Introduction
Surveillance of antibiotic resistance is the most
effective method for obtaining accurate data regarding bacterial
resistance transitions and epidemiology. This data informs the
formulation of guidelines for the use of antibiotics, and helps
control the spread of resistant bacteria (1). In recent years, microbial resistance to
antibiotics has been increasing, particularly in China (2–5). If this
trend continues there will be fewer antibiotics to choose from when
treating patients (6,7). Formulating policy for the use of
antibiotics and the improved management of patients depends on
up-to-date knowledge of the prevalent strains of bacteria in
particular locales or regions of a country, and their likely
patterns of antibiotic resistance. To this end, in the present
retrospective study, enabled by the routine practice of monitoring
clinical bacterial isolates and recording corresponding
antibiograms, the clinical bacterial profiles of patients were
collected and analyzed between 2009 and 2011 at Shanghai First
People's Hospital (Shanghai, China). Patterns of antibiotic
resistance were determined in order to ascertain an accurate
representation of local bacterial resistance and to help improve
the efficacy of empirical antibiotic therapy.
Materials and methods
Clinical survey
The present study retrospectively investigated the
antibiotic susceptibility of bacteria between 2009 and 2011 at
Shanghai First People's Hospital affiliated with Shanghai Jiaotong
University. The study included a total of 5,209 patients (63%
male), from whom were collected sputum, blood and wound culture
samples. The present study was approved by the ethics committee of
Shanghai First People's Hospital. Written informed consent was
obtained from all participants.
Bacterial strains and tests for
antimicrobial susceptibility
The quality control bacterial strains included
Staphylococcus aureus (ATCC25923), Escherichia coli
(ATCC25922), Pseudomonas aeruginosa (ATCC27853),
Enterococcus faecalis (ATCC29212), and Klebsiella
pneumoniae (ATCC700603; Shanghai Center for Clinical
Laboratory, Shanghai, China). Mueller-Hinton agar, which was used
for susceptibility testing, and antibiotic susceptibility disks
were purchased from Oxoid (Thermo Fisher Scientific Inc., Waltham,
MA, USA). Antimicrobial susceptibility testing data were generated
as part of a surveillance program conducted by the Shanghai First
People's Hospital between 2009 and 2011. Drug susceptibility tests
were performed using the Kirby-Bauer antibiotic testing disk
diffusion method (8), and the data
were interpreted in accordance with the 2010 guidelines outlined by
the Clinical and Laboratory Standards Institute (8).
Statistical analyses
WHONET 5.5 software, which was provided by the
Antimicrobial Resistance Monitoring Center of the World Health
Organization (Geneva, Switzerland), was utilized for statistical
analyses.
Results
Distribution of the pathogenic
bacteria
Between 2009 and 2011, E. coli was the most
prevalent bacterial pathogen detected in the present hospital, with
the following detection rates: 2009, 305/1883 (16.2%); 2010,
346/1730 (20%); 201, 408/2082 (19.6%) (Fig. 1). The next most prevalent was
Acinetobacter baumannii: 2009, 225/1875 (12.0%); 2010,
216/1728 (12.5); and 2011, 253/1497 (16.9%), and S. aureus
was the third most prevalent pathogenic bacterium by 2011.
