Introduction
Rotavirus (RV) infection is the leading cause of
diarrheal diseases in infants and children worldwide. In developing
countries, RV-induced diarrhea accounts for 20–70% of diarrheal
diseases in hospitalized children <5-years of age (1,2).
Infection with RV causes an estimated 600,000 deaths per year
worldwide, with >85% of RV-associated mortality occurring in
developing or resource-limited countries (3). This high incidence of RV-induced
diarrhea has caused great economic burden.
Lactadherin, also known as human breast antigen 46
or milk-fat globule-EGF factor 8, is a secreted glycoprotein of the
milk-fat globule membrane. In milk, lactadherin functions as an
antiviral protein inhibiting the symptoms of RV infection; however,
little is known regarding its physiological function (4–6).
Lactadherin binds to ligands in epithelial cells, which may
indicate it has a protective function on intestinal cells (5). RV infection is capable of inducing
diarrhea via various mechanisms, including the destruction of
intestinal epithelial cells, intestinal villus ischemia and enteric
nervous system activation (3). The
authors of the present study have previously demonstrated that
paracellular leakage induced by damage to the intracellular
junctions may be associated with the pathogenesis of RV-induced
diarrhea (5). Intracellular
junctions form part of the intestinal epithelial barrier, and
prevent the invasion of microorganisms and their toxins from the
intestinal tract (7). Therefore, RV
infection may be associated with the intestinal epithelial
barrier.
The present study was conducted to investigate the
effects of lactadherin on the intestinal epithelial barrier.
Various parameters associated with the intestinal epithelial
barrier, including plasma D-lactic acid, intestinal mucin (MUC) 2
and intracellular junction claudin-1 protein were investigated in
order to investigate the mechanism underlying lactadherin
intervention. The results of the present study may provide evidence
for the prevention and treatment of patients with RV-associated
diarrhea.
Materials and methods
Reagents and equipment
A group A RV diagnostic kit was purchased from
Beijing Cosmos Biological Pharmaceutical Co., Ltd., (Beijing,
China), and the plasma D-lactic acid assay kit was purchased from
BioVision, Inc. (Milpitas, CA, USA). Rabbit anti-MUC2 polyclonal
antibody (ab76774) was purchased from Abcam (Cambridge, UK), and
rabbit anti-claudin-1 antibody (NBP1-67515) was purchased from
Novus Biologicals, Ltd. (Milan, Italy). SYBR® Premix Ex Taq™, ROX
reference dye II and PrimeScript™ reverse transcription (RT)
reagent kit were purchased from Takara Biotechnology Co., Ltd.
(Dalian, China). Forward and reverse primers and human lactadherin
DNA were purchased from Sangon Biotech Co., Ltd., (Shanghai,
China).
Laboratory animals and model
preparation
Seven-day-old, specific pathogen-free, healthy
Sprague-Dawley rats (n=75) were purchased from Shanghai SLAC
Laboratory Animal Co., Ltd. (Shanghai, China). Experimental
protocols were approved by the ethics committee of Xinhua Hospital
Affiliated to Shanghai Jiaotong University School of Medicine
(XHEC-F-2014-024; Shanghai, China). Rats were allocated at random
into five groups (n=15 each), including: Control (C), RV infection
(RVI), lactadherin before RV infection (LBRI), lactadherin after RV
infection (LARI) and blank (B) groups. Human RV Wa strains were
provided by the Institute of Virology of the Chinese Academy of
Preventive Medicine (Beijing, China). Rats were intragastrically
fed formula milk without lactadherin from seven-days-old (8). On day 4 of artificial feeding,
10-day-old rats in group RVI were intragastrically administered
1×106 PFU RV. The LBRI group was intragastrically administered 0.25
mg lactadherin daily for the first three days of artificial feeding
and received 1×106 PFU RV, via the same route, on day 4. The LARI
group was intragastrically administered 1×106 PFU RV on day 4 of
artificial feeding and 0.25 mg lactadherin thereafter for three
consecutive days. Group C received an equal volume of maintenance
solution from the RV supernatant (without RV), which contained
Dulbecco's modified Eagle's medium supplemented with 1 ug/ml
trypsin (both Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA), on day 4 of artificial feeding; whereas group B received an
equal volume of normal saline on day 4 of artificial feeding. On
days 1, 4 and 7 following RV administration, five rats were
randomly selected and anesthetized with 100 mg/kg ketamine (Fujian
Gutian Pharma Co., Ltd., Fujian, China) and 10 mg/kg diazepam
(Jilin Province Tat Animal Pharmaceutical Co., Ltd., Jilin, China)
prior to sacrifice via decapitation. Blood samples were harvested
and plasma specimens were collected following centrifugation of
0.3–0.5 ml blood at 950 × g at 4°C for 10 min, which was
subsequently stored at −20°C prior to D-lactic acid determination.
