Introduction
Pavlovian eyelid conditioning is an important method
for tracing the pathway of the projection of fibers, examining the
acquisition or retention of memory and verifying the process of
learning (1,2). A conditioned response (CR) may be
established by pairing a conditioned stimulus (CS), which does not
elicit the eyelid reflex spontaneously, with an unconditioned
stimulus (US) that naturally elicits the eyelid reflex (3). To date, studies on eyelid conditioning
using lesions, inactivation, stimulation and neural tract tracing
have provided information on the pathways in the cerebellum,
cerebrum and brain stem.
Usually, peripheral stimuli are used as a CS; for
example, light CS to the eye, or a tone CS to the ear.
Alternatively, somatic stimuli may be used to establish a CR.
Further investigations were performed by directly stimulating the
central nervous system. Knowlton et al (4) used an auditory stimulus to establish an
eyelid CR. An eyelid CR was also observed in the experiment
performed by Green et al (5),
where the CS and US were transduced directly into the interpositus
nucleus (5). The results from the
study by Knowlton and Thompson (6)
demonstrated that the average length of time to establish eyelid
conditioning by a central CS was shorter compared with a peripheral
CS (6). In addition, a number of
previous studies (5,6) have demonstrated that central CS can
establish eyelid conditioning faster and more effectively compared
with peripheral CS.
Central CS differ from peripheral CS, as a central
CS does not stimulate the peripheral receptor directly, but affects
central areas through which peripheral messages are transduced into
the central nervous system, such as lateral pons (7). Other CS stimulate offsets of
projections, including the projection pathway in the cerebellum and
parallel fibers.
However, whether stimulating the transduction
terminal in the cerebral cortex is able to establish eyelid
conditioning and influence the rate of acquisition is yet to be
elucidated. Furthermore, differences between the effects of delay
eyelid conditioning and trace eyelid conditioning are yet to be
determined, as well as the underlying mechanisms. Investigating
these areas is important to establish mechanisms of CS. Although a
number of associations have been demonstrated between the medial
prefrontal cortex (mPFC) and delay eyelid conditioning (8,9), the
present study, for the first time, investigated the effects of
microcurrent electrical stimulation of the mPFC on the induction of
delay eyelid conditioning in guinea pigs.
Materials and methods
Animals
In total, eight male guinea pigs (weight, 330–430 g;
Animal Center of Jilin University, Changchun, China) were used in
this study. The animals were housed in standard plastic cages and
maintained on a 12-h light/dark cycle with light onset at 7:00 a.m.
Food and water were provided ad libitum and the temperature
was maintained at 25±1°C. All experiments were performed between
8:00 a.m. and 6:00 p.m., during the light portion of the cycle.
Experimental procedures were approved by the Animal Care Committee
of Jilin University (Changchun, China) and were in accordance with
the principles outlined in the National Institutes of Health Guide
for the Care and Use of Laboratory Animals (Bethesda, MD, USA).
Surgery
The guinea pigs were allowed to acclimatize to the
cages for one week prior to surgery. The animals were anesthetized
with an intraperitoneal (i.p.) injection of a mixture of ketamine
(0.3 ml/kg; Jiangsu Hengrui Medicine Co., Ltd., Lianyungang, China)
and sumianxin II (0.05 ml/kg; Veterinarian Institute of Military
Medical Science Academy, Beijing, China). The head of the
anesthetized animal was secured to a stereotaxic apparatus (SR-6N;
Narishige International, Ltd., Tokyo, Japan) with the lambda
positioned 1.0 mm ventral to the bregma. Next, a stimulating
electrode was implanted and the coordinates obtained from the
bregma were 2.0 mm posterior and 0.7 mm lateral. Four stainless
steel screws connected by one conductor were attached to the skull
as a reference electrode. A small metal probe (Tiangen, Beijing,
China), which was used to attach the left upper eyelid to a
movement-measuring device, was sutured into, but not through, the
edge of the left upper eyelid. Following surgery, the animals were
injected with gentamycin sulfate (5 mg/kg i.p.; North China
Pharmaceutical Corporation, Shijiazhuang, China) and
benzylpenicillin sodium (10 mg/kg i.p.; North China Pharmaceutical
Corporation) every 12 h for five days and allowed one week of
recovery. In addition, the mPFC sites were examined under a light
microscope (CX31; Olympus Corp., Tokyo, Japan) following Nissl
staining (6,8).