Sensitivity and resistance rates of
the bacterial strains E. coli
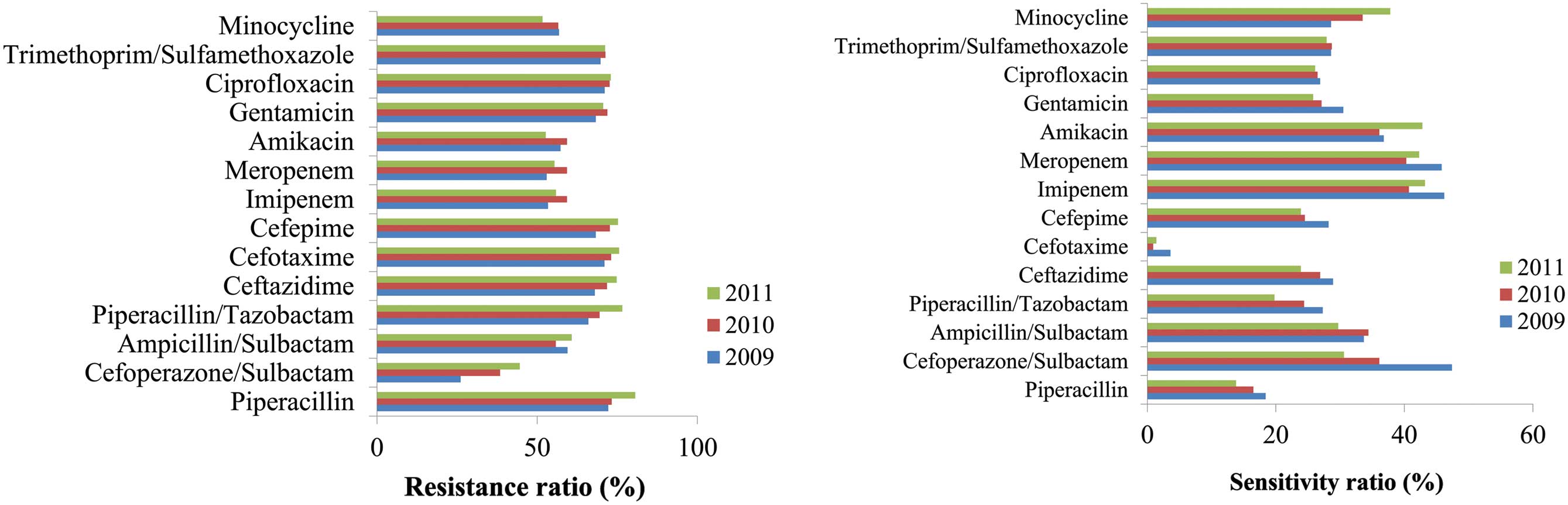
Susceptibility testing of E. coli indicated
>60% resistance against the following antibiotics: Penicillins,
including ampicillin, piperacillin and ampicillin/sulbactam;
certain cephalosporins, including cefuroxime, cefotaxime and
cefaclor; and other antibiotics, including ciprofloxacin,
trimethoprim, sulphamethoxazole and gentamicin. Imipenem and
meropenem were demonstrated to be the most effective antibiotics
against E. coli. By 2011, the resistance rate of E.
coli against meropenem had increased to 0.2% and since then the
trend for resistance against imipenem and meropenem has increased,
with the susceptibility break point increasing from 14–15 to 20–22
(Fig. 2).
S. aureus
The resistance of S. aureus to the most
commonly used antibiotics, including: Penicillin, cefazolin,
gentamicin, clindamycin, erythromycin and levofloxacin, was
demonstrated to be >70%. S. aureus exhibited minimal
resistance to linezolid, vancomycin and teicoplanin in the three
consecutive years (Fig. 3).
