Introduction
Cerebral venous malformations (CVMs) are the most
frequently encountered type of cerebral vascular malformation, with
an incidence of 0.26 per year worldwide (1). Patients with CVMs may present with
serious symptoms, including seizures and subarachnoid and
intraparenchymal bleeds; however, ~33% of patients with CVMs are
asymptomatic (2) and thus CVMs are
not easily diagnosed, being frequently identified during autopsy
(3). CVM lesions are commonly
located in the white matter in the brain, such as in the cerebral
hemisphere, cerebellum or corpora quadrigemina, and may
additionally occur under the pia mater. In the present study, a
typical case of CVM is reported. The patient was admitted to The
First Hospital of Jilin University (Changchun, China) with a mild
and discontinuous headache and a left quadrantanopia. The authors
of the present study hypothesized that the impaired vision may have
been associated with CVMs, which in turn were caused by a
meningioma. If the surface veins of a territory fail to meet the
drain, the blood must drain deeply or farther into an unblocked
escape channel. Therefore, a number of collecting veins may
coalesce deeply to form a draining vein (4); thus suggesting that meningiomas may be
considered a contributing factor to the formation of CVMs. It has
been shown that obstruction during venous development may result in
embryonal medullary veins draining into a few expended draining
veins (4). The present study
hypothesized that the impaired vision was associated with CVMs, and
aimed to raise awareness and to better understand their hemodynamic
effects on adjacent areas. The majority of patients with CVMs
typically recover well following conservative treatment.
Case report
A 43-year-old woman with a 3-year history of
discontinuous whole-brain headache and a 1-year history of impaired
vision and memory deterioration accompanied by right facial
numbness was admitted to The First Hospital of Jilin University on
1st October 2011 for treatment following aggravation of the
headache for 10 days. Physical examination indicated no
neurological deficits. Following admission, the patient underwent
