Introduction
Diabetes mellitus (DM) is a group of metabolic
disorders characterized by hyperglycemia over a prolonged period.
The primary causes of DM are insulin deficiency or dysfunction,
which impair the metabolism of carbohydrates, lipids and proteins,
leading to oxidative stress and resulting in chronic health
complications such as diabetic nephropathy, neuropathy,
hypertension and cardiovascular disease (1,2). It has
been estimated that the number of individuals with DM will increase
to 360–380 million between 2025 and 2030 (3). The World Health Organization previously
reported that DM was one of the ten leading causes of mortality
globally between 2000 and 2012 (4).
Although cardiovascular diseases, such as ischemic heart disease,
have been the most significant causes of mortality over the past
decade, the mortality rate associated with diabetic patients is
continuously rising at an increased rate compared with
cardiovascular diseases (4).
Therefore, it has been clearly shown that DM is currently among the
most challenging global health problems. Extensive interventions
have been recommended for the treatment of DM, including diet
control, exercise and hypoglycemic drugs, particularly insulin.
However, diet control and exercise may not be successful
interventions due to the modern lifestyle. Numerous factors may
lead to patient noncompliance, including the undesirable side
effects of the drug treatments coupled with the difficult treatment
schedules and the expense and safety of long-term use (5). Therefore, the search for medicinal
plants with anti-hyperglycemic and anti-oxidative activities and
limited side effects remains a major challenge.
Numerous medicinal plants have been identified that
exhibit anti-oxidative and anti-hyperglycemic activities (6–8). Among
them, Ocimum sanctum L. (OS) is promising as it is routinely
used in cooking vegetables, and also has been recommended for the
treatment of a number of diseases by local people in various
countries (9,10). Previous studies have shown that OS is
safe for consumption as it contains no genotoxic or organotoxic
effects (11,12). In addition, OS has been reported to
possess therapeutic value for the treatment of a number of
diseases, including bronchitis, diarrhea and dysentery (9,13). Our
previous study showed that a diet containing 2% OS leaf powder
resulted in a significant reduction in blood glucose levels in
streptozotocin-induced DM rats (14). The administration of aqueous and
alcoholic extracts of OS leaves also resulted in reduced blood
glucose levels in streptozotocin-induced DM rats (15,16), and
prevented insulin resistance in normal rats fed with a fructose
diet (17). Prior studies have
suggested that OS leaf extracts possess anti-hyperglycemic
activity, in addition to protecting organs against various stress
conditions such as hyperlipidemia (6), inflammation (18), cancer (19) and heavy metal toxicity (20,21).
Furthermore, an aqueous extract of OS leaves has been shown to
promote antioxidative activity, thus protecting various organs
against DM (16,22). OS leaves are known to be a rich
source of volatile and fixed oils. Our previous study showed that
volatile and fixed oils extracted from OS leaves decreased the
serum lipid profile and protected the hearts of rats fed with a
high fat diet (23,24). However, the anti-hyperglycemic,
anti-hyperlipidemic and organ protective effects of the fixed oil
extracted from OS leaves against DM have not yet been investigated.
It is known that the liver and kidney are the primary organs at
risk in patients with DM. Therefore, the majority of previous
studies have focused on the hepatic and renal protective activities
of various medical plants in DM (8,16,20,25).
However, DM may damage the heart in addition to the liver and
kidneys (26). Furthermore, DM has a
high correlation with cardiovascular diseases (27).
Therefore, the present study was conducted to
elucidate the anti-hyperglycemic, anti-hyperlipidemic and
anti-oxidative activities of the fixed oil extract of OS leaves,
and to evaluate the ability of this extract to protect various
vital organs including the liver, kidneys and heart in DM rats. In
addition, the chemical composition of fixed oil was determined.
Materials and methods
Extraction of fixed oil from OS
leaves
Fresh OS leaves were obtained from the National
Institute of Thai Traditional Medicine, the Ministry of Public
Health (Nonthaburi, Thailand), and were cut into small pieces (1
cm). The separated leaves were washed and air-dried at room
temperature, and ground to powder using a blender (Phillips Avance
HR2097/00; Phillips, Amsterdam, Netherlands). Fixed oil was
extracted from the OS leaves using a Soxhlet extractor (Soxtherm
Multistat/SX PC; C. Gerhardt GmbH & Co., Königswinter, Germany)
with hexane as the solvent, as previously described by the
Association of Official Analytical Chemists (28). The sample was filtered and
evaporated. The extraction solvent was removed by rotary
evaporation (Buchi R-124; Buchi Labortechnik, Flawil, Switzerland).
In cases where the extraction solvent contained OS powder, the
extraction solvent was filtered through a Whatman paper no. 1 (GE
Healthcare Life Sciences, Little Chalfont, UK) prior to
evaporation. From 100 g of dried OS leaf powder, the percentage
yield of OS fixed oil was 1.046 g%. The OS fixed oil was collected
and stored at 4°C.
Identification of fatty acids in OS
fixed oil using gas chromatography-mass spectrometry (GC-MS)
Fatty acids were transformed into methyl esters
according to the ISO procedure (29). The fatty acid methyl esters (FAMEs)
were analyzed by GC-MS, using a 5840A gas chromatograph
(Hewlett-Packard, Palo Alto, CA, USA) equipped with a flame
ionization detector for electron-impact mass spectrometry, and an
integrator. The 1.5-µl FAME sample was injected, and separation was
conducted using a Agilent J&W DM-23 capillary column (30 m
length, 0.25 mm i.d., and 0.25 mm film thickness of 5%; DEGS;
Agilent Technologies, Santa Clara, CA, USA). The carrier gas was
helium (pressure, 19 psi) with a split ratio of 50:1. The carrier
gas and the column flow rate was 62.9 ml/min. The oven temperature
was initially maintained at 80°C, then increased to 180°C at
10°C/min and finally maintained at 220°C for 7 min. The temperature
of the injection port and the detector were set at 300°C. The fatty
acids were identified by comparing their retention times with those
of standards (Sigma-Aldrich, St. Louis, MO, USA). The content of
each fatty acid was expressed as a percentage of the total fatty
acid profile.
