture. The inclusion
criteria for patients with active TB were as follows: i) Had not
received any anti-TB treatment prior to entering the study; ii)
Culture or molecular confirmation of infection with
drug-susceptible M. tuberculosis; and iii) human immunodeficiency
virus (HIV) negative. The symptoms of pulmonary TB include fever,
productive cough, night sweats, weight loss, chest pain and
malaise. According to the severity of clinical presentation,
patients with active TB were divided in three groups (mild,
moderate and severe). Subjects with latent TB were those who had
contact with a person with confirmed active TB and had a positive
tuberculin skin test; none of them showed clinical symptoms or
chest X-ray signs suggesting active TB. The non-TB controls were
those who had not contacted with any person with confirmed active
TB and had a negative tuberculin skin test. All subjects in this
study were HIV negative. Baseline characteristics of all subjects
are shown in Table I. The study was
approved by the Ethics Committee of the Second Xiangya Hospital.
Written informed consent was obtained from all subjects.
 | Table I.Baseline characteristics of study
subjects. |
Table I.
Baseline characteristics of study
subjects.
| Characteristic | Active TB (n=56) | Latent TB (n=43) | Non-TB (n=56) | P-value |
|---|
| Age
(years)a | 59.7±16.9 | 55.2±18.5 | 57.1±16.4 | 0.83 |
| Age group
(years) |
|
|
|
|
|
15–29 | 6 (10.7) | 6 (14.0) | 6 (13.3) | 0.95 |
|
30–44 | 7 (12.5) | 6 (14.0) | 7 (13.3) |
|
|
45–59 | 12 (21.4) | 12 (27.9) | 12 (21.4) |
|
| ≥60 | 31 (55.4) | 19 (44.1) | 31 (55.4) |
|
| Age range
(years) | 18–72 | 17–69 | 18–72 |
|
| Male gender | 39 (69.6) | 27 (62.8) | 39 (69.6) | 0.72 |
| M.
tuberculosis culture positivity | 41 (73.2) | 0 (0) | 0 (0) | 1.00 |
| Clinical
presentation |
|
|
|
|
| Mild | 5 (8.9) | – | – | – |
|
Moderate | 40 (71.4) | – | – |
|
|
Severe | 11 (19.6) | – | – |
|
| Sputum ET-1 level
(pg/ml) | 39.7 (17.9–56.4) | 6.3
(3.1–16.7)a | 5.7
(2.6–15.2)a | <0.01 |
| Plasma ET-1 level
(pg/ml) | 1.5 (1.3–1.8) | 1.3 (1.0–1.8) | 1.2 (1.0–1.7) | 0.08 |
| Co-morbidities |
|
|
|
|
| Hypertension | 27 (48.2) | 19 (44.2) | 24 (42.9) | 0.84 |
| CAD | 23 (41.1) | 16 (37.2) | 21 (37.5) | 0.90 |
| Chronic
bronchitis | 17 (30.4) | 11 (25.6) | 10 (17.9) | 0.30 |
| COPD | 10 (17.9) | 5 (11.6) | 5 (8.9) | 0.36 |
Treatment
All patients with active pulmonary TB received
standard anti-TB chemotherapy with a weight-adjusted fixed-dose of
55 mg/kg Rifafour e-275 (Sanofi-Aventis, Beijing, China) consisting
of isoniazid, rifampin, pyrazinamide, and ethambutol. The treatment
was administered on an inpatient basis.
Sputum sampling
For patients with active TB, first morning sputum
samples were collected at baseline (day 0) and on days 1, 2, 4, 6,
10, and 14 during Rifafour e-275 treatment. For subjects with
latent TB and non-TB controls, induced sputum samples were
collected within 72 h after enrollment, as previously described
(7). The samples were centrifuged at
2,000 × g for 10 min and the supernatant was collected. The ET-1
level in the supernatant was quantified with a sandwich
enzyme-linked immunosorbent assay (ELISA) kit (DET100; R&D
Systems, Minneapolis, MN, USA) according to the manufacturer's
instructions. For patients with active TB, an aliquot of the sputum
sample was subject to log colony-forming unit (CFU) determination
on 7H11 agar with Selectatab (polymyxin B, ticarcillin,
amphotericin B and trimethoprim; Mast Group, Ltd., Bootle,
Merseyside, UK) added. Log CFU determinations were performed on
samples collected on days 0, 1, 2, 4, 6, 10 and 14. A second
aliquot was decontaminated with 1% NaOH-N-acetyl-L-cysteine,
diluted with phosphate-buffered saline (PBS) and centrifuged at 4°C
and 3,000 × g for 15 min. The supernatant was discarded and the
pellet resuspended in 1.5 ml PBS. Then, 500 ml of this suspension
was used to inoculate a Mycobacteria Growth Indicator Tube (MGIT;
BD Biosciences, Sparks Glencoe, MD, USA) supplemented with oleic
acid, albumin, dextrose and catalase (OADC), and polymyxin B,
amphotericin B, nalidixic acid, trimethoprim, azlocillin (PANTA).