Apparatus
Eyelid movements were measured using a
high-resolution spring-return potentiometer (JZJ01; Cheng Yi,
Chengdu, China), attached via a thread lead hooked through the
nylon loop sutured into the left upper eyelid. A stimulator (YC-2;
Chengdu, China), equipped with a constant current and stimulus
isolation, was used to deliver an electronic CS, while a plastic
pipe placed 1.0 cm from the left eyeball was used to deliver a
corneal air puff (US). The CS and US were controlled using a
computer-monitored system. Eyelid movement mechanogram and markers
of the applied stimuli were digitized at a sample rate of 20 kHz
using a data acquisition system (RM6280C; Cheng Yi) and were
acquired using the built-in software (version 4.7; Microsoft Corp.,
Redmond, WA, USA). A Windows PC was used to store and analyze the
behavioral data.
Training
Guinea pigs were allowed to adapt to the
experimental environment for two days (80 min/day) prior to the
training sessions. The daily training sessions included 50 trials
of classical delay eyelid conditioning with an interval of 15–30
sec, during which the animals were restrained in a Plexiglas
container (25×15×15 cm) located in a sound- and light-attenuating
chamber, and their heads were secured with blunt ear-bars pressing
on the head-stages. All 50 trials consisted of paired presentations
of CS and US. The CS, electrical stimulation of the mPFC, was
administered in a 1,000 Hz train of 450 µA monophasic pulses for
250 msec. The US was a mild puff of air to the eyes (3 ψ) for 100
msec.
Resident-intruder (RI) test
Three weeks prior to the start of the experiment, a
male guinea pig from the training group (mPFC operated group) was
housed with a female rat to stimulate territorial behavior. A novel
young male intruder guinea pig was exposed to a male resident rat
(mPFC male guinea pigs) for 15 min. The female was removed 30 min
prior to the test. Behaviors were recorded using a digital video
camera (LS20M; Olympus Corp.). In the present experiments, the
intruder guinea pigs were not aggressive and no intruder guinea
pigs initiated aggression.
Grouping
The pigs were divided into two groups, including the
training (in which medial prefrontal cortex was stimulated by
microcurrent electrical treatment) and sham guinea (in which pigs
did not receive any treatment) groups. Furthermore, the pigs were
divided into the RI test (in which pigs underwent the RI test) and
RI control (in which pigs were not subjected to the RI test)
groups.
Immunohistochemical detection of Fos
protein in the neurons of guinea pigs
For immunocytochemical detection of Fos protein
expression, the guinea pigs were anesthetized with Avertin
(Sigma-Aldrich, Carlsbad, CA, USA) 2 h following the initiation of
the RI test and perfused with paraformaldehyde. The sham and
training groups of the mPFC-operated guinea pigs were housed alone
until perfusion. The brains were processed for immunocytochemical
detection of Fos protein expression, as previously described by
Wang et al (10).
Western blot analysis
Tissue samples (20 mg) were homogenized in 200 µl
ice-cold lysis buffer (Sigma-Aldrich), supplemented with
phenylmethylsulfonyl fluoride, and centrifuged at 12,000 × g for 10
min at 4°C, following which the supernatants were collected. Equal
quantities of protein from each sample were subjected to SDS-PAGE
(10% polyacrylamide) and transferred onto polyvinylidene difluoride
membranes (Millipore, Bedford, MA, USA). The membranes were blocked
for 2 h with 1% bovine serum albumin, then incubated overnight at
4°C with a mouse monoclonal anti-Fos antibody (1:2,000; sc-52;
Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). The membranes
were washed and incubated with a polyclonal goat anti-mouse IgG
secondary antibody conjugated to horseradish peroxidase (1:1,000;
sc-395763; Santa Cruz Biotechnology, Inc.). Immunoreactive bands
were visualized with a SuperSignal West Pico enhanced
chemiluminescence kit (Pierce Biotechnology, Inc., Rockford, IL,
USA), according to the manufacturer's instructions. Band
intensities were quantified using Quantity One software (Bio-Rad
Laboratories, Hercules, CA, USA).