A 5.5-cm section was harvested from the small intestinal tissue
along with a 5-cm section via the ileocecal valve, which were
immediately washed with phosphate-buffered saline (PBS; Thermo
Fisher Scientific, Inc.). Prior to sectioning for electron
microscopy, a 2-mm specimen segment was fixed with 2%
glutaraldehyde (Novon Scientific Co., Pleasanton, CA, USA) and a
2-cm specimen segment was frozen in liquid nitrogen and stored at
−80°C prior to RT-quantitative polymerase chain reaction
(qPCR) analysis. In preparation for the pathological
sections, a 2-cm specimen segment was fixed with 10% neutral
formalin buffer (Sinopharm Chemical Reagent Co., Ltd., Shanghai,
China).
Lactadherin expression
Lactadherin was provided by Zhangjiang Biotechnology
Co. Ltd., (Shanghai, China). The human lactadherin DNA fragment was
amplified by PCR from human lactadherin cDNA prior to cloning into
the BamHI and EcoRI restriction sites of the pFastBac™ HTA vector
(Invitrogen; Thermo Fisher Scientific Inc.). Once the recombinant
bacmid was confirmed, Sf9 insect cells (Zhangjiang Biotechnology
Co. Ltd.) were transfected, as previously described (9).
Lactadherin western blot
identification and purification
Sf9 cells were detected as polygonal following
transfection, as previously described (9), and the supernatant of medium was
collected and amplified. Following protein extraction and
separation by sodium dodecyl sulfate-polyacrylamide gel
electrophoresis, as previously described (9), 50 µg protein was incubated with 200
µg/ml rabbit MFG-E8 (H-60) polyclonal immunoglobulin G (1:200;
sc-33545; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at 4°C
for 12–14 h. Subsequently, the recombinant protein was further
purified using S-Sepharose, Q-Sepharose ion-exchange
chromatography, and Sephacryl S-100 gel filtration chromatography
(GE Healthcare Life Sciences, Shanghai, China), according to the
manufacturer's protocol, to 95% purity, as demonstrated by
densitometric scanning (Image Lab Gel Doc XR+ system; Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The purity and molecular
weight of lactadherin were consistent with native lactadherin.
Diarrhea model establishment
RV antigen detection in feces
RV antigen rapid detection card (Beijing Cosmos
Biological Pharmaceutical Co., Ltd.) was used according to the
colloidal gold method (10) with the
following criteria: (i) Positive, two red lines; (ii) negative, one
control line; and (iii) invalid, no control line, invalid
experiment or repetitive detection required.
Feces characteristics
Rat feces were characterized according to four
grades as follows: 1, Normal color (brown) and nature; 2, abnormal
color (green or yellow-green) and normal nature; 3, normal color
and abnormal nature (thin or watery); and 4, abnormal color (green
or yellow-green) and nature (thin or watery) (11). Diarrhea was diagnosed if the total
points were ≥2 (Table I).
 | Table I.Feces scoring of pups among the five
groups at various time points. |
Table I.
Feces scoring of pups among the five
groups at various time points.
| Day | Control | RVI | LBRI | LARI | Blank |
|---|
| D1 |
1.0±0.0a |
2.0±0.5b |
1.0±0.5a,b |
2.0±0.5b |
1.0±0.0a |
| D2 |
1.0±0.0a |
3.0±1.0b |
2.0±0.5a,b |
2.0±1.0a,b |
1.0±0.0a |
| D3 |
1.0±0.0a |
3.0±.0.5b |
2.0±1.0a,b |
2.0±0.5a,b |
1.0±0.0a |
| D4 |
1.0±0.0a |
4.0±1.0b |
3.0±1.0a,b |
3.0±0.0a,b |
1.0±0.0a |
| D5 |
1.0±0.0a |
3.0±0.5b |
1.0±1.0a |
2.0±1.0a |
1.0±0.0a |
| D6 |
1.0±0.0a |
3.0±0.5b |
1.0±0.5a |
1.0±1.0a |
1.0±0.0a |
| D7 |
1.0±0.0 |
1.0±1.0b |
1.0±0.0 |
1.0±0.0 |
1.0±0.0 |
Ultraviolet spectrophotometry of enzyme
activity
Plasma D-lactic acid levels were determined using a
commercial kit according to the manufacturer's protocol. Standard
samples of 0, 5, 10, 15, 20 and 25 µl, were added to a 96-well
plate, with the volume adjusted to 50 µl using Tris-buffered saline
(Thermo Fisher Scientific, Inc.). A total of 50 µl plasma specimen
was added to the blank wells and 50 µl enzyme reaction mixture was
added to all sample wells, in addition to standard and specimens.