non-contrast computed tomography, which showed a round, slightly
high-density shadow with well-defined margins (2.5×3.0×4.1 cm) in
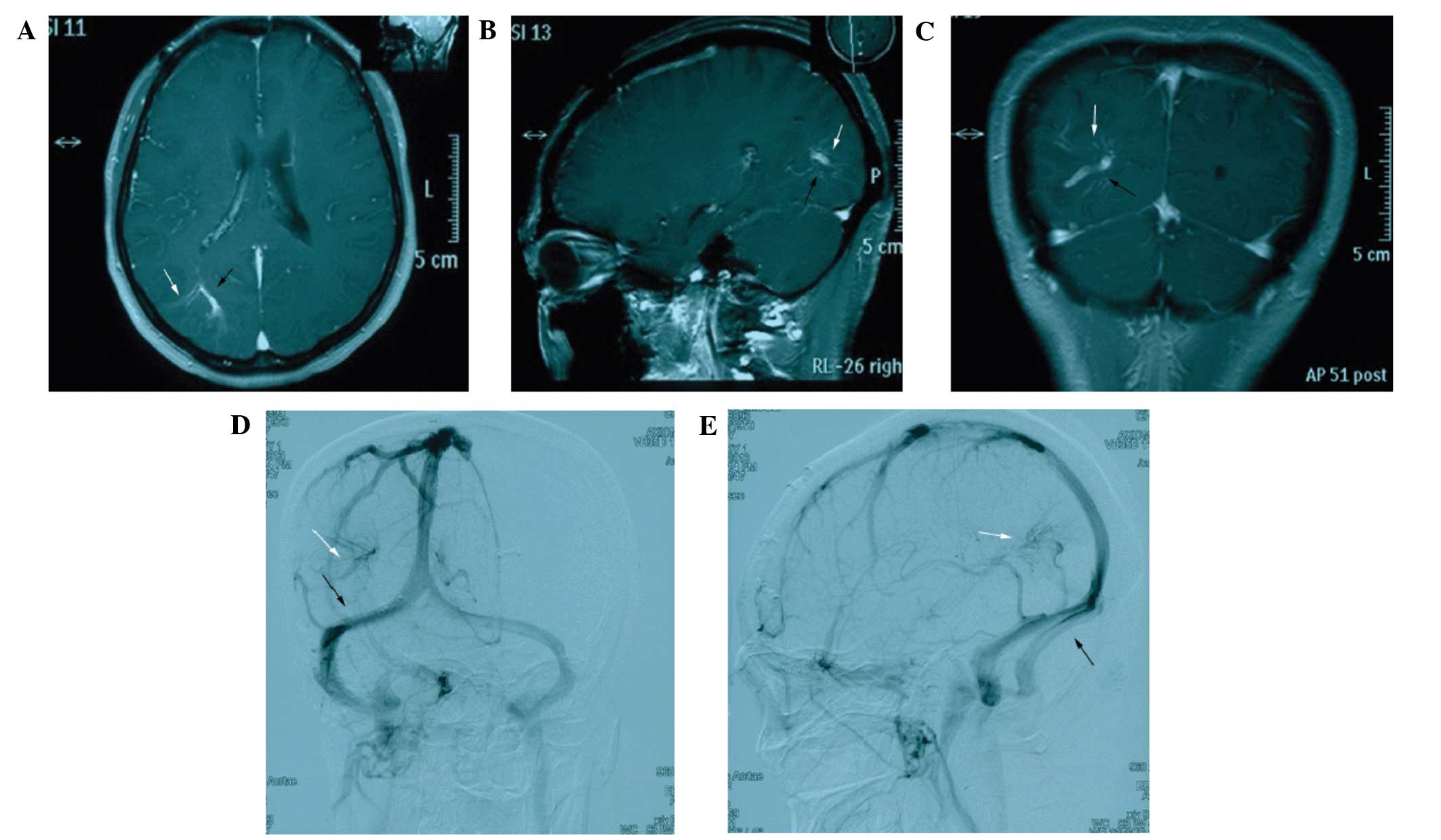
the right frontal cortex. Routine enhanced head magnetic resonance
imaging (MRI) showed a markedly enhanced lesion adhering to the
dura mater, causing mild compression of the right cerebral
ventricle (Fig. 1). Several dilated
medullary veins in the right occipital lobe converged in a fan
shape to form two dilated central veins, with an umbrella- or
medusa-like appearance. The diagnosis upon admission was
intracranial meningioma of the right frontal lobe with concomitant
ipsilateral occipital venous malformation. Subsequently, the
patient underwent microsurgery for meningioma resection, during
which the entire tumor and affected dura mater were resected, and
electrocoagulation was repeatedly performed on the basilar dura
mater. An MRI demonstrated an ill-defined hyperdensity in the right
cerebellar hemisphere. The patient was then discharged from the
hospital, with no further treatment required for CVM.
The patient was readmitted to the hospital at 1 year
after surgery for follow-up and continued to complain of a mild,
discontinuous headache. Enhanced head MRI was evaluated with
gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid and
digital subtracting angiography (DSA) was performed, which revealed
enhancement of the dilated medullary veins and draining veins,
presenting in a typical caput medusa-like appearance with a number
of small dilated medullary veins converging into a single draining
vein in a radial pattern. These draining veins drained into the
right transverse sinus, which in turn drained into the cavernous
sinus through the superficial middle cerebral veins. Furthermore,
the DSA showed prolonged vein phase. Fig. 2 shows the drainage direction of the
draining veins. No treatment was performed for the CVM due to the
high risks of treatment and the paucity of symptoms experienced by
the patient. The patient returned to the clinic 8 weeks later and
had remained asymptomatic. Written informed consent was obtained
from the patient for the publishing of the present study.