MGITs were incubated at 37°C in a BACTEC MGIT 960 instrument (BD
Biosciences) until they were flagged positive, or for a maximum of
42 days if no growth was detected. Time to positivity (TTP) in MGIT
culture was recorded. Contamination was excluded by placing one
drop of positive liquid culture on a blood agar plate (NHLS, Cape
Town, South Africa) and by incubating for 48 h at 37°C without
visible growth.
Statistical analysis
Statistical analyses were performed using SPSS for
Windows, version 13.0 (SPSS, Inc., Chicago, IL, USA). ET-1 levels
were expressed as the median with interquartile range. Other
continuous variables were expressed as mean ± standard deviation.
Comparisons of sputum ET-1 levels among subject groups were
performed with nonparametric Kruskal-Wallis H tests followed by
pairwise comparisons using Nemenyi tests. Categorical variables
were compared with Chi-square tests. Correlation analyses between
the changes in sputum ET-1 level and the changes in log CFU or TTP
results were examined using Spearman's rank tests. Multivariate
logistic regression was performed to assess the odds ratio (OR) and
its 95% confidence interval (CI). A two-tailed P<0.05 was
considered statistically significant.
Results
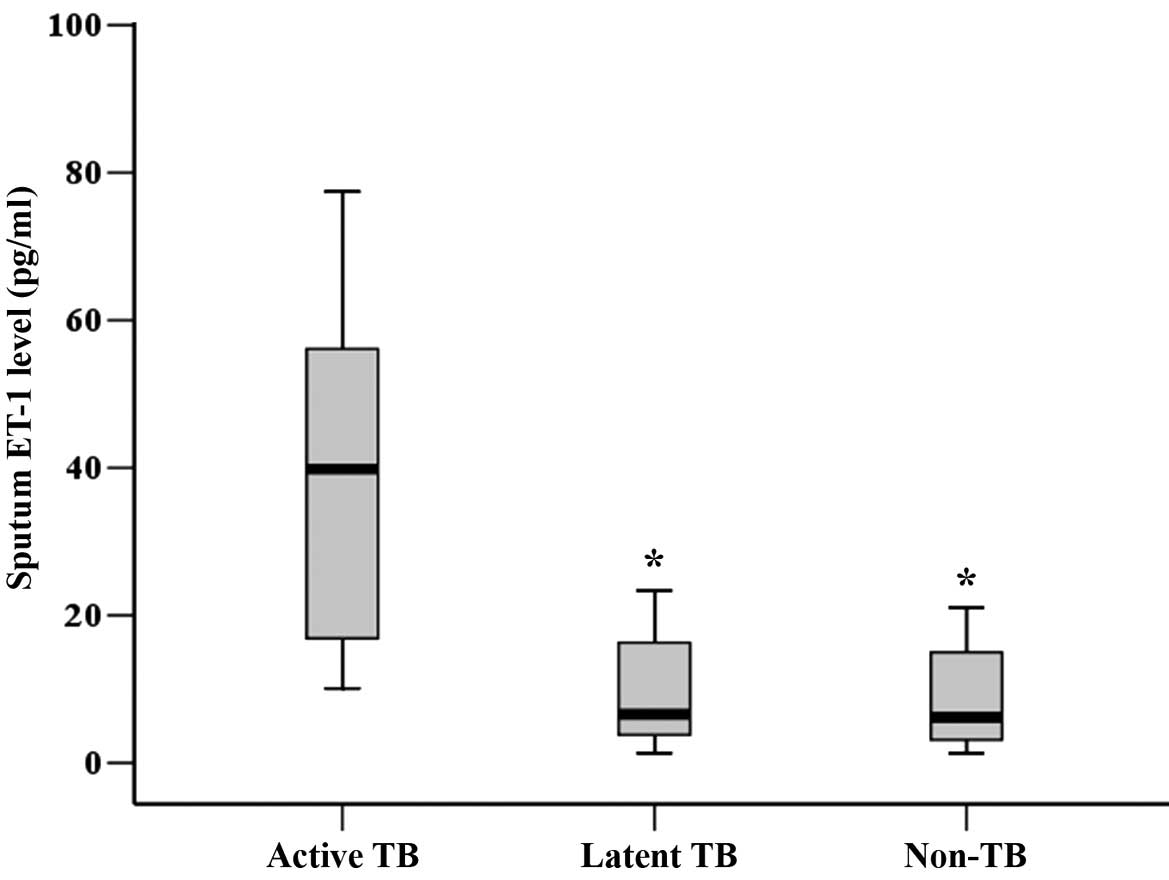
Elevated sputum ET-1 level is
associated with active pulmonary TB
As shown in Table I,
there were no significant differences in age, gender and the
prevalence of the co-morbidities hypertension, coronary artery
disease, chronic bronchitis and COPD among the subject groups at
baseline. The active TB group had a significantly higher sputum
ET-1 level, but not plasma ET-1 level than the latent TB and the
non-TB groups at baseline (Table I).