Following incubation at room temperature for 30 min, optical
density (OD) was measured at 450 nm using a Synergy™ H4
spectrophotometer (BioTek Instruments, Inc., Winooski, VT, USA).
Standard curve was obtained in order to calculate plasma D-lactic
acid levels as follows: y=0.062×+0.616.
Hematoxylin and eosin (HE) staining
In order to examine intestinal mucosal morphology, 5
cm small intestinal tissue specimens were harvested from the
ileocecal valve on day 4 post-infection. Tissue specimens were
fixed with 10% neutral formalin buffer were subjected to gradient
ethanol dehydration, xylene transparency, paraffin embedding,
sectioning and HE staining (Changdao Biological Technology Co.,
Ltd., Shanghai, China) prior to light microscopy (Olympus CX21;
Corporation, Tokyo, Japan) to observe the morphology of the small
intestine mucosa.
Electron microscopy
In order to examine the ultrastructure and
intracellular conjunctions of the intestinal epithelial cells, 5 cm
small intestinal tissue specimens were harvested from the ileocecal
valve on day 4 post-RV infection. Intestinal tissues were fixed
with 2% glutaraldehyde and pure epoxy resin 618 embedding (China
National Chemical Co., Ltd., Beijing, China), sectioning, lead
citrate e-dyeing and electron microscopy analysis was performed by
the Electron Microscopy Division at Shanghai Jiaotong University
School of Medicine.
Immunohistochemical streptavidin-peroxidase
analysis
Immunohistochemical analysis of small intestinal
MUC2 and intracellular junction claudin-1 protein was performed
using intestinal tissue specimens harvested on day 4
post-infection. Tissue specimens were fixed, embedded, sectioned
and de-waxed using conventional methods (12) and high-pressure antigen retrieval of
specimens was subsequently performed by incubating the specimens in
3% H2O2 at room temperature for 10 min. Subsequently, the specimens
were soaked twice in PBS for 5 min, and incubated with normal goat
serum (Shanghai Haoran Biological Technology Co., Ltd., Shanghai,
China) at room temperature for 20 min. The serum was discarded and
the specimens were subsequently incubated with rabbit primary
polyclonal anti-MUC2 (1:100) and anti-claudin-1 (1:100) antibodies
4°C overnight. Following this, the specimens were rinsed three
times with PBS for 5 min and incubated with horseradish
peroxidase-conjugated goat anti-rabbit secondary antibody (1:100;
111-035-003; Jackson ImmunoResearch Laboratories, Inc., West Grove,
PA, USA) working solution at room temperature for 60 min. Specimens
were subsequently rinsed three times with PBS for 5 min and
developed using 3,3′-diaminobenzidine reagent (Wuhan Boster
Biological Technology, Ltd., Wuhan, China). Hematoxylin
counterstaining was performed and the specimens were mounted using
the conventional method (12). Five
fields of view were randomly selected and images of the intestinal
mucosal layer were captured using a light microscope
(magnification, ×40). Image Pro Plus 6.0 (Media Cybernetics, Inc.,
Rockville, MD, USA) was used to compare the average OD of the brown
yellow particles amongst the various rat groups, which was
calculated according to the area of brown yellow particles and
their OD values, as follows: A=OD/area.
RT-qPCR
The mRNA expression levels of intestinal MUC2 mucin
and intracellular junction claudin-1 protein expression levels were
analyzed using RT-qPCR, using the following primers: MUC2, forward
5′-AGACCGTAGTGCTGTTGACTGA-3′, and reverse
5′-GGTAGGAGGAGGGTTTGAAGAT-3′; claudin-1, forward
5′-AAAAGATGTGGATGGCTGTCA-3′, and reverse
5′-GTGGTGTTGGGTAAGAGGTTGT-3′; and β-actin, forward
5′-CCCATCTATGAGGGTTACGC-3′ and reverse 5′-TTTAATGTCACGCACGATTTC-3′.
Total RNA was extracted using TRIzol® (Invitrogen; Thermo Fisher
Scientific, Inc.) and 0.2 ml chloroform (Sinopharm Chemical Reagent
Co., Ltd.) per sample. Following incubation at 37°C for 15 min, the
100 µg RNA was reverse-transcribed to cDNA at 85°C for 5 sec using
a Veriti 96-well thermal cycler (Applied Biosystems; Thermo Fisher
Scientific, Inc.). To a final volume of 20 µl, the PCR reaction
mixture contained 10 µl 1X SYBR Premis Ex Taq II™, 0.8 µl forward
primer (0.4 µM), 0.8 µl reverse primer (0.4 µM), 0.4 µl 1X ROX
reference dye II, 2 µl DNA template and 6 µl RNase-free dH2O.