Statistical analysis
All data are expressed as the mean ± standard error
of the mean. Statistical significance was determined using the
t-test with SPSS software package version 18.0 (SPSS, Inc.,
Chicago, IL, USA), where P<0.05 was considered to indicate a
statistically significant difference.
Results
Acquisition rate of CR and SR
After seven days of CS-US paired training, the
acquisition rate of the CR exhibited an unstable increase and
reached 11.24±1.40%, which was significantly higher compared with
the guinea pigs that had not received training (P<0.01; Fig. 1A). Furthermore, the acquisition rate
of the SR also exhibited a slight increase (Fig. 1B).
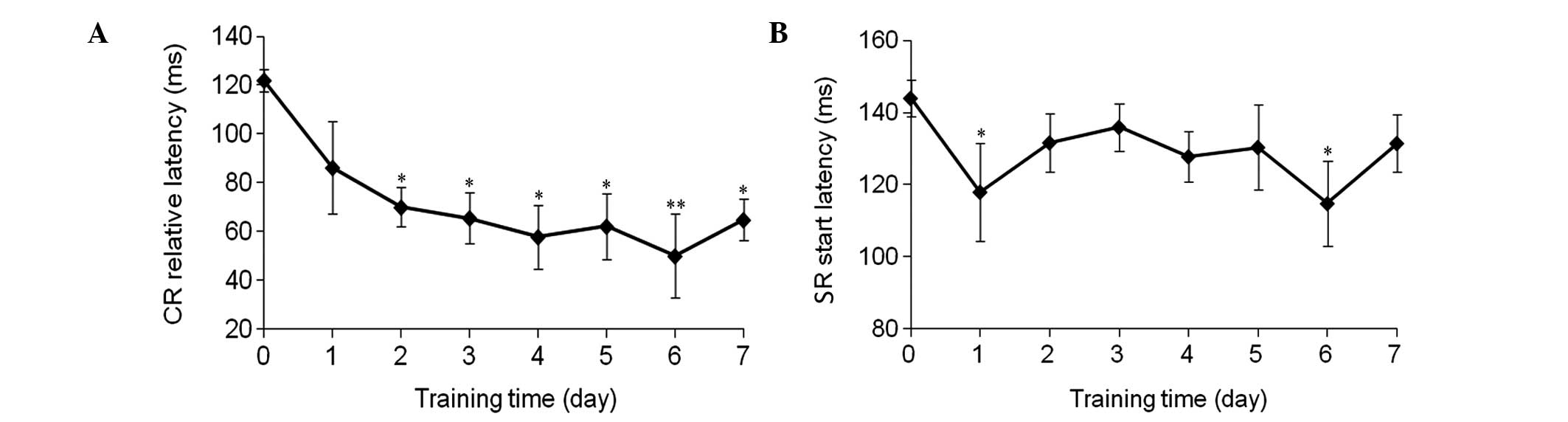
Relative latency period of the CR and
SR
The relative latency period of the CR significantly
decreased between 122.0±4.5 and 64.8±8.5 msec (P<0.01; Fig. 2A), and a decrease in the start
latency period of the SR was also observed (Fig. 2B).