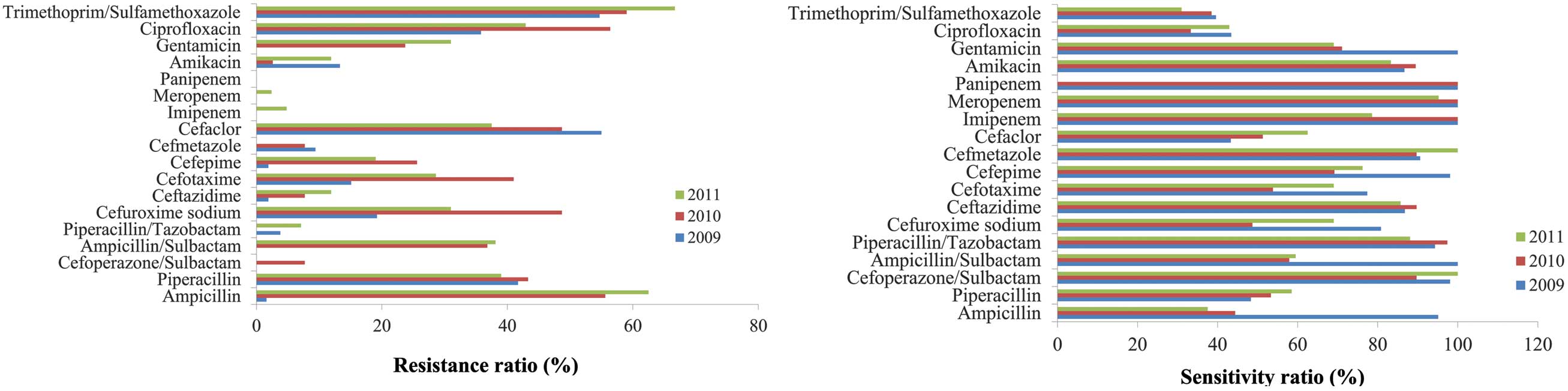
A. baumannii
For the majority of the antibiotics investigated,
the resistance rates of A. baumannii were >60%.
Cefoperazone/sulbactam was demonstrated to be the most effective
antibiotic treatment against this bacterium; however, its
resistance rate increased from 26.1 to 44.6% between 2009 and 2011
(Fig. 4).
P. aeruginosa
For the majority of the antibiotics investigated
against P. aeruginosa, the resistance rates were ~20%,
including cefoperazone/sulbactam and piperacillin, whereas the
resistance rates against amikacin were ~10% (Fig. 5).
K. pneumoniae
With sensitivity rates of 100% to imipenem and
meropenem in 2009 and 2010, K. pneumoniae was the most
highly sensitive bacterium; however, K. pneumoniae had
developed a small degree of resistance to these antibiotics by 2011
(Fig. 6).
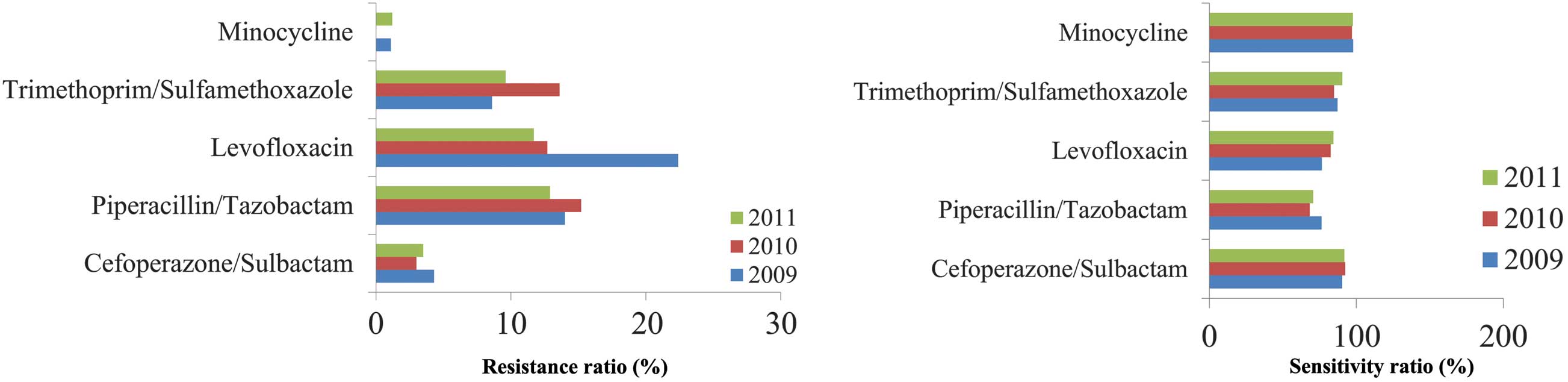
Stenotrophomonas maltophilia
monicamide
S. maltophilia monicamide was demonstrated to
be sensitive to cefoperazone/sulbactam (Fig. 7), with no change demonstrated over
the three years.