Amplification conditions were as follows: 1 cycle of 95°C for 30
sec; followed by 40 cycles of 95°C for 5 sec and 60°C for 34 sec;
and one cycle of 95°C for 15 sec, 60°C for 1 min, and 95°C for 15
sec. Results were automatically computed and analyzed using ABI
7500 software (Applied Biosystems; Thermo Fisher Scientific, Inc.).
cDNA expression levels were transformed to mRNA expression level,
and the sample values were normalized against the β-actin reference
values.
Statistical methods
Data were analyzed using the SAS statistical
software package (version 8.02; SAS Institute Inc., Cary, NC, USA),
and presented as the mean ± standard deviation. Multi-group and
pairwise comparisons were performed using single factor analysis of
variance and Student-Newman-Keuls tests, respectively. P<0.05
was considered to indicate a statistically significant
difference.
Results
Influence of lactadherin on the course
and degree of diarrhea
The feces of rats in groups B and C demonstrated
negative RV antigen detection results, which was consistent with
their lack of diarrheal symptoms. Positive results were detected in
groups RVI, LBRI and LARI, which was consistent with their clinical
symptoms of diarrhea 24 h after intragastric administration of RV,
including increased water content or abnormal fecal color. The
severity of diarrhea peaked 4 days after intragastric
administration of RV.
Among the five rat groups, the most severe clinical
symptoms of diarrhea were detected in the RVI group, including
substantially increased fecal water content, high defecation
frequency and long disease duration, with normal feces
predominantly observed by day 7. By comparison, groups LBRI and
LARI demonstrated less severe clinical symptoms of diarrhea,
including slightly increased fecal water content and short disease
duration, with normal feces predominantly observed by day 6.
Influence of lactadherin on mucosal