Animal preparation
In total, 21 male Wistar rats (age, 8 weeks; weight,
200–250 g) were purchased from the Animal Center of Salaya Campus,
Mahidol University (Bangkok, Thailand). The rats were cared for in
accordance with the principles and guidelines of the Institutional
Animal Ethics Committee of Rangsit University (Pathumtani,
Thailand), which is under the National Council of Thailand for
Laboratory Animal Care. The rats were housed under a 12-h
light-dark cycle at 25±2°C and fed with standard rat food and tap
water ad libitum. DM was induced with an intraperitoneal
injection of streptozotocin (STZ; Sigma-Aldrich) dissolved in
citrate buffer (pH 4.5) at a dose of 65 mg/kg. At 5 days after STZ
injection, fasting blood glucose was measured, and only rats with a
blood glucose level of ≥200 mg/dl were included in the study.
Experimental design
Three groups of 7 rats each were established as
follows: i) Normal control rats; ii) normal untreated DM rats; and
iii) DM + fixed oil rats.
In our previous study it was observed that
supplementation with 2% dried OS leaf powder in the diet for three
weeks induced anti-hyperglycemic and lipid-lowering effects in DM
rats (14). The average dried OS
leaf powder consumption was 4.45 g/kg/day. Therefore, the daily
dose of OS fixed oil administered in the present study, calculated
based on our previous data, was ~46.54 mg/kg/day.
Following diabetic induction, DM rats were
administered OS fixed oil by intragastric intubation once a day for
three weeks. Blood was collected weekly from rat tail veins to
determine blood glucose levels. Body weight and food consumption
were determined once a week.
Determination of serum lipid profile,
serum insulin and biochemical evaluation of liver, kidney and
cardiac injury
Following the OS fixed oil administration period,
the rats were fasted overnight and anesthetized by an
intraperitoneal injection of zolitil (40 mg/kg; Virbac, Carros,
France) and xylazine (3 mg/kg; L.B.S. Laboratory Ltd., Bangkok,
Thailand). Blood was collected from the abdominal vein to determine
serum insulin and lipid profile levels, including total
cholesterol, triglyceride, low-density lipoprotein cholesterol
(LDL-C), and high-density lipoprotein cholesterol (HDL-C). Liver
function was evaluated by determining the serum levels of alanine
aminotransferase (ALT) and aspartate aminotransferase (AST). In
addition, cardiac injury was evaluated by measuring serum lactate
dehydrogenase (LDH) and creatine kinase MB subunit (CK-MB) levels.
Kidney function was evaluated by measuring the serum levels of
creatinine and blood urea nitrogen (BUN).
Determination of lipid peroxide and
the activity of antioxidative enzymes in the liver, kidney and
heart
Following the experimental period, the rats were
fasted overnight and anesthetized using zolitil (40 mg/kg) and
xylazine (3 mg/kg). Subsequently, the jugular vein was cannulated
and perfused with ice-cold normal saline to remove the red blood
cells. When all organs looked pale, the liver, kidney and heart
were excised, cleaned and weighed. All organs were stored at −80°C
until required for further analysis.
Determination of tissue lipid peroxide
content
All organs were homogenized with 0.1 M phosphate
buffer (pH 7.4). Lipid peroxides in the liver, kidney and heart
were assessed using thiobarbituric acid reactive substances
(TBARS), as previously described (30). TBARS was expressed in nmol
malondialdehyde (MDA)/mg protein, using 1,1,3,3-tetraethoxy propane
as a standard. Tissue protein levels were determined using Lowry's
method, as previously described (31).
Determination of the activity of
antioxidative enzymes in the various tissue samples
The levels of a number of antioxidative enzymes,
including glutathione peroxidase (GPx), catalase (CAT) and
superoxide dismutase (SOD) were determined. The liver, kidney and
cardiac tissue homogenates were prepared by homogenizing the
tissues in 0.1 M phosphate buffer (pH 7.4). The homogenate was then
centrifuged at 832 × g at 4°C for 10 min. The supernatant was
collected and centrifuged again at 7,800 × g at 4°C for 30 min. The
supernatant fraction was collected and further centrifuged at
136,000 × g at 4°C for 60 min. The final supernatant was analyzed
to estimate the GPx, CAT and SOD activities, using the procedures
described by Tapple (32), Luck
(33), and Winterbourn et al
(34), respectively.
Evaluation of tissue morphology
According to the experimental results, OS fixed oil
only protected the kidney against DM. Therefore, only the
histopathological appearance of renal tissue was evaluated.
Following the study, kidneys were removed and washed with normal
saline. All kidneys were longitudinally sectioned and fixed in 10%
neutral-buffered formalin. Subsequently, the kidney samples were
dehydrated and prepared under routine paraffin embedding protocol
(35). The samples in paraffin
blocks were serially sectioned at 5 µm, deparaffinized and stained
with conventional hematoxylin and eosin (H&E) stain (Bio-Optica
Milano SpA, Milan, Italy).
Biochemical assay for blood glucose,
total cholesterol, triglyceride, HDL-C, LDL-C, AST, ALT,
creatinine, BUN, LDH, CK-MB and serum insulin
Blood glucose levels were determined using a blood
glucose strip (Abbott Laboratories Ltd., Maidenhead, UK). The
concentrations of total cholesterol, triglyceride and HDL-C were
assayed by using the cholesterol (cat. no. 10028) and triglyceride
(cat. no. 10724) enzymatic assay kits (Gesellschaft für Biochemica
und Diagnostica GmbH, Wiesbaden, Germany). In order to measure the
serum cholesterol and triglyceride levels, 10 µl serum was added to
1 ml of assay reagent, the samples were incubated at 37°C for 5
min, and then the absorbance was measured at 500 nm (for serum
lipid profile: Genesys 20 spectrophotometer, model 4001/4, Thermo
Fisher Scientific, Inc., Haverthill, MA, USA; for other serum
levels: UV spectrophotometer, model UV-2501PC, Shimadzu Co., Ltd.,
Kyoto, Japan). The serum levels of the various parameters were
calculated according to the manufacturer's instructions in the
assay kits.
For HDL-C determination, 200 µl serum was added in
500 µl of precipitating reagent, mixed and incubated at room
temperature for 10 min. The samples were then centrifuged at 4,000
× g for 10 min and 200 µl supernatant was collected and used to
determine the HDL-C using assay reagent. Subsequently, LDL-C was
calculated using the following equation: LDL-C = [TC-(HDL-C)] -
(triglyceride/5).
The serum levels of AST, ALT, creatinine, BUN, LDH
and CK-MB were measured using various enzymatic kits. In order to
determine the AST and ALT levels (AST assay kit, cat. no. 12011;
ALT assay kit, cat. no. 120120; Gesellschaft Für Biochemica und
Diagnostica GmbH), 200 µl serum was added to 1 ml of working
reagent, the samples were incubated at 37°C for 1 min, and then the
absorbance was measured at 340 nm every 1 min for 3 min.