As shown in Fig. 1, the sputum ET-1
level in the active TB group was significantly higher than those in
the latent TB and the non-TB groups at baseline (P<0.01).
In order to identify the factors that significantly
affected the sputum M tuberculosis culture positivity,
multivariate logistic regression analysis was performed using
sputum culture results (M. tuberculosis negative=0, M.
tuberculosis positive=1) as the dependent variable. Age, gender
(female=0, male=1), severity of clinical presentation (mild=1,
moderate=2, severe=3), sputum ET-1 level, plasma ET-1 level, and
co-morbidities (hypertension and/or coronary artery disease, no=0,
yes=1; chronic bronchitis and/or COPD, no=0, yes=1) were used as
independent variables. As shown in Table II, the severity of clinical
presentation and the sputum ET-1 level entered the logistic
regression model. The results indicated that the severity of
clinical presentation (OR=2.74, 95% CI=1.04–7.22, P<0.01) and
the sputum ET-1 level (OR=6.50, 95% CI=1.32–32.02, P=0.04) were
significantly associated with sputum M. tuberculosis culture
positivity, which suggests that these two factors are independent
indicators of active pulmonary TB.
 | Table II.Logistic regression analysis of
factors significantly associated with sputum M. tuberculosis
culture positivity. |
Table II.
Logistic regression analysis of
factors significantly associated with sputum M. tuberculosis
culture positivity.
| Factor | Point estimate | Standard error | Wald Chi-square | Odds P-value | 95% CI ratio for odds
ratio |
|---|
| Severity of clinical
presentation | 1.01 | 3.68 | 9.61 | <0.01 | 2.74 | 1.04–7.22 |