morphology in rat small intestine
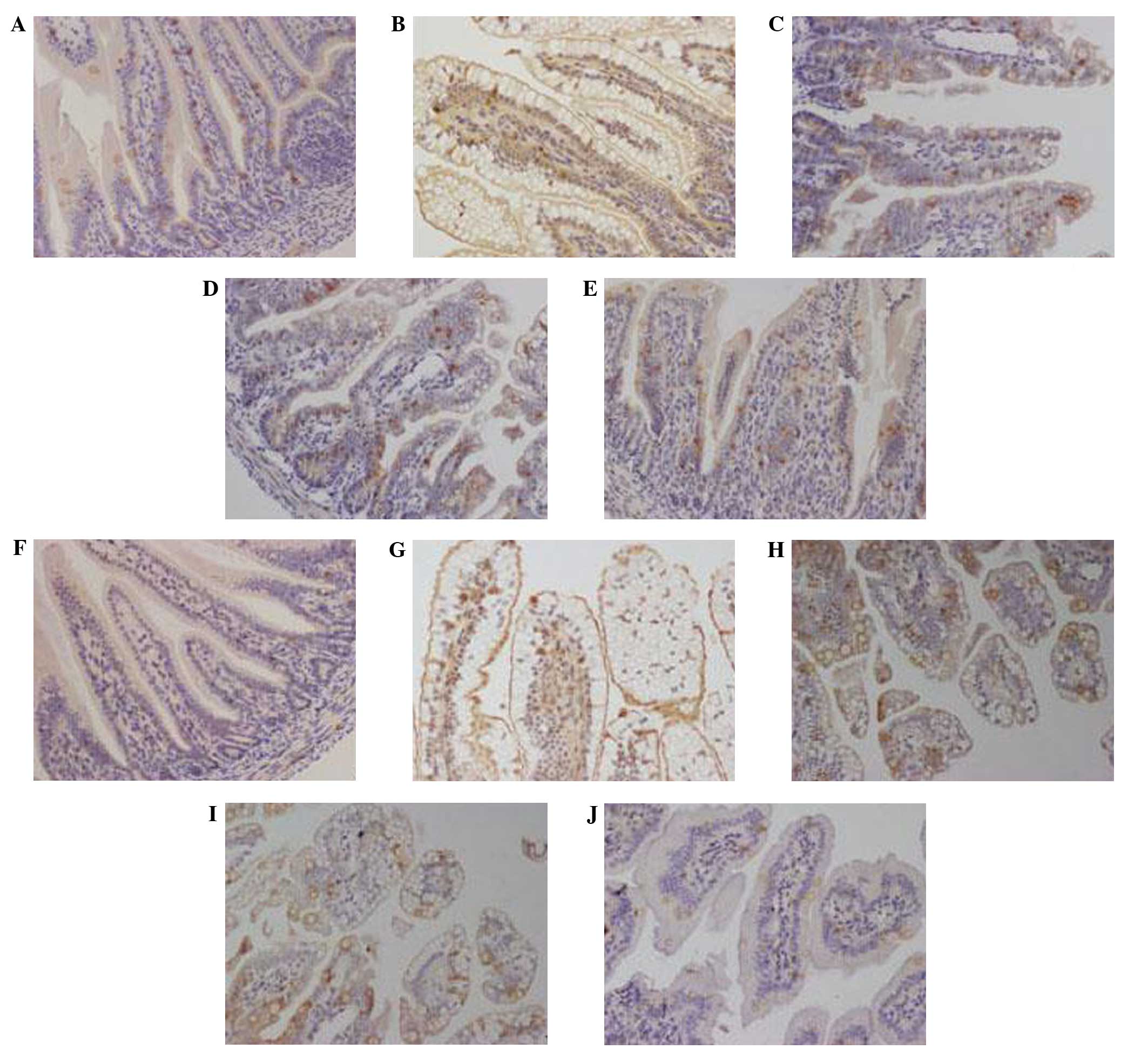
At 4 days post-RV infection, rats in groups C and B
exhibited intact intestinal villi without swelling, degeneration,
necrosis or inflammatory cell infiltration, as detected by light
microscopy (Fig. 1A and E). By
contrast, swelling and congested intestinal villi with fractures,
substantial vacuolation of epithelial cells, changes in the
position of the nucleus, non-obvious inflammatory cell infiltration
and minimal lymphocytic infiltration was detected in the RVI group
(Fig. 1B). In the LBRI and LARI
groups, light microscopy demonstrated relatively intact intestinal
villi with partial vacuolation of epithelial cells and partial
alterations in the position of the nucleus (Fig. 1C and D).
As detected by electron microscopy, groups C and B
demonstrated compact, neat and long intestinal microvilli with
closed intracellular junctions at 4 days post-RV infection
(Fig. 1A and E); as compared with
the sparse, shortened and disordered intestinal microvilli with
broadened intracellular junctions and swollen mitochondria detected
in the RVI group (Fig. 1B). As
compared with groups B and C, groups LBRI and LARI demonstrated
longer intestinal microvilli with slightly broadened intercellular
junctions and swollen mitochondria (Fig.
1C and D).
Influence of lactadherin on rat plasma
D-lactic acid levels
On days 1, 4 and 7 following RV infection, plasma
D-lactic acid levels were significantly elevated in groups RVI,
LBRI and LARI, as compared with group C (P<0.05). Notably,
plasma D-lactic acid levels were highest in the RVI group,
particularly on day 4 post-infection (Fig. 2).
Effects of lactadherin on MUC2 and
claudin-1 expression levels in rat small intestine
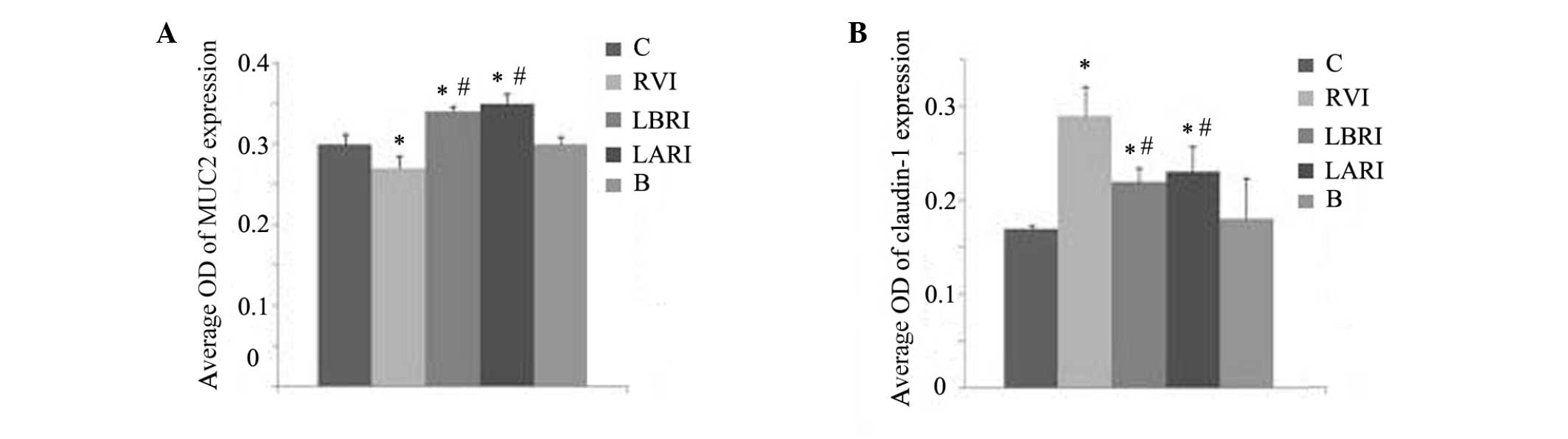
In groups C and B at 4 days post-RV infection, MUC2
expression was evenly distributed (Fig.