E. faecalis
In 2011, E. faecalis exhibited a resistance
rate of 55.6% against levofloxacin, which was a notable increase
compared with previous years. E. faecalis was demonstrated
to be 100% sensitive to linezolid, vancomycin and teicoplanin
(Fig. 8).
Proteus. mirabilis
P. mirabilis was initially 100% sensitive to
cefoperazone/sulbactam, imipenem, meropenem and gentamicin;
however, this sensitivity decreased by 0.0, 21.4, 4.8 and 31.0%,
respectively, over three years (Fig.
9).
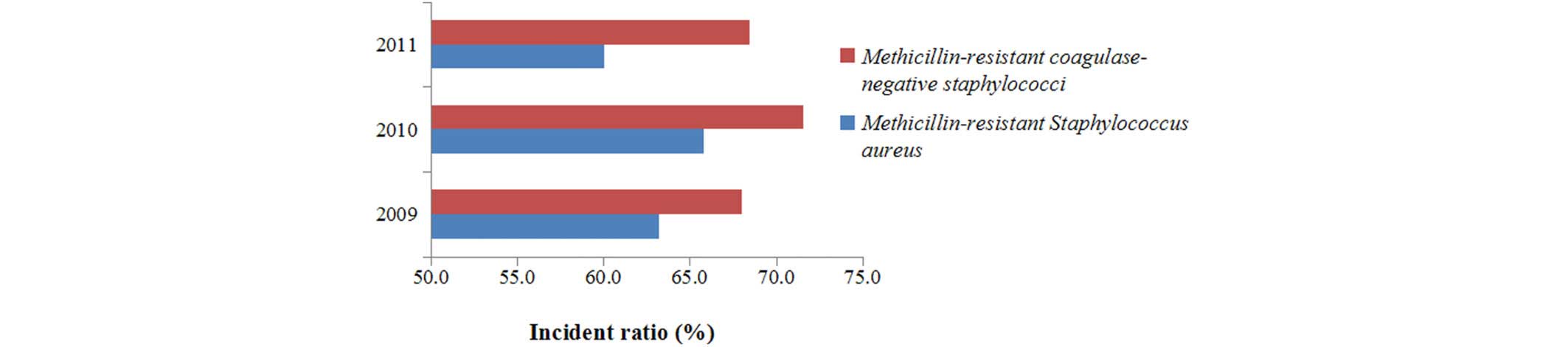
Methicillin-resistant S. aureus
Between 2009 and 2011, the detection rates for
methicillin-resistant S. aureus were 63.2, 65.8 and 60.1%,
respectively (Fig. 10); whereas the
detection rates for methicillin-resistant coagulase-negative
staphylococci were 68.0, 71.6 and 68.5%, respectively between 2009
and 2011.
Discussion
The results of the present study demonstrated that
the most common pathogen detected between 2009 and 2011 at Shanghai
First People's Hospital was E. coli, followed by A.
baumannii, P. aeruginosa, S. aureus, K.
pneumoniae, S. maltophilia, E. faecium, E.
faecalis, P. mirabilis and E. cloacae. The
percentage of Gram-negative bacteria detected was significantly
higher, as compared with that of Gram-positive bacteria.
Furthermore, non-fermenting Gram-negative bacteria were more
prevalent than Enterobacteriaceae, and A. baumannii
was more prevalent than P. aeruginosa, which was
demonstrated to be the most prevalent pathogen at the hospital
since 2011.