Discussion
CVM is a congenital cerebral vascular disease, which
is also known as a brain development venous abnormality (1). It is a type of cerebral vascular
malformation histologically composed of venous components,
including small dilated medullary veins and one or more draining
veins (5). Abnormal dilation of the
cerebral veins may be observed, and blood vessel walls contain
internal elastic fibers with normal brain tissues between them
(6). CVM may occur in any region of
the brain, but most commonly originates adjacent to the
anterior-inferior cerebellar artery, middle cerebral artery and
galenic venous system. CVM is a rare congenital disease, the
underlying mechanism of which is not clear (4,7). It is
generally considered to be caused by obstruction during venous
development, which occurs following the development of the arterial
system, resulting in embryonal medullary veins draining into a
single thick draining vein (2).
Normal island-like areas of brain tissue may exist among these
abnormally dilated plexiform veins without feeding arteries or
direct arteriovenous shunts (4).
As a CVM develops as venous hypoplasia during the
embryonic period, its impact on brain blood circulation is a slow
process, allowing for an occult clinical presentation (8). Patients with a CVM may exhibit symptoms
including seizure, subarachnoid hemorrhage or intracerebral
hemorrhage (9); however, ~33% of CVM
patients are asymptomatic (10). A
previous study reported that the incidence of intracranial
hemorrhage is increasing, primarily due to the coexistence of mixed
venous-cavernous hemangiomas and arteriovenous malformations
(11). Furthermore, previous autopsy
reports indicate that CVMs account for 2.5–2.6% of cerebral
vascular malformations, which is 3–4 times more common than
arteriovenous malformations (8,10,12).
CVMs are divided into two types based on the type of
draining vein, namely superficial or deep CVM (13), with the present case belonging to the
superficial type. Certain researchers indicate that CVM should be
considered when MRI reveals a jellyfish head-shaped medullary vein,
with a long T1 and long or short T2 signal, or when a large
strip-shaped area of no signal appears (12). The use of enhanced MRI scanning to
identify these malformed vessels based on their jellyfish or
umbrella shape may completely substitute DSA examination (1,14). Under
MRI, the T1-weighted image exhibits a flow void signal in patients
without ischemic or hemorrhagic complications within the drainage
territory of CVMs, while the vessels parallel to the scanning plane
exhibit a high signal, combined with a low signal in the
T2-weighted image, which is associated with the echo phase reunion
phenomenon (12). Vessels in other
directions predominantly exhibit a low signal, the draining veins
are quite thick, and the detection rate of MRI is high, which are
necessary conditions for CVM diagnosis (15). In enhanced MRI scanning, CVM mainly
presents with a markedly high signal and its structure is clear.
Enhanced scanning is particularly crucial if the lesion is small,
and there is no edema or mass effect around the lesion under MRI
(14). A recent publication
suggested that three types of ‘arterialized developmental venous
anomalies (DVAs)’ may exist (6), and
that DSA is required to adequately characterize arterialized DVAs
(14). The standards of DSA
diagnosis of CVM are as follows (14,16): i)
Vascular lesion appears in the venous phase, with no feeding
artery; ii) numerous small dilated medullary veins; and iii)
drainage through a dilated brain-penetrating vein (superficial
type) or subependymal vein (deep type). Although MRI is able to
diagnose the majority of CVMs, the DSA remains the best imaging
module for investigating the hemodynamic behavior of CVMs.
Active surgical treatment is recommended only for
CVMs with posterior fossa bleeding (15). For patients with bleeding, craniotomy
hematoma evacuation or intraventricular hematoma evacuation may be
performed, and patients typically recover well following surgery
(17,18). However, the CVM itself is not
directly treated as the risk of further bleeding following surgery
is high, and resection of the lesion may immediately cause venous
infarction of cerebral tissues, leading to edema and congestion of
cerebral tissues, or even cerebral necrosis (4).
In conclusion, CVM may be diagnosed using MRI, but
DSA remains the best imaging module for investigating the
hemodynamic behavior of DVAs. For patients exhibiting bleeding,
only craniotomy hematoma evacuation or intraventricular hematoma
evacuation is required, while treatment for the CVM may not be
necessary in other cases. The majority of patients with CVMs have
no clinical symptoms, and prognosis is typically favorable.