The assay kits used for creatinine (cat. no. CR
510), urea (cat. no. UR 446), LDH (cat. no. LD 401) and CK-MB (cat.
no. CK 1296) were purchased from Randox Laboratories Ltd. (London,
UK). For serum creatinine and urea determination, 10 µl serum was
added to 1 ml of working reagent and the samples were incubated at
37°C for 30 sec. The absorbance of the creatine sample was read
immediately and 2 min later at 492 nm, while that of urea was read
immediately and 1 min later at 340 nm. The serum BUN level was then
calculated by dividing the serum urea level by 2.14, which is the
conversion factor derived based on the molecular weights of BUN and
urea. For serum LDH and CK-MB determination, 20 and 40 µl serum,
respectively, was added to 1 ml of working reagent, and the samples
were incubated at 37°C for 30 sec and 10 min, respectively. The
absorbance at 340 nm was measured immediately and every 1 min for 3
min for LDH, and immediately and after 5 min for CK-MB.
Serum insulin was assayed using a radioimmunoassay
kit (cat. no. TKIN1; Diagnostic Product Co., Ltd., Los Angeles, CA,
USA). Briefly, 200 µl serum was added to the Coat-A-count tube
(included in the kit), followed by 1 ml 125I-Insulin,
and then the sample was mixed and incubated overnight at 15–28°C.
The remaining insulin was decanted, the sample tubes were inverted
and then counted in a γ counter for 1 min (Genesys Gamma 1,
Laboratory Technologies Inc., Elburn IL, USA), according to the
manufacturer's instructions.
Statistical analysis
Values are presented as the mean ± standard error of
the mean. The results were analyzed by one-way analysis of
variance. Duncan multiple rank test was performed to determine
statistical significance among groups using SPSS software, version
11.5 (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Fixed oil composition, body weight and
food intake
Saturated and unsaturated fatty acids were
identified in the OS fixed oil, as shown in Fig. 1 and Table
I. The predominant fatty acid identified in the fixed oil was
α-linolenic acid (60.60%). Fig. 2
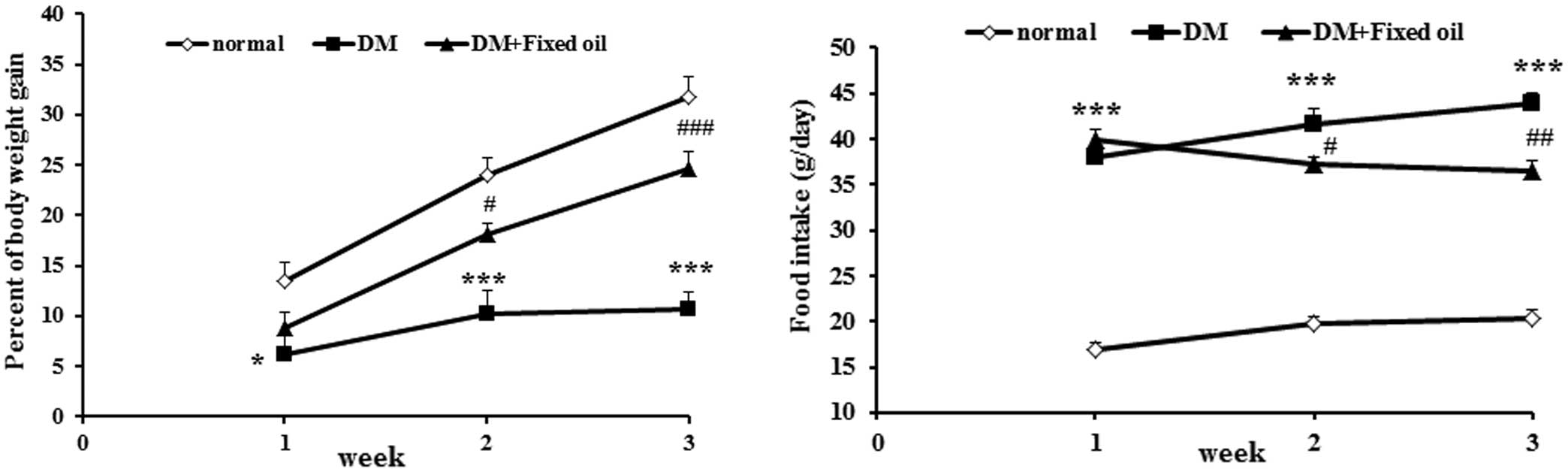
shows the percentage body weight gain and food intake of all
groups. Body weight gain of the untreated DM rats was significantly
reduced compared with the normal control rats throughout three
weeks (P<0.05 at the first week and P<0.001 at the second and
third weeks). The reduction in body weight gain was more prominent
in the DM rats treated with the OS fixed oil. Food intake was
significantly increased in DM rats (P<0.001), and was slightly
lowered at the second and third week in the DM rats treated with
the OS fixed oil.
 | Table I.Fatty acid compositions in the fixed
oil extracted from Ocimum sanctum L. leaves. |
Table I.
Fatty acid compositions in the fixed
oil extracted from Ocimum sanctum L. leaves.