| Sputum ET-1 level
(pg/ml) | 1.87 | 0.49 | 4.17 | 0.04 | 6.50 | 1.32–32.02 |
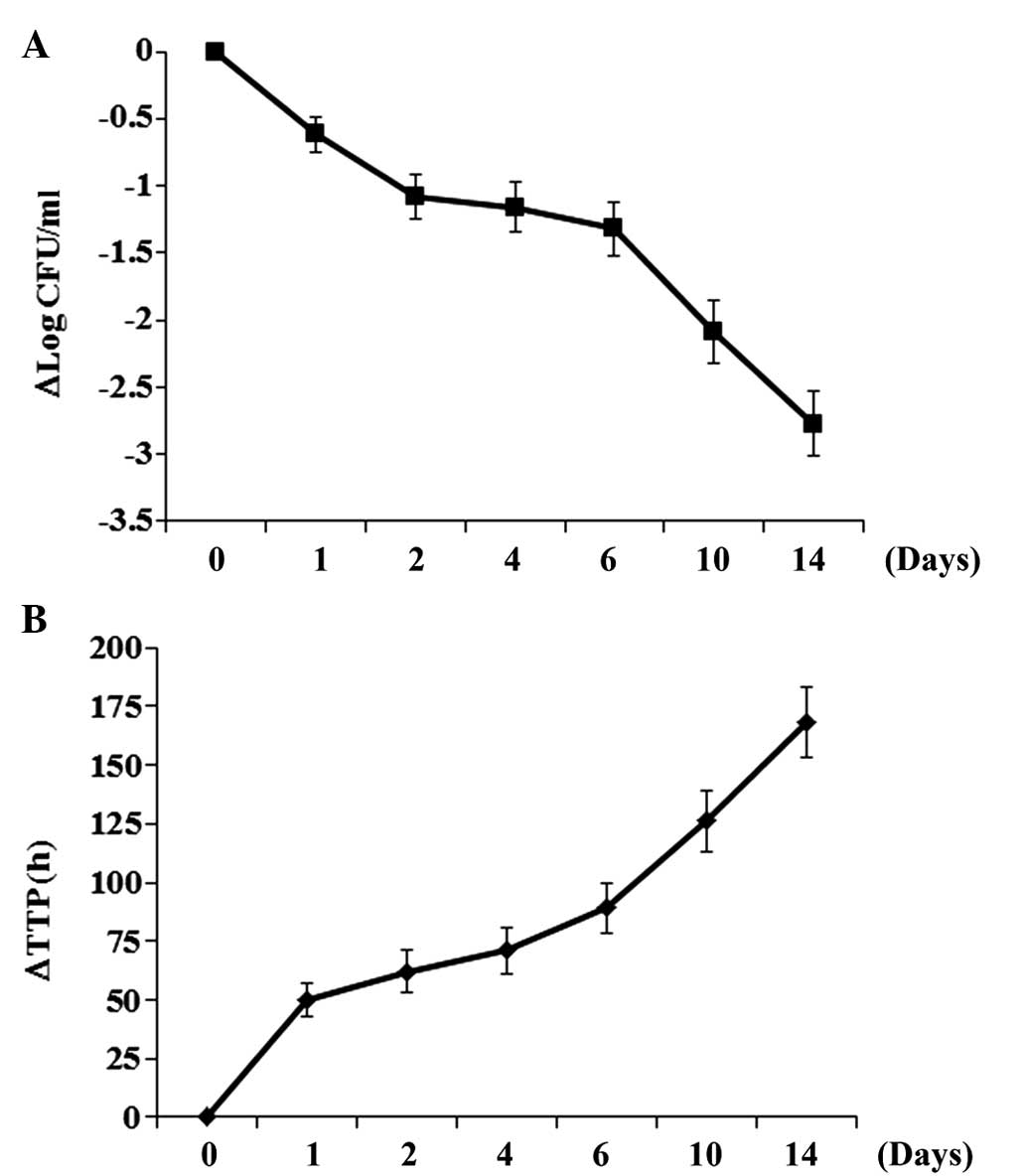
Change in sputum ET-1 level correlates
with patient response to anti-TB chemotherapy
In order to determine the association between the
level of ET-1 in the sputum and the patient response to anti-TB
chemotherapy, patients in the active TB group were treated with a
weight-adjusted fixed-dose of 55 mg/kg Rifafour e-275 and the
sputum ET-1 level, the number of CFU/ml and TTP were measured at
baseline (day 0) and on days 1, 2, 4, 6, 10 and 14. As shown in
Fig. 2, the number of CFU and TTP
decreased and increased, respectively, over the time of treatment.
The sputum ET-1 level decreased over the time of treatment
(Fig. 3), with a trend similar to
that of the number of CFU. As shown in Table III, correlation analyses with
Spearman rank tests revealed that decrements (from baseline) in
sputum ET-1 level were in significant positive correlation with
decrements (from baseline) in the number of CFU at each time point
during the treatment, with the correlation coefficient ranging from
0.31 on day 4 to 0.54 on day 14 (all P<0.05). By contrast,
decrements (from baseline) in the sputum ET-1 level were in
significant negative correlation with increments (from baseline) in
TTP at each time point during the treatment, with the correlation
coefficient ranging from −0.42 on day 2 to −0.56 on day 14 (all
P<0.01; Table IV).
 | Table III.Correlation between changes in sputum
ET-1 level and changes in CFU/ml in patients receiving
anti-tuberculosis chemotherapy. |
Table III.