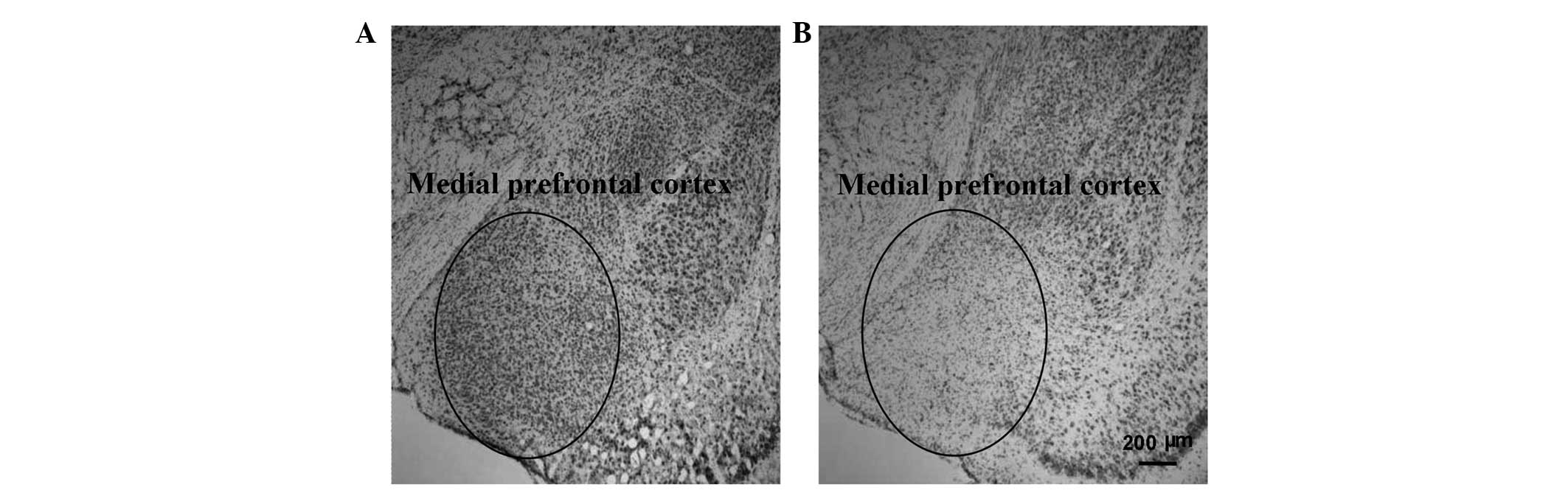
Examination of the mPFC in the
training and control guinea pigs
Examination of the mPFC with Nissl staining revealed
lesions in the mPFC regions in the training group when compared
with the control group (Fig. 3).
This observation indicated that training may trigger changes in the
mPFC region of guinea pigs.
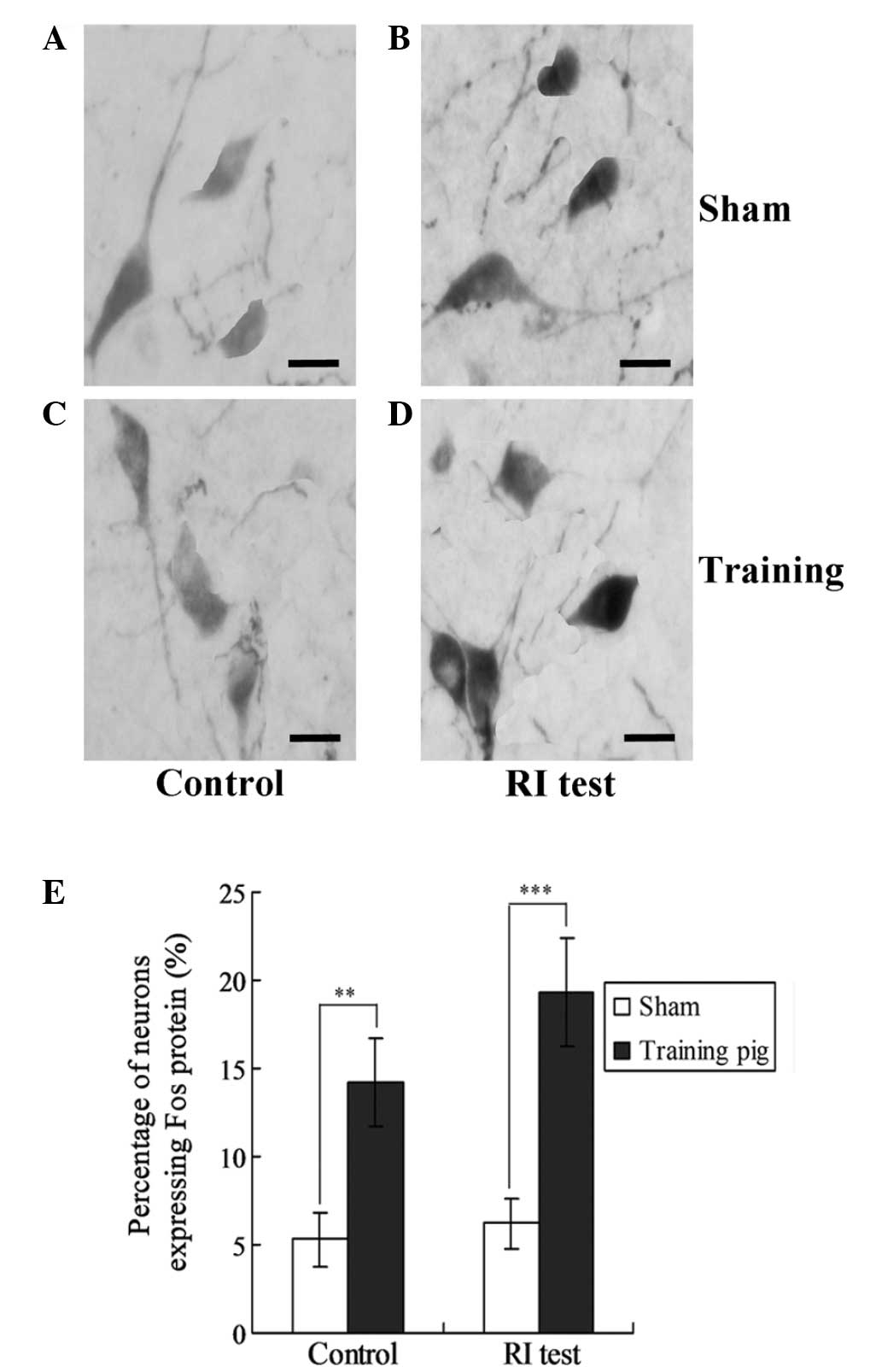
Effect of mPFC lesions on the
expression levels of Fos in the neurons of the guinea pigs in the
training group
To examine the effects of mPFC lesions on the
neurons in the training group guinea pigs, the expression levels of
Fos protein were investigated in the training and control guinea
pigs. As shown in Fig. 3, the
expression of Fos protein in the training group guinea pigs was
significantly higher compared with the sham group, for the control
pigs and RI test guinea pigs (P<0.01; Fig. 4).
Fos protein expression increases
following training in guinea pigs
In order to confirm Fos expression in the training
group guinea pigs, Fos protein was detected in the neurons using
western blot analysis. The results revealed that Fos expression was
significantly increased in the training group compared with the
sham group (P<0.01; Fig. 5).
Furthermore, for the RI test pigs, Fos expression increased in the
training group when compared with sham group (P<0.01; Fig. 5).
Discussion
The understanding of the mechanisms underlying
eyelid conditioning, including delay eyelid conditioning and trace
eyelid conditioning, has increased significantly in recent decades.
Eyelid conditioning is primarily associated with cerebellar
interpositus nuclei and brainstem nuclei (11,12);
however, it has also been shown to be involved with the mPFC
(13,14). Therefore, in the present study, CS
was performed on unreported regions, including the mPFC, to
investigate different neural circuits. Notably, it was found that
following CS-US paired training, microcurrent electrical
stimulation of the mPFC may be used as a special CS to induce delay
eyelid conditioning. However, the amplitude and acquisition speed
were lower compared with those of a conditioned reflex by auditory
CS.