| Fatty acid | Molecular
formula | % |
|---|
| Palmitic | 16:0 | 15.65 |
| Stearic | 18:0 |
3.08 |
| Oleic | 18:1 (n-9) cis |
2.81 |
| Linoleic | 18:2 (n-6) cis | 17.86 |
| α-Linolenic | 18:3 (n-3) | 60.60 |
Blood glucose, serum insulin, lipid
profile, and organ weight and function
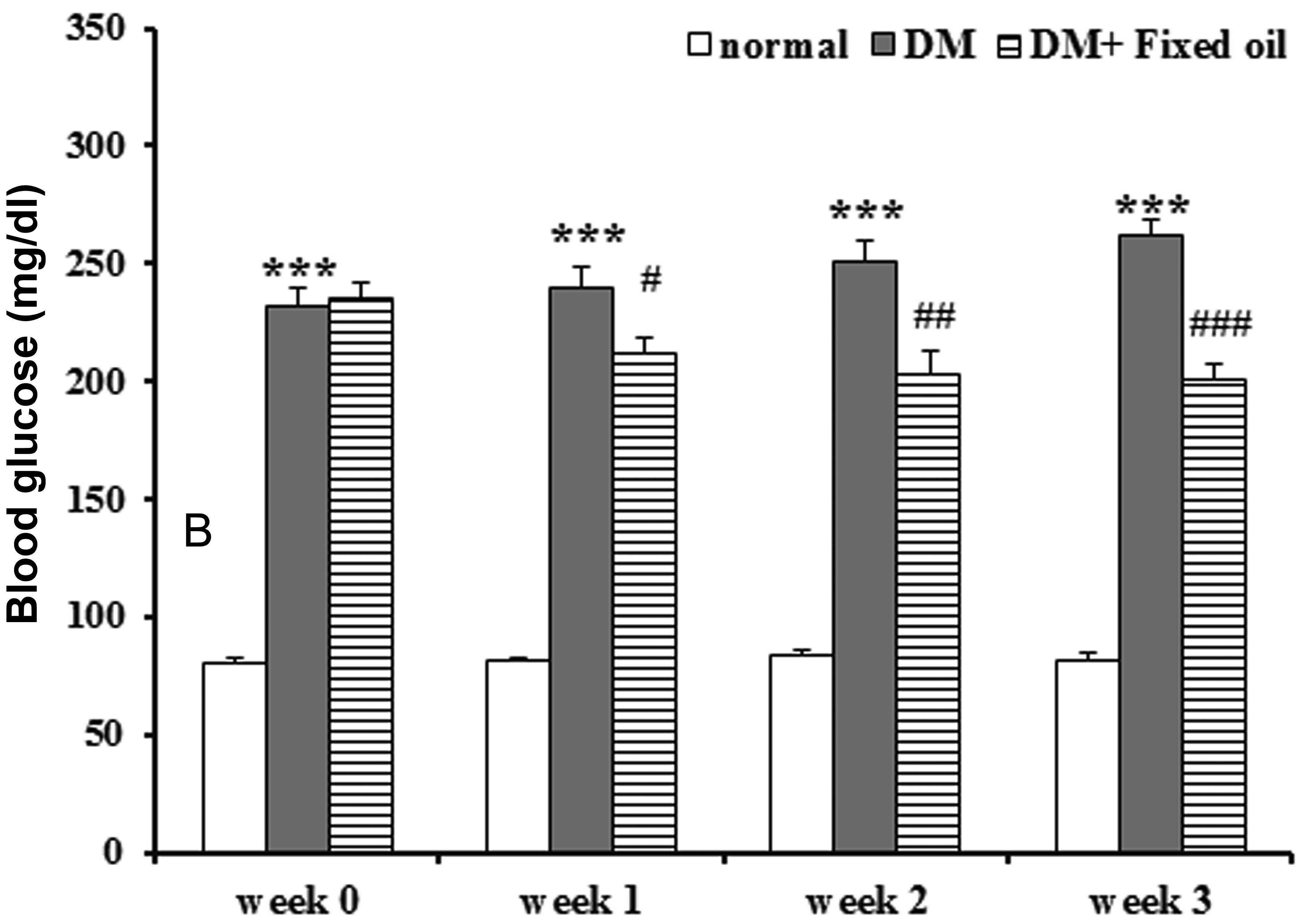
Fixed oil significantly reduced high blood glucose
of DM rats throughout three weeks of treatment (Fig. 3; P<0.001). Serum insulin levels
were significantly decreased in the DM rats (P<0.001), an effect
which was more marked in DM rats treated with the OS fixed oil
(Table II). Liver, kidney and heart
weight were significantly increased in the untreated DM rats
(Table II; P<0.001). Only the
high level of kidney weight was attenuated in the DM rats treated
with the OS fixed oil. Total cholesterol, triglyceride and LDL-C
were significantly increased (P<0.001), whereas HDL-C was
reduced in the untreated DM rats (Table III; P<0.05). The high serum
lipid profile was markedly reduced, whereas HDL-C was slightly
increased in the DM + OS fixed oil rats. Serum levels of AST, ALT,
LDH, CK-MB, creatinine and BUN in the untreated DM rats were
significantly increased compared with the normal control rats
(Table IV). Only high serum levels
of creatinine and BUN were reversed in DM + fixed oil rats.
 | Table II.Alterations of serum insulin, and
liver, kidney and heart weight in the three groups. |
Table II.
Alterations of serum insulin, and
liver, kidney and heart weight in the three groups.
| Group | Serum insulin
(µU/ml) | Liver weight
(g/kg) | Kidney weight
(g/kg) | Heart weight
(g/kg) |
|---|
| Normal | 5.98±0.26 | 34.9±1.3 |
6.43±0.11 |
3.5±0.07 |
| DM |
3.22±0.18a |
52.3±2.0a |
13.3±0.7a |
4.0±0.1a |
| DM + fixed oil |
4.50±0.24a,b |
51.0±1.4a |
11.8±0.2a,b |
4.1±0.1a |
 | Table III.Differences in the serum lipid
profile in the three groups. |
Table III.
Differences in the serum lipid
profile in the three groups.
| Group | Total cholesterol
(mg/dl) | Triglyceride
(mg/dl) | HDL-C (mg/dl) | LDL-C (mg/dl) |
|---|
| Normal | 49±1 | 55±3 | 26±1 | 12±1 |
| DM | 93±7a | 92±7a | 20±1a | 55±6a |
| DM + fixed oil | 70±4a,b | 45±8b | 23±2 | 39±3a,b |
 | Table IV.Differences in ALT, AST, LDH, CK-MB,
creatinine and BUN in serum of rats in the three groups. |
Table IV.
Differences in ALT, AST, LDH, CK-MB,
creatinine and BUN in serum of rats in the three groups.
| Group | AST (U/l) | ALT (U/l) | LDH (U/l) | CK-MB (U/l) | Creatinine
(mg/dl) | BUN (mg/dl) |
|---|
| Normal |
82±7 | 36±3 | 279±46 | 420±49 | 0.92±0.07 | 14.4±0.5 |
| DM |
132±12a |
75±11a | 545±34a | 593±47a |
2.18±0.22a |
25.2±0.5a |
| DM + fixed oil | 138±6a | 79±5a | 452±63a | 603±40a |
1.27±0.18b |
20.2±0.7a,b |
Lipid peroxide and antioxidant
levels
The high level of tissue lipid peroxide as indicated
by TBARS was significantly increased (P<0.001), whereas GPx
(P<0.05), CAT (P<0.001) and SOD (P<0.001) activities were
significantly decreased, in the liver tissue of the untreated DM
rats (Table V). TBARS and CAT were
increased, whereas no significant changes in GPx and SOD activity
were observed in the cardiac tissue of the untreated DM rats. The
OS fixed oil treatment had no effect on TBARS or the activities of
any antioxidative enzymes in the liver and cardiac tissues.