Correlation between changes in sputum
ET-1 level and changes in CFU/ml in patients receiving
anti-tuberculosis chemotherapy.
| Day | Correlation
coefficient (r) | P-value |
|---|
| 1 | 0.44 | <0.01 |
| 2 | 0.51 | <0.01 |
| 4 | 0.31 | 0.02 |
| 6 | 0.36 | <0.01 |
| 10 | 0.42 | <0.01 |
| 14 | 0.54 | <0.01 |
 | Table IV.Correlation between changes in sputum
ET-1 level and changes in TTP in patients receiving
anti-tuberculosis chemotherapy. |
Table IV.
Correlation between changes in sputum
ET-1 level and changes in TTP in patients receiving
anti-tuberculosis chemotherapy.
| Day | Correlation
coefficient (r) | P-value |
|---|
| 1 | −0.52 | <0.01 |
| 2 | −0.42 | <0.01 |
| 4 | −0.43 | <0.01 |
| 6 | −0.46 | <0.01 |
| 10 | −0.49 | <0.01 |
| 14 | −0.56 | <0.01 |
Discussion
The present study, to the best of our knowledge,
provides the first evidence that the sputum ET-1 level is
significantly associated with active pulmonary TB and the
effectiveness of anti-TB chemotherapy.
ET-1, produced by airway epithelial and endothelial
cells and macrophages (8–10), functions as a pro-inflammatory factor
in the airways, where it acts as a chemoattractant and upregulates
other important inflammatory mediators such as IL-6 and GM-CSF
(5,11). A systemic rise of ET-1 levels occurs
in response to a variety of factors, including sepsis and ischemia
(5,12). In the present study, it was observed
that the sputum ET-1 level was significantly elevated in patients
with active pulmonary TB compared with patients with latent TB and
TB-free controls. Following adjustment for confounders such as age,
gender, severity of clinical presentation, plasma ET-1 level and
comorbidities that might affect the sputum ET-1 level, multivariate
logistic regression analysis revealed that the sputum ET-1 level
was an independent indicator for active pulmonary TB. Since the
plasma ET-1 level was not significantly increased, it is likely
that the elevation of sputum ET-1 levels in patients with active
pulmonary TB was due to the pulmonary, not systemic, inflammatory
responses to active M. tuberculosis infection.
The viability of bacilli and the susceptibility to
anti-TB therapy is usually monitored by culture (13), which remains the gold standard in the
diagnosis and follow-up of mycobacterial infections. However, it is
a time-consuming process, since M. tuberculosis grows slowly
and several weeks or months are required for its detection in
clinical samples (14).
Inflammation-related factors have been suggested as potential
biomarkers for active TB (15,16).
Travar et al (15) reported
that the sputum level of interferon λ-2 was significantly higher in
patients with active pulmonary TB than in patients with latent TB
and healthy controls. Cai et al (16) reported that the expression level of
complement C1q in the peripheral blood was able to discriminate
patients with active TB from those with latent TB infection and
healthy controls. The results of the present study show that the
sputum ET-1 level was significantly higher in the patients with
active pulmonary TB than in those with latent TB and the TB-free
controls. Whether these factors are connected in active pulmonary
TB and how remain to be explored in our future studies.
In this study, decrements in the sputum ET-1 level
significantly correlated with decrements in CFU and increments in
TTP during anti-TB chemotherapy. This corroborates the finding that
the sputum ET-1 level is significantly associated with active
pulmonary TB, and also suggests that decrements in the sputum ET-1
level could be a potential indicator of the effectiveness of
anti-TB chemotherapy. Determination of the sputum ET-1 level by
ELISA is fast (<5 h) and easy, which supports the feasibility of
using the sputum ET-1 level as a biomarker for active pulmonary TB
and the effectiveness of anti-TB chemotherapy. We plan to explore
the clinical application value of the sputum ET-1 level for
patients with active pulmonary TB in a future study with a large
patient sample.
The present study has several limitations: i) Only
HIV-negative subjects were enrolled to minimize the potential
effects of immunodeficiency on the sputum ET-1 level, since ET-1 is
profoundly involved in inflammatory responses in the airways. ii)
Only newly diagnosed patients infected with drug-susceptible M.
tuberculosis and without previous anti-TB treatment were
enrolled in the active TB group to exclude possible confounding
effects of drug-resistant M. tuberculosis on the
effectiveness of anti-TB chemotherapy in this study (17). Nevertheless, the findings of this
study provide a solid basis for future studies with a more
extensive patient sample.