3A and E) with no substantial variations in the average OD
values (Fig. 4A). In the RVI group,
MUC2 expression was predominantly detected in the perinuclear space
and cytoplasm with low expression levels detected in the nucleus
(Fig. 3B); whereas MUC2 expression
was concentrated to specific areas in groups LBRI and LARI
(Fig. 3C and D). Furthermore,
significantly increased average OD values were demonstrated in the
LBRI and LARI groups, as compared with groups B and C (P<0.05).
The lowest average OD was detected in the RVI group, as compared
with the other groups (Fig. 4A).
In groups C and B 4 days post-RV infection,
claudin-1 expression was predominantly detected in the cell
membrane (Fig. 3F and J), with no
differences in the average OD values (Fig. 4B). In groups RVI, LBRI and LARI,
claudin-1 expression was predominantly detected in the cell
membrane, with low expression levels detected in the nucleus and
cytoplasm (Fig. 3G–I). Claudin-1
expression was greatest in the RVI group. The associated average OD
values were increased in groups RVI, LBRI and LARI, as compared
with groups B and C, with the greatest values detected in the RVI
group (Fig. 4B).
Results of MUC2 and claudin-1 mRNA
assays
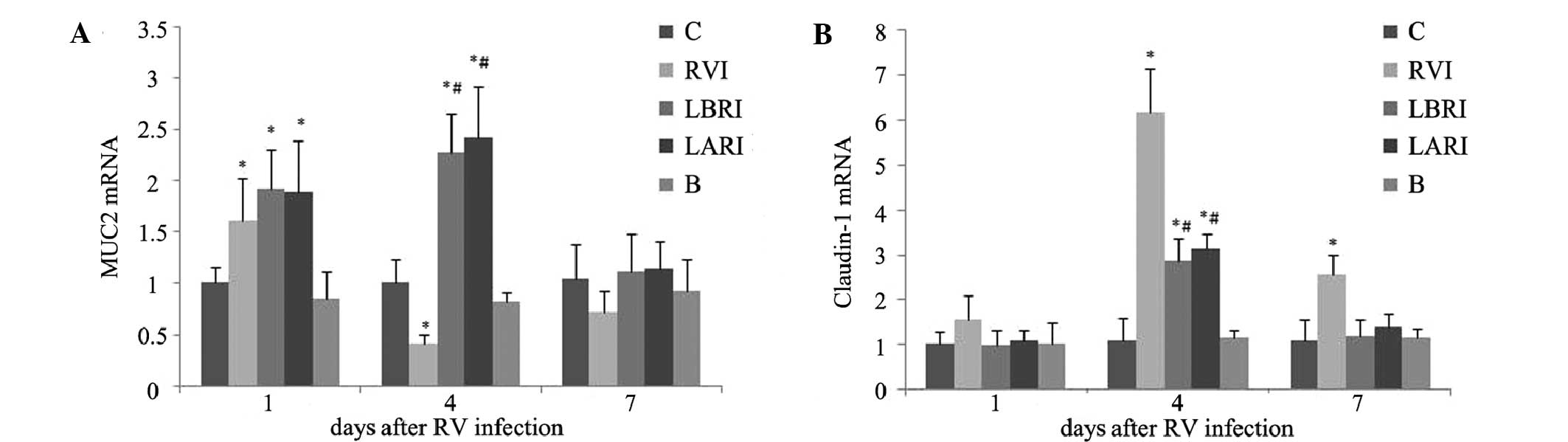
On day 1 post-RV infection, MUC2 mRNA expression
levels were significantly increased in groups RVI, LBRI and LARI,
as compared with groups B and C (P<0.05). On day 4
post-infection, MUC2 mRNA expression levels were elevated in groups
LBRI and LARI and group RVI had significantly decreased, as
compared with groups B and C. By day 7 post-infection, no
substantial differences in MUC2 mRNA expression levels were
detected amongst the various groups (Fig. 5A).
No substantial differences in claudin-1 mRNA
expression levels were detected amongst the various groups on day 1
post-infection. However, on day 4 post-infection, claudin-1 mRNA
expression levels were significantly increased in groups RVI, LBRI
and LARI, as compared with groups B and C (P<0.05). Claudin-1
mRNA expression levels were highest in the RVI group. On day 7
post-infection, claudin-1 mRNA expression levels were significantly
elevated in the RVI group, as compared with the other groups
(P<0.05) (Fig. 5B).
Discussion
The RV genus belongs to the Reoviridae family, the
members of which have genomes containing 11 segments of
double-stranded (ds)RNA and primarily infect infants <2 years
old (13). Lactadherin is crucially
involved in reducing the incidence of RV-associated diarrhea and
relevant diarrheal symptoms (4,14,15).