Although the circuit involved in this CR has not
been investigated experimentally, it is considered to differ from
the common CR circuit. The present study hypothesized that the
former is a long-loop circuit composed of multi-synapses, while the
latter is a short-loop circuit with fewer synapses. This hypothesis
was proposed firstly since delay eyelid conditioning has been
demonstrated to be largely unaffected by forebrain lesions,
engaging the cerebellum directly (15). Secondly, the output structure of
delay eyelid conditioning and the nucleus of the facial nerve dose
were not connected to the cerebellar interpositus nuclei. Finally,
the mPFC is not the direct input terminal of sensory signals.
Therefore, mPFC may develop an indirect association with the delay
eyelid conditioning pathway via multi-synapses. Thus, the synaptic
plasticity in a long-loop circuit is more difficult to construct
and there is less signal attenuation in a short-loop circuit, which
is consistent with the phenomena of the low amplitude and
acquisition speed. However, the results demonstrated that this
special CR was not stable during the training and test session. The
reason for this is unclear, however, it was hypothesized that this
may due to the tiny movement of electrodes; for example, the same
currents form different electrode positions, which could result in
significant difference (16).
This special delay eyelid conditioning appears to be
more accurate and efficient in timing than auditory CR, as the
distance between the CR peak and the start of the US (relative CR
latency) under the stimuli of the mPFC was shorter compared with
auditory stimulation. The cerebellar cortex is considered to be
responsible for the accurate timing mechanism of eyelid
conditioning (17); however, the
signal by direct CS on the mPFC promotes the accuracy of CR timing.
Further investigation into the underlying mechanism is
required.
The delay conditioned reflex developed in the
present study is the easiest eyelid conditioning to learn, while
other harder eyelid conditioning, including trace eyelid
conditioning and long delay eyelid conditioning, are yet to be
investigated under the same conditions. In addition, the CS current
used was 450 µA, and a smaller current may not result in the same
effect. Overall, the observations of the present study may aid
further investigation into the formation of eyelid conditioning and
the mechanism underlying the circuit in various conditions; thus,
contribute to an improved understanding of conditioned reflexes.
Following the establishment of different distant cortex-induced
cortical conditioning, inactivation and impairment may then be used
to investigate the problem of this type of specific conditioning of
neural pathways.
As aforementioned, the mechanism of eyelid
conditioning has been extensively studied and the ‘double parts
hypothesis’ has been generally acknowledged. However, in this
hypothesis, the establishment of conditioning is not consistent
with the ‘temporal connection’ hypothesis proposed by Lachnit
(18). Therefore, the acquisition of
eyelid conditioning does not require temporal connection to be
established between the auditory cortex and body movement cortex.
The afferent signals caused by an auditory stimulus and/or somatic
pain stimulus reach the cerebellum concomitantly and subsequently
cause synaptic plasticity changes in the cerebellar cortex and
interpositus nucleus; thus, eyelid conditioning is established.
Lavond and Steinmetz (19)
previously reported acquisition of classical conditioning without
the cerebellar cortex. Furthermore, Kelly et al (20) demonstrated that when the cerebral and
cerebellar cortex of rabbits were damaged, eyelid conditioning was
able to be established, although more training was required.
Therefore, as the means of support of animals has
been obtained over millions of years of evolution, the conditioning
reflex has simple functions, but complicated mechanisms. However,
further investigation is required. In the present study, a CS was
exerted on the mPFC to train guinea pigs to acquire eyelid
conditioning. The results may further the understanding of
different neural circuits that multiple eyelid conditioning
establishment is based on. In addition, the results be used to
further investigate the common mechanism of conditioning.
Eyelid conditioning is a form of associative
learning and has been used extensively to study neural structures
and mechanisms of learning and memory (21,22).