References
|
1
|
San Millán Ruíz D and Gailloud P: Cerebral
developmental venous anomalies. Childs Nerv Syst. 26:1395–1406.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jagadeesan BD, Almandoz Delgado JE, Moran
CJ and Benzinger TL: Accuracy of susceptibility-weighted imaging
for the detection of arteriovenous shunting in vascular
malformations of the brain. Stroke. 42:87–92. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sarwar M and McCormick WF: Intracerebral
venous angioma. Case report and review. Arch Neurol. 35:323–325.
1978. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pereira VM, Geibprasert S, Krings T,
Aurboonyawat T, Ozanne A, Toulgoat F, Pongpech S and Lasjaunias PL:
Pathomechanisms of symptomatic developmental venous anomalies.
Stroke. 39:3201–3215. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vogl TJ, Bergman C, Viliringer A, Einhäupl
K, Lissner J and Felix R: Dural sinus thrombosis: Value of venous
MR angiography for diagnosis and follow up. AJR Am J Roentgenol.
162:1191–1198. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
San Millán Ruíz D, Delavelle J, Yilmaz H,
Gailloud P, Piovan E, Bertramello A, Pizzini F and Rüfenacht DA:
Parenchymal abnormalities associated with developmental venous
anomalies. Neuroradiology. 49:987–995. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bisdorff A, Mulliken JB, Carrico J,
Robertson RL and Burrows PE: Intracranialvascular anomalies in
patients with periorbital lymphatic and lymphaticovenous
malformations. AJNR Am J Neuroradiol. 28:335–341. 2007.PubMed/NCBI
|
|
8
|
Leblanc GG, Golanov E, Awad IA and Young
WL: Biology of Vascular Malformations of the Brain NINDS Workshop
Collaborators: Biology of vascular malformations of the brain.
Stroke. 40:e694–e702. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ruíz DS, Yilmaz H and Gailloud P: Cerebral
developmental venous anomalies: Current concepts. Ann Neurol.
66:271–283. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vattoth S, Purkayastha S, Jayadevan ER and
Gupta AK: Bilateral cerebral venous angioma associated with
varices: A case report and review of the literature. AJNR Am J
Neuroradiol. 26:2320–2322. 2005.PubMed/NCBI
|
|
11
|
Numaguchi Y, Nadell JM, Mizushima A and
Wilensky MA: Cerebral venous angioma and a varix: A rare
combination. Comput Radiol. 10:319–323. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Saeed O, Khan AA, Herial NA and Qureshi
AI: Exercise Induced Transient Neurological Deficit in a Patient
with Cerebellar Developmental Venous Anomaly. J Vasc Interv Neurol.
8:17–20. 2015.PubMed/NCBI
|
|
13
|
Dross P, Raji MR and Dastur KJ: Cerebral
varix associated with a venous angioma. AJNR Am J Neuroradiol.
8:373–374. 1987.PubMed/NCBI
|
|
14
|
Ostertun B and Solymosi L: Magnetic
resonance angiography of cerebral developmental venous anomalies:
Its role in differential diagnosis. Neuroradiol. 35:97–104. 1993.
View Article : Google Scholar
|
|
15
|
Santucci GM, Leach JL, Ying J, Leach SD
and Tomsick TA: Brain parenchymal signal abnormalities associated
with developmental venous anomalies: Detailed MR imaging
assessment. AJNR Am J Neuroradiol. 29:1317–1323. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guclu B, Ozturk AK, Pricola KL, Seker A,
Ozek M and Gunel M: Cerebral venous malformations have distinct
genetic origin from cerebral cavernous malformations. Stroke.
36:2479–2480. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wilums G, Demaerel P, Marchal G, Baert AL
and Plets C: Gadolinium-enhanced MRI of cerebral venous angiomas
with emphasis on their drainage. J Comput Assist Tomogr.
15:199–206. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cakirer S: De novo formation of a
cavernous malformation of the brain in the presence of a
developmental venous anomaly. Clin Radiol. 58:251–256. 2003.
View Article : Google Scholar : PubMed/NCBI
|