However, renal TBARS was increased, while the activities of GPx and
SOD were decreased, without significant changes of CAT activity, in
the untreated DM rats. The OS fixed oil treatment appeared to
reduce the elevated level of TBARS to below normal levels, and
normalized the low levels of GPx and SOD activities, while markedly
increasing CAT activity in the renal tissue of the DM + fixed oil
rats.
 | Table V.Effect of fixed oil of Ocimum
sanctum L. leaves on lipid peroxide and antioxidative enzymes
activity in the rat liver, heart and renal tissues. |
Table V.
Effect of fixed oil of Ocimum
sanctum L. leaves on lipid peroxide and antioxidative enzymes
activity in the rat liver, heart and renal tissues.
| Group | TBARS | GPx | CAT | SOD |
|---|
| Liver |
|
|
|
|
|
Normal |
1.05±0.05 |
1.01±0.06 |
254±27 |
112±10 |
| DM |
1.34±0.03a |
0.61±0.06a |
138±9a |
51±7a |
| DM +
fixed oil |
1.29±0.03a |
0.72±0.02a |
166±10a |
54±4a |
| Heart |
|
|
|
|
|
Normal |
0.72±0.03 |
0.27±0.01 |
9.2±0.8 |
41±5 |
| DM |
0.98±0.03a |
0.25±0.01 |
19.9±0.8a |
40±5 |
| DM +
fixed oil |
0.93±0.02a |
0.28±0.02 |
21.0±0.9a |
45±4 |
| Kidney |
|
|
|
|
|
Normal |
1.02±0.03 |
1.02±0.02 |
4.99±0.38 |
52±4 |
| DM |
1.20±0.05a |
0.80±0.06a |
4.71±0.24 |
31±4a |
| DM +
fixed oil |
0.89±0.02a,b |
1.13±0.11b |
76.4±9.5a,b |
45±5b |
Histopathological examination
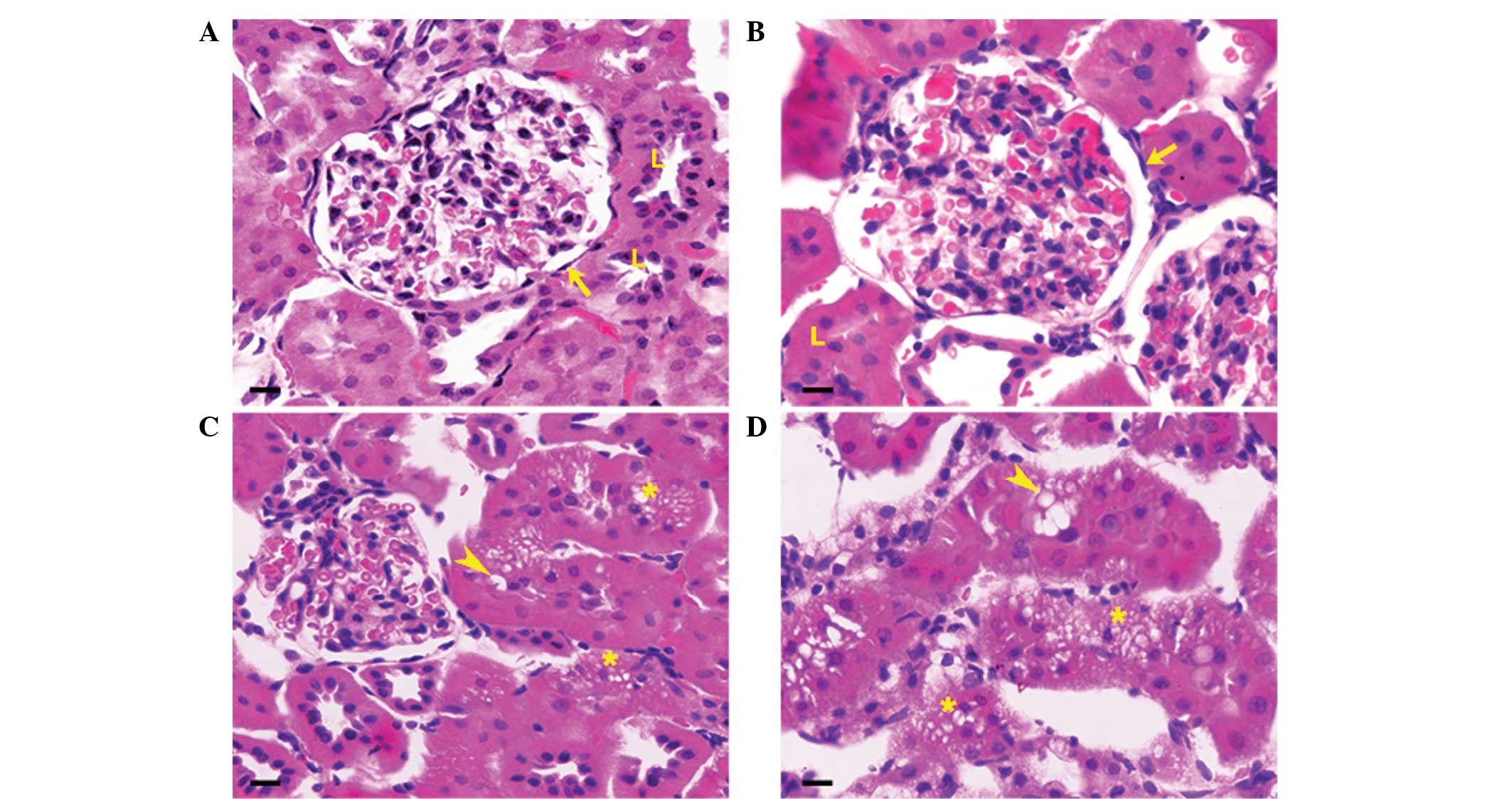
Fig. 4 shows the
histopathological examination of renal tissues in all groups. The
renal tissue of normal rats exhibited normal cortex and medullar
features (Fig. 4A). The renal
glomeruli and intact lining epithelium of the Bowman's capsule were
normal. The glomerular capillary loops were lining with normal
mesangial cells, which exhibited basic appearance of the loops. The
quantities of vascular endothelial and mesangial cells were within
normal limits. Furthermore, the proximal convoluted tubules were
preserved and contained with the luminal spaces indicated by
well-differentiated columnar epithelial cells. The renal tissue of
the DM rats showed moderate to severe multifocal necrosis, with
interstitial infiltration of mononuclear cells (Fig. 4C and D). The glomerular membranes
were thickened, with interposition of mesangial cells between two
layers. There was an increased mesangial cellularity and matrix.