Although differing in the timing of the training stimuli, variants,
including trace eyelid conditioning and delay eyelid conditioning,
involve training by a CS paired with a reinforcing US to develop a
CR (23). While auditory and visual
stimuli are usually applied to the formation of conditioned eyelid
(24), direct stimuli to the central
neural pathway are also employed to induce a successful CR. Freeman
and Rabinak (25) demonstrated that
electrical stimulation of the pontine nuclei may be used as a CS in
rodents, from which the similarity of the CS pathway in rats,
rabbits and ferrets was identified. Furthermore, Freeman and Duffel
(26) demonstrated that
microstimulation of the cochlear nucleus may be used as a CS to
assess developmental changes in projections to other auditory
nuclei or the pontine nuclei in rats. Kalmbach et al
(13) used electrical stimulation of
mossy fibers as a CS to investigate the interactions between the
mPFC and cerebellum. CS in the central neural pathway perform more
rapid enhancement of eyelid conditioning (27). The stimulated regions in the central
nerve pathway CS include brain regions, such as lateral pons, where
the signal from a traditional CS passes through, and the signal
transduction pathway, including parallel fibers.
However, whether eyelid conditioning is formed by a
direct CS of the mPFC is yet to be demonstrated. A number of
correlations have been reported between delay eyelid conditioning
and mPFC mechanically; however, the present study, for the first
time, revealed that microcurrent electrical stimulation of the mPFC
may be used as a special CS to induce delay eyelid conditioning in
guinea pigs. This observation may expand the current understanding
of conditioned reflexes.
Acknowledgements
The study was supported by a grant from the National
Natural Science Foundation of China (no. 81070875/H0902).
References
|
1
|
Vogel EH: Reinstatement of short-latency
responses after asymptotic pavlovian conditioning training by the
presentation of an extraneous stimulus. Biol Res. 45:61–65. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen Y, Huang F, Wang D, Weng Z and Deng
Z: Upregulation of heme oxygenase-1 expression may facilitate
memory and learning in mice. Exp Ther Med. 5:1491–1495.
2013.PubMed/NCBI
|
|
3
|
Thürling M, Galuba J, Thieme A, Burciu RG,
Goricke S, Beck A, Wondzinski E, Siebler M, Gerwig M, Bracha V and
Timmann D: Age effects in storage and extinction of a naturally
acquired conditioned eyeblink response. Neurobiol Learn Mem.
109:104–112. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Knowlton BJ, Lavond DG and Thompson RF:
The effect of lesions of cerebellar cortex on retention of the
classically conditioned eyelid response when stimulation of the
lateral reticular nucleus is used as the conditioned stimulus.
Behav Neural Biol. 49:293–301. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Green JT, Johnson TB, Goodlett CR and
Steinmetz JE: Eyelid classical conditioning and interpositus
nucleus activity are disrupted in adult rats exposed to ethanol as
neonates. Learn Mem. 9:304–320. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Knowlton BJ and Thompson RF:
Microinjections of local anesthetic into the pontine nuclei reduce
the amplitude of the classically conditioned eyelid response.
Physiol Behav. 43:855–857. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ding G, Shao J, Ding Q, Fang Z, Wu Z, Xu J
and Gao P: Comparison of the characteristics of mesenchymal stem
cells obtained from prostate tumors and from bone marrow cultured
in conditioned medium. Exp Ther Med. 4:711–715. 2012.PubMed/NCBI
|
|
8
|
Wu GY, Yao J, Zhang LQ, Li X, Fan ZL, Yang
Y and Sui JF: Reevaluating the role of the medial prefrontal cortex
in delay eyeblink conditioning. Neurobiol Learn Mem. 97:277–288.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang C, Hong T, Shen J, Ding J, Dai XW,
Zhou ZQ and Yang JJ: Ketamine exerts antidepressant effects and
reduces IL-1β and IL-6 levels in rat prefrontal cortex and
hippocampus. Exp Ther Med. 5:1093–1096. 2013.PubMed/NCBI
|
|
10
|
Wang Y, Liu JH, Li XH and Li L: Medial
prefrontal cortex in modifying intermale aggression via oxytocin
released in bed nucleus stria terminalis. J Neurol Sci Turk.