In conclusion, this study indicates that an elevated
sputum ET-1 level is an independent indicator of active pulmonary
TB and suggests that decrements in the sputum ET-1 level may
reflect the effectiveness of anti-TB chemotherapy.
References
|
1
|
Global tuberculosis control: Surveillance,
planning and financing. World Health Organization (Geneva,
Switzerland). 2009.
|
|
2
|
Parashar D, Chauhan DS, Sharma VD and
Katoch VM: Applications of real-time PCR technology to
mycobacterial research. Indian J Med Res. 124:385–398.
2006.PubMed/NCBI
|
|
3
|
Dorman SE: New diagnostic tests for
tuberculosis: Bench, bedside, and beyond. Clin Infect Dis. 50(Suppl
3): S173–S177. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zheng L, Tipoe G, Lam WK, Ho JC, Shum I,
Ooi GC, Leung R and Tsang KW: Endothelin-1 in stable
bronchiectasis. Eur Respir J. 16:146–149. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Roland M, Bhowmik A, Sapsford RJ,
Seemungal TA, Jeffries DJ, Warner TD and Wedzicha JA: Sputum and
plasma endothelin-1 levels in exacerbations of chronic obstructive
pulmonary disease. Thorax. 56:30–35. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chalmers GW, Macleod KJ, Sriram S, Thomson
LJ, McSharry C, Stack BH and Thomson NC: Sputum endothelin-1 is
increased in cystic fibrosis and chronic obstructive pulmonary
disease. Eur Respir J. 13:1288–1292. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bhowmik A, Seemungal TA, Sapsford RJ,
Devalia JL and Wedzicha JA: Comparison of spontaneous and induced
sputum for investigation of airway inflammation in chronic
obstructive pulmonary disease. Thorax. 53:953–956. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ehrenreich H, Anderson RW, Fox CH,
Rieckmann P, Hoffman GS, Travis WD, Coligan JE, Kehrl JH and Fauci
AS: Endothelins, peptide with potent vasoactive properties, are
produced by human macrophages. J Exp Med. 172:1741–1748. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nakano J, Takizawa H, Ohtoshi T, Shoji S,
Yamaguchi M, Ishii A, Yanagisawa M and Ito K: Endotoxin and
proinflammatory cytokines stimulate endothelin-1 expression and
release by airway epithelial cells. Clin Exp Allergy. 24:330–336.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Giaid A, Polak JM, Gaitonde V, Hamid QA,
Moscoso G, Legon S, Uwanogho D, Roncalli M, Shinmi O, Sawamura T,
et al: Distribution of endothelin-like immunoreactivity and mRNA in
the developing and adult human lung. Am J Respir Cell Mol Biol.
4:50–58. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mullol J, Baraniuk JN, Logun C, Benfield
T, Picado C and Shelhamer JH: Endothelin-1 induces GM-CSF, IL-6 and
IL-8 but not G-CSF release from a human bronchial epithelial cell
line (BEAS-2B). Neuropeptides. 30:551–556. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Warner TD and Klemm P: What turns on the
endothelins? Inflamm Res. 45:51–53. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takahashi T and Nakayama T: Novel
technique of quantitative nested real-time PCR Assay for
Mycobacterium tuberculosis DNA. J Clin Microbiol.
44:1029–1039. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Montenegro RA, Guarines KM, Montenegro LM,
Lira LA, Falcão J, Melo FL, Santos FC, Nascimento AL, Zuzarte MS,
Leite RC and Schindler HC: Assessment of messenger RNA (mRNA) of
Mycobacterium tuberculosis as a marker of cure in patients
with pulmonary tuberculosis. J Appl Microbiol. 117:266–272. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Travar M, Vucic M and Petkovic M:
Interferon lambda-2 levels in sputum of patients with pulmonary
Mycobacterium tuberculosis infection. Scand J Immunol.
80:43–49. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cai Y, Yang Q, Tang Y, Zhang M, Liu H,
Zhang G, Deng Q, Huang J, Gao Z, Zhou B, et al: Increased
complement C1q level marks active disease in human tuberculosis.
PLoS One. 9:e923402014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mdivani N, Li H, Akhalaia M, Gegia M,
Goginashvili L, Kernodle DS, Khechinashvili G and Tang YW:
Monitoring therapeutic efficacy by real-time detection of
Mycobacterium tuberculosis mRNA in sputum. Clin Chem.
55:1694–1700. 2009. View Article : Google Scholar : PubMed/NCBI
|