Furthermore, the proximal convoluted tubules exhibited numerous
cellular vacuolizations within the cytoplasm of the epithelial
cells lining the proximal convoluted tubules. Predominant clusters
of large vacuoles were observed in certain tubular epithelial
cells. Cellular swelling of the tubular epithelium is noticeable
without its luminal space. In the DM + fixed oil rat tissues,
general renal appearance was improved (Fig. 4B). Glomeruli remained defined,
mesangial cells showed reduced proliferation and the cellular
matrix showed decreased accumulation. The cytoplasmic vacuolization
was markedly reduced in the epithelium of the proximal convoluted
tubules.
Discussion
In addition to cancer and cardiovascular diseases,
diabetes mellitus (DM) has become a major global health problems in
the 21st century, due to the gradually and continuous increased in
the number of diabetic patients (3,4). Due to
the wide range of affected organs and its influence on multiple
organ systems, DM may lead to serious health complications and
potentially mortality. Although a number of interventions are
recommended for the management of DM, patients are often
unsuccessful at following the regimes due to lifestyle and
undesirable side effects, cost and the safety of the long-term use
of synthetic drugs (5,36). Therefore, novel treatments that
fulfil the required safety and efficiency of life-long treatments
are needed. It has been widely accepted that medicines of herbal
origin may be useful in treating various diseases, as many contain
bioactive photochemical ingredients that may function as effective,
safe and cheap therapeutic compounds (7,16).
Ocimum sanctum L. (OS), commonly used as a cooking
vegetable, has shown its potency as a therapeutic herb and has
already been proven to be safe for long term consumption, as
mentioned earlier. A number of experimental studies have evaluated
the anti-diabetic activity of OS using alcoholic and aqueous
extracts of its leaves (15–17). However, OS leaves are enriched in
volatile and fixed oils. To the best of our knowledge, no previous
studies have investigated the anti-diabetic and anti-oxidative
effects of fixed oil extracted from OS leaves. The present study
showed that the fixed oil extract of OS decreased the elevated
blood glucose levels and serum lipid profile in DM rats (Fig. 3 and Table III). These results are consistent
with those of our previous study, which demonstrated the
anti-hyperlipidemic activity of OS fixed oil in rats fed with a
high fat diet (24). The
lipid-lowering effect of OS may be a beneficial property for the
prevention of cardiovascular disease in DM.
β-cells are known to be highly susceptible to
cytotoxic agents such as streptozotocin (STZ) (37). STZ induces DM by the rapid depletion
of β-cells, leading to a reduction in insulin secretion. Insulin is
a vital hormone for the maintenance of normal levels of blood
glucose and lipids. The present results indicated that the low
level of serum insulin in DM rats could be raised by treatment with
OS fixed oil. Therefore, the anti-hyperglycemic and lipid-lowering
abilities of OS fixed oil may be associated with its ability to
improve pancreatic β-cell function. α-Linolenic acid, the primary
fatty acid contained in OS fixed oil, is speculated to be the
active ingredient underlying these activities, which is supported
by several studies (38,39). In a previous study, α-linolenic acid
agonist administration significantly decreased plasma glucose and
augmented insulin release in mice (38). Furthermore, a diet rich in
α-linolenic acid has been shown to improve insulin sensitivity by
increasing GLUT4 protein content in the gastrocnemius muscle
membranes of STZ-induced diabetic rats (38).
Lipid peroxidation is a free radical-mediated
process that occurs following oxidative stress. If this process is
localized in biological membranes it may cause a variety of types
of cellular membrane damage, including change in membrane fluidity,
increased membrane permeability and finally membrane rupture
(40). Therefore, free radicals
serve a crucial function in the induction of a variety of
stress-related diseases. In order to prevent the cellular
deterioration induced by free radicals, cells possess an
antioxidant defense system. An elevation of blood glucose induces
oxidative stress, resulting in an increased production of
oxygenated free radicals and decreased antioxidant enzyme
activities (6,41). This may result in intracellular
structure modification, and ultimately affect normal cellular
function, leading to pathogenesis and the development of diabetic
complications (41,42). The present results show that DM
impaired the liver, kidney and cardiac functions of the DM rats, as
indicated by the augmentation of serum levels of AST, ALT,
creatinine, BUN, LDH and CK-MB (Table
IV). In addition, TBARS levels were significantly increased,
whereas the activities of various antioxidative enzymes were
significantly suppressed in the liver, heart and renal tissues of
untreated DM rats (Table V). These
results demonstrate that three weeks of diabetic induction is
sufficient to cause oxidative stress and damage to the liver, heart
and renal tissues of rats.
Notably, the TBARS level was increased without
significant differences in GPx and SOD, whereas CAT was markedly
increased in the cardiac tissue of untreated DM rats. It has been
reported that during periods of elevated oxidative stress, cells
protect themselves by increasing activity of various antioxidative
enzymes (6,43). Therefore, the augmentation of cardiac
CAT activity without changes in GPx and SOD activity is potentially
a compensatory response against the oxidative stress induced by DM
(43,44). However, the enhancement of cardiac
CAT activity may not markedly protect the heart against oxidative
stress, as levels of cardiac TBARS remained elevated. This suggests
that numerous free radicals were generated in the rat cardiac
tissue, which could not be eliminated through increasing CAT
activity alone.
The OS fixed oil exerted no hepatoprotective or
cardioprotective effects against DM, as indicated by the unchanged
elevations in the serum levels of AST, ALT, LDH and CK-MB, in
addition to TBARS levels and the activities of the various
anti-oxidative enzymes (Tables IV
and V). The results of our previous
study suggested that OS fixed oil exerted a cardioprotective effect
in rats fed with a high fat diet (24); however, fixed OS oil appeared to have
no effect in diabetic rats in the present study. This may be due to
the difference in the severity of stress exposure. The hearts of
the DM rats in the present study were exposed to hyperglycemic and
hyperlipidemic conditions, whereas the hearts of the high fat-fed
rats were exposed to hyperlipidemia only (24,45). In
contrast to the liver and heart tissues, OS fixed oil normalized
GPx and SOD activities and markedly promoted CAT activity, which
subsequently decreased the high level of TBARS, in renal tissue.