29:768–777. 2012.
|
|
11
|
Thompson RF and Steinmetz JE: The role of
the cerebellum in classical conditioning of discrete behavioral
responses. Neuroscience. 162:732–755. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Timmann D, Drepper J, Frings M, Maschke M,
Richter S, Gerwig M and Kolb FP: The human cerebellum contributes
to motor, emotional and cognitive associative learning. A review.
Cortex. 46:845–857. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kalmbach BE, Ohyama T, Kreider JC, Riusech
F and Mauk MD: Interactions between medial prefrontal cortex and
cerebellum revealed by trace eyelid conditioning. Learn Mem.
16:86–95. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yoshizawa K, Emoto Y, Kinoshita Y, Yuri T
and Tsubura A: N-methyl-N-nitrosourea-induced cerebellar hypoplasia
in rats: Effect of arachidonic acid supplementation during the
gestational, lactational and post-weaning periods. Exp Ther Med.
6:627–634. 2013.PubMed/NCBI
|
|
15
|
McLaughlin J, Skaggs H, Churchwell J and
Powell DA: Medial prefrontal cortex and pavlovian conditioning:
trace versus delay conditioning. Behav Neurosci. 116:37–47. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Im CH, Jung HH, Choi JD, Lee SY and Jung
KY: Determination of optimal electrode positions for transcranial
direct current stimulation (tDCS). Phys Med Biol. 53:N219–N225.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Attwell PJ, Ivarsson M, Millar L and Yeo
CH: Cerebellar mechanisms in eyelid conditioning. Ann NY Acad Sci.
978:79–92. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lachnit H: Transswitching and contextual
conditioning. Relevant aspects of time. Pavlov J Biol Sci.
21:160–172. 1986.PubMed/NCBI
|
|
19
|
Lavond DG and Steinmetz JE: Acquisition of
classical conditioning without cerebellar cortex. Behav Brain Res.
33:113–164. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kelly TM, Zuo CC and Bloedel JR: Classical
conditioning of the eyelid reflex in the decerebrate-decerebellate
rabbit. Behav Brain Res. 38:7–18. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cotterill RM: Cooperation of the basal
ganglia, cerebellum, sensory cerebrum and hippocampus: possible
implications for cognition, consciousness, intelligence and
creativity. Prog Neurobiol. 64:1–33. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li B, Wang L, Liu Y, Chen Y, Zhang Z and
Zhang J: Jujube promotes learning and memory in a rat model by
increasing estrogen levels in the blood and nitric oxide and
acetylcholine levels in the brain. Exp Ther Med. 5:1755–1759.
2013.PubMed/NCBI
|
|
23
|
Wang H, Hu Y and Tsien JZ: Molecular and
systems mechanisms of memory consolidation and storage. Prog
Neurobiol. 79:123–135. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Steinmetz AB, Edwards CR, Steinmetz JE and
Hetrick WP: Comparison of auditory and visual conditioning stimuli
in delay eyelid conditioning in healthy young adults. Learn Behav.
37:349–356. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Freeman JH and Rabinak CA: Eyelid
conditioning in rats using pontine stimulation as a conditioned
stimulus. Integr Physiol Behav Sci. 39:180–191. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Freeman JH and Duffel JW: Eyelid
conditioning using cochlear nucleus stimulation as a conditioned
stimulus in developing rats. Dev Psychobiol. 50:640–646. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wei B, Guo Y, Zhai J, Su J, Han L, Kang C
and Zhang Q: A study of the relationship between the Wnt/β-catenin
signaling pathway and the gastrointestinal development of rat
embryonic and perinatal periods. Exp Ther Med. 5:1598–1602.
2013.PubMed/NCBI
|