At present, antibiotic consumption in China is high,
with various quantities and classes of antibiotics being
prescribed, depending on the type of hospital (9–11). Given
that the worldwide spread of multidrug-resistant bacteria is
threatening the availability of safe and effective antibiotic
treatment for patients (12), strong
infection control measures and alternative therapeutic agents are
required. Methicillin-resistant S. aureus remains the
primary Gram-positive bacterium of concern in public hospitals
(13,14). Strains of this bacterium are
resistant to β-lactams, macrolides, fluoroquinolones and
aminoglycosides; therefore, glycopeptide antibacterial agents
remain the last line of antimicrobial defense.
Antibiotic consumption and the development of
bacterial resistance are closely associated, and antibiotic
resistance rates are increasing in developing countries, as
compared with those in developed countries (15). Extensive use of third-generation
cephalosporins has caused bacteria under this selective pressure to
generate extended-spectrum β-lactamases and AmpC enzymes, which are
capable of overcoming the anti-bacterial activities of
cephalosporins (16). In the present
study, the susceptibility tests demonstrated that the majority of
bacteria were highly sensitive to linezolid, vancomycin and
teicoplanin; therefore, these remain effective antimicrobial agents
against otherwise resistant bacteria. Furthermore, the present
study demonstrated that Gram-negative bacilli may be more sensitive
to imipenem. All bacteria were 100% sensitive to imipenem and
meropenem in 2009 and 2010; however, a small increase in resistance
to imipenem was detected in 2011.
In order to mitigate the development of antibiotic
resistance and reduce nosocomial infections, clinicians should pay
particular attention to clinical indications. Furthermore,
clinicians should understand the dynamics of bacterial resistance,
the results of bacterial susceptibility tests, and develop
appropriate prevention measures that seek to reduce the incidence
of nosocomial infections and the spread of resistant bacteria
(17,18). E. coli remains the most common
pathogen at Shanghai First People's Hospital, and the resistance
rates of bacteria to conventional and emerging antibiotics are
increasing. Previous studies investigating similar control
strategies supported the effectiveness of targeted active
surveillance (19–21). The present study suggested that
clinicians should seek to prescribe medications based on
antimicrobial susceptibility results, in order to avoid the misuse
of antibiotics and to reduce the probability of resistant strains
emerging under the selection pressure.
Acknowledgements
This study was supported by grants from the Natural
Science Foundation of China (No. 81470852), the Science and
Technology Commission of Shanghai Science and Technology Support
Project (No. 13431900503), the Medical and Technology Across
Project of Shanghai Jiaotong University (No. YG2012MS02) and Young
Talents Project of Shanghai Health System (No. XYQ2013091).
References
|
1
|
Linder JA, Bates DW and Platt R:
Antivirals and antibiotics for influenza in the United States, 1995
– 2002. Pharmacoepidemiol Drug Saf. 14:531–536. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xie DS, Xiong W, Lai RP, Liu L, Gan XM,
Wang XH, Wang M, Lou YX, Fu XY, Wang HF, et al:
Ventilator-associated pneumonia in intensive care units in Hubei
Province, China: A multicentre prospective cohort survey. J Hosp
Infect. 78:284–288. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chuang JH, Huang AS, Huang WT, Liu MT,
Chou JH, Chang FY and Chiu WT: Nationwide surveillance of influenza
during the pandemic (2009-10) and post-pandemic (2010-11) periods
in Taiwan. PLoS One. 7:e361202012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
You JH, Chan ES, Leung MY, Ip M and Lee
NL: A cost-effectiveness analysis of “test” versus “treat” patients
hospitalized with suspected influenza in Hong Kong. PLoS One.
7:e331232012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee N and Ison MG: Diagnosis, management
and outcomes of adults hospitalized with influenza. Antivir Ther.
17(1 Pt B): 143–157. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Linder JA, Chan JC and Bates DW:
Appropriateness of antiviral prescribing for influenza in primary
care: A retrospective analysis. J Clin Pharm Ther. 31:245–252.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sarkar P and Gould IM: Antimicrobial
agents are societal drugs: How should this influence prescribing?