These results indicate that the OS fixed oil exerted a free radical
scavenging activity, providing renal protection against DM. This
hypothesis is supported by the normal general appearance of the rat
renal tissue, as shown by histopathological analysis (Fig. 4).
Notably, the OS fixed oil exerted a marked
anti-oxidative capacity in the renal tissue of the DM rats; by
normalizing the reduced levels of GPx and SOD, in addition to
enhancing the CAT activity to a higher than normal level. This may
be the reason why the OS fixed oil suppressed the renal TBARS
levels to below normal levels (Table
V). The α-linolenic acid contained in the OS fixed oil may be
responsible for the renal protective activity against DM. It has
been shown that a high α-linolenic acid diet ameliorated diabetic
nephropathy in rats (46) and
prevented high glucose-induced renal tubular cell damage by
reducing the production of reactive oxygen species (47). The present results suggest that fixed
oil extracted from OS leaves may be of therapeutic use as an
anti-hyperglycemic and lipid-lowering agent, in addition to being a
cytoprotective agent for the prevention of renal injury as a result
of DM. As the present study was performed in rats with STZ-induced
DM, which is type 1 DM, the effect of OS fixed oil in type 2 DM
requires further investigation.
In conclusion, treatment with the fixed oil
extracted from OS leaves for three weeks significantly lowered the
diabetically-elevated blood glucose levels and serum lipid profile,
while increasing serum insulin levels in STZ-induced DM rats. The
OS fixed oil exhibited a free radical scavenging activity, which
provided renal protection against DM via the suppression of high
TBARS levels, and by enhancing the activity of various
antioxidative enzymes in the rat renal tissue. Histopathological
studies showed that the OS fixed oil protected rat renal tissues
against DM. The α-linolenic acid contained in OS fixed oil may be
mechanistically involved in its anti-hyperglycemic,
anti-hyperlipidemic and renal protective activities against DM.
Acknowledgements
The authors thank the Thailand Research Fund for
supporting this project (grant no. RMU5080016).
References
|
1
|
American Diabetes Association: Diagnosis
and classification of diabetes mellitus. Diabetes Care. 33(Suppl
1): 62–69. 2010.
|
|
2
|
Rochette L, Zeller M, Cottin Y and Vergely
C: Diabetes, oxidative stress and therapeutic strategies. Biochim
Biophys Acta. 1840:2709–2729. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wild S, Roglic G, Green A, Sicree R and
King H: Global prevalence of diabetes: estimates for the year 2000
and projections for 2030. Diabetes Care. 27:1047–1053. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
World Health Organization: The top 10
causes of death. Fact sheet No 310. http://www.who.int/mediacentre/factsheets/fs310/en/Accessed.
May. 2014
|
|
5
|
Emilien G, Maloteaux JM and Ponchon M:
Pharmacological management of diabetes: Recent progress and future
perspective in daily drug treatment. Pharmacol Ther. 81:37–51.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Suanarunsawat T, Ayutthaya WD, Songsak T,
Thirawarapan S and Poungshompoo S: Lipid-lowering and antioxidative
activities of aqueous extracts of Ocimum sanctum L. leaves
in rats fed with a high-cholesterol diet. Oxid Med Cell Longev.
2011:9620252011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Taleb-Senouci D, Ghomari H, Krouf D,
Bouderbala S, Prost J, Lacaille-Dubois MA and Bouchenak M:
Antioxidant effect of Ajuga iva aqueous extract in
streptozotocin-induced diabetic rats. Phytomedicine. 16:623–631.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sarkhail P, Rahmanipour S, Fadyevatan S,
Mohammadirad A, Dehghan G, Amin G, Shafiee A and Abdollahi M:
Antidiabetic effect of Phlomis anisodonta: Effects on
hepatic cells lipid peroxidation and antioxidant enzymes in
experimental diabetes. Pharmacol Res. 56:261–266. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pattanayak P, Behera P, Das D and Panda
SK: Ocimum sanctum Linn. A reservoir plant for therapeutic
applications: An overview. Pharmacogn Rev. 4:95–105. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cohen MM: Tulsi - Ocimum sanctum: A
herb for all reasons. J Ayurveda Integr Med. 5:251–259. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chandrasekaran CV, Srikanth HS, Anand MS,
Allan JJ, Viji MM and Amit A: Evaluation of the mutagenic potential
and acute oral toxicity of standardized extract of Ocimum
sanctum (OciBest™). Hum Exp Toxicol. 32:992–1004. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gautam MK and Goel RK: Toxicological study
of Ocimum sanctum linn leaves: Hematological, biochemical
and histopathological studies. J Toxicol.
2014:1356542014.PubMed/NCBI
|
|
13
|
Mahajan N, Rawal S, Verma M, Poddar M and
Alok S: A phytopharmacological overview on Ocimum species
with special emphasis on Ocimum sanctum. Biomed Prevent
Nutr. 3:185–192. 2013. View Article : Google Scholar
|
|
14
|
Suanarunsawat T and Songsak T:
Anti-hyperglycemic and anti-hyperlipidemic effect of dietary
supplement of white Ocimum sanctum L. before and after
STZ-induced diabetes mellitus. Inter J Diabetes Metab. 13:18–23.
2005.
|
|
15
|
Narendhirakannan RT, Subramanian S and
Kandaswamy M: Biochemical evaluation of antidiabetogenic properties
of some commonly used Indian plants on streptozotocin-induced
diabetes in experimental rats. Clin Exp Pharmacol Physiol.
33:1150–1157. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Suanarunsawat T, Ayutthaya WD,
Thirawarapan S and Poungshompoo S: Anti-oxidative,
anti-hyperglycemic and lipid-lowering effects of aqueous extracts
of Ocimum sanctum L. leaves in diabetic rats. Food Nutr Sci.
5:801–811. 2014. View Article : Google Scholar
|
|
17
|
Reddy SS, Karuna R, Baskar R and
Saralakumari D: Prevention of insulin resistance by ingesting
aqueous extract of Ocimum sanctum to fructose-fed rats. Horm
Metab Res. 40:44–49. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shetty S, Udupa S and Udupa L: Evaluation
of antioxidant and wound healing effects of alcoholic and aqueous
extract of Ocimum sanctum linn in rats. Evid Based
Complement Alternat Med. 5:95–101. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bhattacharyya P and Bishayee A: Ocimum
sanctum Linn. (Tulsi): An ethnomedicinal plant for the
prevention and treatment of cancer. Anticancer Drugs. 24:659–666.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Banu Sharmila G, Kumar G and Murugesan AG:
Effects of leaves extract of Ocimum sanctum L. on
arsenic-induced toxicity in Wistar albino rats. Food Chem Toxicol.