Previous studies have demonstrated that breast milk contains
lactadherin with a specific sequence, i.e., N'-Arg-Gly-Asp (RGD)
crosslinked with integrin αVβ3 and αVβ5, thus competitively
inhibiting the RV combination with host cells (12,14–17). The
results of the present study demonstrated that, following infection
with RV, rats in the groups treated with lactadherin, LBRI and
LARI, exhibited less severe diarrheal symptoms, as compared with
the RVI group. Furthermore, light and electron microscopy analysis
demonstrated that rats in the LBRI and LARI groups suffered less
damage to intestinal tissue, as compared with the RVI group,
suggesting that lactadherin may have a role in the prevention and
treatment of RV-associated diarrhea.
It has previously been demonstrated that intestinal
mucosal permeability increases prior to the substantial changes in
intestinal mucosal morphology (18,7).
Therefore, intestinal mucosal permeability reflects early damage to
the intestinal mucosa barrier, and D-lactic acid levels may be used
to quantitatively assess intestinal mucosal barrier function
(18,7). The present study demonstrated that
D-lactic acid levels were elevated in rats in groups RVI, LBRI and
LARI, as compared with those in groups B and C on days 1, 4 and 7
following RV infection. RV infection may have caused damage to the
intestinal mucosal barrier and increased intestinal permeability,
allowing the transport of bacterially produced D-lactic acid from
the intestine to the plasma via the damaged mucosa, leading to
increased plasma D-lactic levels. D-lactic acid levels peaked on
day 4 post-infection, suggesting that the intestinal lesions became
most severe at this point, which is consistent with the peak of
clinical diarrheal symptoms. Furthermore, plasma D-lactic acid
levels decreased in groups LBRI and LARI, as compared with the RVI
group. Analyses of the mucosal morphology of the rat's small
intestines using light and electron microscopy demonstrated that
the damage to the small intestinal tissues was less severe in the
LBRI and LARI groups, as compared with the RVI group on day 4
post-infection. These results suggested that lactadherin may have
counteracted the increased intestinal permeability induced by RV
infection.
The intestinal epithelial barrier is the first-line
of mucosal immune defense, and includes the mucus layer, intestinal
epithelial cell layer and the tight junctions of intestinal
epithelial cells, which are associated with intestinal permeability
(19). Previous studies have
demonstrated that the intestinal mucus layer, in particular mucin,
is able to inhibit the adhesion of viruses to intestinal epithelial
cells (11,20,21). To
date, 21 mucins have been discovered, of which MUC2, which is most
abundant in the small intestine, is one of the most important
(22). The mucus gel later is an
integral structural component of the intestine, which provides a
medium for protection, lubrication and transport between the
luminal contents and the epithelial lining (23). As such, as a structural component of
the protective mucus layer, MUC2 ensures intestinal mucus has a
high density and viscoelasticity and the synthesis rate of MUC2 is
a potential parameter for intestinal barrier function (24). Boshuizen et al (22) have previously demonstrated that MUC2
mRNA expression levels were reduced in the jejunums of RV-infected
rats, which increased in the ilea of the rats on day 1
post-infection but remained at similar levels at other time points,
as compared with the control. The results of the present study
demonstrated that on day 1 post-infection, small intestinal MUC2
mRNA expression levels were increased in the rats in groups RVI,
LBRI and LARI, as compared with those in groups B and C. The dsRNA
and VP4 outer capsid protein of RV may have respectively activated
corresponding signal transduction pathways for the direct or
indirect upregulation of MUC2 expression (22); therefore, further improving the
capability of the immune system to defend against infection. On day
4 post-infection, the mRNA expression levels of MUC2 in the RVI
group were reduced compared with those detected in groups B and C,
which may have been due to a reduction in MUC2 secretion caused by
a decline of the body's MUC2 reserve and the apoptosis of goblet
cells, which are capable of secreting MUC2. Regarding the increased
MUC2 mRNA expression levels detected in groups LBRI and LARI, as
compared with groups B and C, it is hypothesized that lactadherin
promoted intestinal epithelial cell growth and goblet cell
maturation, thus reducing the apoptosis of intestinal epithelial
cells. MUC2 is a crucial component of the intestinal epithelial
barrier which protects against microbial infection; therefore, this
increase in MUC2 expression levels may enhance the defensive
function of the intestinal epithelial barrier. This may explain why
rats in the LBRI and LARI groups exhibited reduced intestinal
permeability following lactadherin administration, which
counteracted the increased intestinal permeability induced by
infection with RV. Following the removal of inflammatory lesions
and the recovery of epithelial cells, MUC2 expression levels should
return to normal; however, the underlying mechanisms require
further study.