Drugs. 66:893–901. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wayne P: Performance standards for
antimicrobial susceptibility testing. Ninth informational
supplement NCCLS document M100-S9. National Committee for Clinical
Laboratory Standards. 2008.
|
|
9
|
Hvistendahl M: Public health. China takes
aim at rampant antibiotic resistance. Science. 336:795. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li Y, Xu J, Wang F, Wang B, Liu L, Hou W,
Fan H, Tong Y, Zhang J and Lu Z: Overprescribing in China, driven
by financial incentives, results in very high use of antibiotics,
injections, and corticosteroids. Health Aff (Millwood).
31:1075–1082. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Song W, Cao J and Mei YL: Correlation
between cephamycin consumption and the incidence of antimicrobial
resistance in Acinetobacter baumannii at a university
hospital in China from 2001 to 2009. Int J Clin Pharmacol Ther.
49:765–771. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Amadeo B, Dumartin C, Venier A,
Fourrier-Réglat A, Coignard B and Rogues AM: Factors associated
with the prevalence of antibiotic use for the treatment of
hospital-acquired infections at 393 French hospitals: A regional
variation analysis. Infect Control Hosp Epidemiol. 32:155–162.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Collins J, Raza M, Ford M, Hall L, Brydon
S and Gould FK: Review of a three-year meticillin-resistant
Staphylococcus aureus screening programme. J Hosp Infect.
78:81–85. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Waterhouse M, Morton A, Mengersen K, Cook
D and Playford G: Role of overcrowding in meticillin-resistant
Staphylococcus aureus transmission: Bayesian network
analysis for a single public hospital. J Hosp Infect. 78:92–96.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gupta A, Kapil A, Lodha R, Kabra SK, Sood
S, Dhawan B, Das BK and Sreenivas V: Burden of
healthcare-associated infections in a paediatric intensive care
unit of a developing country: A single centre experience using
active surveillance. J Hosp Infect. 78:323–326. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kumarasamy KK, Toleman MA, Walsh TR,
Bagaria J, Butt F, Balakrishnan R, Chaudhary U, Doumith M, Giske
CG, Irfan S, et al: Emergence of a new antibiotic resistance
mechanism in India, Pakistan, and the UK: A molecular, biological,
and epidemiological study. Lancet Infect Dis. 10:597–602. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bouzbid S, Gicquel Q, Gerbier S, Chomarat
M, Pradat E, Fabry J, Lepape A and Metzger MH: Automated detection
of nosocomial infections: Evaluation of different strategies in an
intensive care unit 2000–2006. J Hosp Infect. 79:38–43. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang M, Hu Z and Hu F: Nosocomial
meningitis caused by Acinetobacter baumannii: Risk factors
and their impact on patient outcomes and treatments. Future
Microbiol. 7:787–793. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Harbarth S, Hawkey PM, Tenover F, Stefani
S, Pantosti A and Struelens MJ: Update on screening and clinical
diagnosis of meticillin-resistant Staphylococcus aureus
(MRSA). Int J Antimicrob Agents. 37:110–117. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Harris AD, Furuno JP, Roghmann MC, Johnson
JK, Conway LJ, Venezia RA, Standiford HC, Schweizer ML, Hebden JN,
Moore AC and Perencevich EN: Targeted surveillance of
methicillin-resistant Staphylococcus aureus and its
potential use to guide empiric antibiotic therapy. Antimicrob
Agents Chemother. 54:3143–3148. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vos MC, Behrendt MD, Melles DC, Mollema
FP, de Groot W, Parlevliet G, Ott A, Horst-Kreft D, van Belkum A
and Verbrugh HA: 5 years of experience implementing a
methicillin-resistant Staphylococcus aureus search and
destroy policy at the largest university medical center in the
Netherlands. Infect Control Hosp Epidemiol. 30:977–984. 2009.
View Article : Google Scholar : PubMed/NCBI
|