47:490–495. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ramesh B and Satakopan VN: Antioxidant
activities of hydroalcoholic extract of Ocimum sanctum
against cadmium induced toxicity in rats. Indian J Clin Biochem.
25:307–310. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hussain EH, Jamil K and Rao M:
Hypoglycaemic, hypolipidemic and antioxidant properties of tulsi
(Ocimum sanctum linn) on streptozotocin induced diabetes in
rats. Indian J Clin Biochem. 16:190–194. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Suanarunsawat T, Na Devakul Ayutthaya W,
Songsak T, Thirawarapan S and Poungshompo S: Antioxidant activity
and lipid-lowering effect of essential oils extracted from
Ocimum sanctum L. leaves in rats fed with a high cholesterol
diet. J Clin Biochem Nutr. 46:52–59. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Suanarunsawat T, Boonnak T, Ayutthaya Na
WD and Thirawarapan S: Anti-hyperlipidemic and cardioprotective
effects of Ocimum sanctum L. fixed oil in rats fed a high
fat diet. J Basic Clin Physiol Pharmacol. 21:387–400. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Su J, Zhang P, Zhang JJ, Qi XM, Wu YG and
Shen JJ: Effects of total glucosides of paeony on oxidative stress
in the kidney from diabetic rats. Phytomedicine. 17:254–260. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li CJ, Lv L, Li H and Yu DM: Cardiac
fibrosis and dysfunction in experimental diabetic cardiomyopathy
are ameliorated by alpha-lipoic acid. Cardiovasc Diabetol.
11:732012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mercer BN, Morais S, Cubbon RM and Kearney
MT: Diabetes mellitus and the heart. Int J Clin Prac. 66:640–647.
2012. View Article : Google Scholar
|
|
28
|
AOAC International: Method 948.22.
Official Methods of Analysis of AOAC International (16th).
(Washington, DC). Association of Analytical Communities. 1995.
|
|
29
|
International Organization for
Standardization (ISO): Animal and vegetable fats and oils:
Preparation of methyl esters of fatty acids (Method ISO 5509). ISO
(Geneva). 1–6. 1978.
|
|
30
|
Ohkawa H, Ohishi N and Yagi K: Assay for
lipid peroxides in animal tissues by thiobarbituric acid reaction.
Anal Biochem. 95:351–358. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lowry OH, Rosebrough NJ, Farr AL and
Randall RJ: Protein measurement with the Folin phenol reagent. J
Biol Chem. 193:265–275. 1951.PubMed/NCBI
|
|
32
|
Tapple AL: Glutathione peroxidase and
hydroperoxidase methods. Methods in Enzymology. 2:Sidney F and
Lester P: (New York, NY). Academic Press. 506–513. 1978. View Article : Google Scholar
|
|
33
|
Luck H: Catalase. Method for Enzymatic
Analysis. 3:Bergmeyer HU: (New York and London). Academic Press.
885–894. 1965. View Article : Google Scholar
|
|
34
|
Winterbourn CC, Hawkins RE, Brian M and
Carrell RW: The estimation of red cell superoxide dismutase
activity. J Lab Clin Med. 85:337–341. 1975.PubMed/NCBI
|
|
35
|
Hewitson TD, Wigg B and Becker GJ: Tissue
preparation for histology: Fixation, embedding, and antigen
retrieval for light microscopy. Methods Mol Biol. 611:3–18. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Reichard P and Pihl M: Mortality and
treatment side-effects during long-term intensified conventional
insulin treatment in the Stockholm Diabetes Intervention Study.
Diabetes. 43:313–317. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Elsner M, Guldbakke B, Tiedge M, Munday R
and Lenzen S: Relative importance of transport and alkylation for
pancreatic beta-cell toxicity of streptozotocin. Diabetologia.
43:1528–1533. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Moran BM, Abdel-Wahab YH, Flatt PR and
McKillop AM: Evaluation of the insulin-releasing and
glucose-lowering effects of GPR120 activation in pancreatic
β-cells. Diabetes Obes Metab. 16:1128–1139. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Khamaisi M, Rudich A, Beeri I, Pessler D,
Friger M, Gavrilov V, Tritschler H and Bashan N: Metabolic effects
of gamma-linolenic acid-alpha lipoic acid conjugate in
streptozotocin diabetic rats. Antioxid Redox Signal. 1:523–535.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Halliwell B and Chirico S: Lipid
peroxidation: Its mechanism, measurement and significance. Am J
Clin Nutr. 57(5 Suppl): 715S–725S. 1993.PubMed/NCBI
|
|
41
|
Rochette L, Zeller M, Cottin Y and Vergely
C: Diabetes, oxidative stress and therapeutic strategies. Biochim
Biophys Acta. 1840:2709–2729. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
King GL and Loeken MR:
Hyperglycemia-induced oxidative stress in diabetic complications.
Histochem Cell Biol. 122:333–338. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kumar S, Prasad S and Sitasawad SL:
Multiple antioxidants improve cardiac complications and inhibit
cardiac cell death in streptozotocin-induced diabetic rats. PLoS
One. 8:e670092013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zobali F, Avci A, Canbolat O and Karasu C:
Effects of vitamin A and insulin on the anti-oxidative state of
diabetic rat heart: A comparison study with combination treatment.
Cell Biochem Funct. 20:75–80. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Suanarunsawat T, Ayutthaya WD, Songsak T
and Rattanamahaphoom J: Anti-lipidemic actions of essential oil
extracted from Ocimum sanctum L. leaves in rats fed with
high cholesterol diet. J Appl Biomed. 7:45–53. 2009.
|
|
46
|
Barcelli UO, Weiss M, Beach D, Motz A and
Thompson B: High linoleic acid diets ameliorate diabetic
nephropathy in rats. Am J Kidney Dis. 16:244–251. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Jiang M, Zheng J, Yu W, Lv G, Xu Q, Zhou Y
and Zhai C: Protective effect of ALA on high glucose induced
cellular injury of LLC-PK1 cell. Wei Sheng Yan Jiu. 41:191–194.
2012.(In Chinese). PubMed/NCBI
|