The integrity of the intestinal epithelial barrier
is maintained by intestinal epithelial cells and intracellular
junctions, which ensure that the areas between two cells are
impermeable (25–27). Previous studies have demonstrated
that paracellular leakage caused by damage to the intracellular
junctions may be an important mechanism underlying RV-associated
diarrhea (20,28). Tight junctions have a key role in the
association of cells (25–27,29) and
in recent years, claudin-1 has been proposed as an important
component of the tight junctions in the intestinal barrier
(30,31). However, claudin-1 expression levels
increase, rather than decrease, with increasing inflammatory
intestinal permeability (25,26). In
the present study, no substantial variations in claudin-1 mRNA
expression levels were detected amongst the rat groups receiving
various treatments on day 1 post-RV infection. However, claudin-1
mRNA expression levels were elevated in the RVI, LBRI and LARI
groups, as compared with groups B and C; furthermore, the RVI group
demonstrated increased levels on day 4 post-infection, as compared
with the LBRI and LARI groups. On day 7 post-infection, claudin-1
mRNA expression levels increased in the rats in group RVI, as
compared with the other groups. Furthermore, claudin-1 mRNA
expression was predominantly detected in the cell membrane;
however, low expression levels were detected in the cytoplasm and
nuclei of rats in the RVI, LBRI and LARI groups. These results were
consistent with a previous study investigating inflammatory bowel
disease, conducted by Poritz et al (32). Furthermore, the present study
demonstrated that claudin-1 mRNA expression levels were increased
in the RVI, LBRI and LARI groups, as compared with groups B and C
on day 4 post-infection; whereas these levels were increased in the
RVI group, as compared with groups B and C on day 7 post-infection.
Similarly, plasma D-lactic acid levels were elevated in the RVI,
LBRI and LARI groups, as compared with groups B and C on days 4 and
7. Claudin-1 may function as a channel protein in the regulation of
tight junctions and its upregulation is likely to have increased
the permeability between epithelial cells, resulting in increased
intestinal permeability in the rats in groups RVI, LBRI and LARI.
Furthermore, the claudin-1 mRNA expression and plasma D-lactic acid
levels were reduced in the LBRI and LARI groups, as compared with
the RVI group on days 4 and 7 following infection with RV. These
results suggested that lactadherin may reduce the upregulated
expression of claudin-1 mRNA associated with RV infection in order
to maintain the intestinal epithelial barrier and improve
intestinal permeability.
In conclusion, the present study demonstrated that
lactadherin increased the expression levels of small intestinal
MUC2 and reduced the expression levels of claudin-1 intracellular
junction protein, thus protecting the intestinal epithelial barrier
and counteracting the increased intestinal permeability caused by
infection with RV. These results suggested that lactadherin may be
used in the prevention and treatment of RV-associated diarrhea.
Acknowledgements
The authors of the present study would like to thank
Dr Tongxin Chen at the Shanghai Children's Medical Center
Affiliated to Shanghai Jiao Tong University School of Medicine
(Shanghai, China) for technical support; Professor Luying Peng at
Tongji University School of Medicine and Life Science Experimental
Teaching Center of Pathogenic Biology Laboratory (Shanghai, China)
for providing P2 laboratory; Dr Zhaojun Duan at the China Disease
Prevention and Control Center for Disease Prevention Control
(Beijing, China) and Professor Haimei Tian at Peking Union Medical
College Tumor Hospital (Beijing, China) for providing experimental
materials; and Dr Haiqin Shen at Shanghai Jiao Tong University
School of Medicine for data collection.
Glossary
Abbreviations
Abbreviations:
|
RV
|
rotavirus
|
|
MFG-E8
|
milk-fat globule-EGF factor 8
|
|
OD
|
optical density
|
|
SD
|
standard deviation
|
|
RGD
|
Arg-Gly-Asp
|
|
PBS
|
phosphate buffered saline
|
|
C
|
control group
|
|
RVI
|
RV infection group
|
|
LBRI
|
lactadherin before rotavirus infection
group
|
|
LARI
|
lactadherin after rotavirus
infection
|
|
B
|
blank group
|
References
|
1
|
Walker CL, Rudan I, Liu L, Nair H,
Theodoratou E, Bhutta ZA, O'Brien KL, Campbell H and Black RE:
Global burden of childhood pneumonia and diarrhoea. Lancet.
381:1405–1416. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lanata CF, Fischer-Walker CL, Olascoaga
AC, Torres CX, Aryee MJ and Black RE: Child Health Epidemiology
Reference Group of the World Health Organization and, UNICEF.
Global causes of diarrheal disease mortality in children <5
years of age: A systematic review. PLoS one. 8:e727882013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen SC, Tan LB, Huang LM and Chen KT:
Rotavirus infection and the current status of rotavirus vaccines. J
Formos Med Assoc. 111:183–193. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kvistgaard AS, Pallesen LT, Arias CF,
López S, Petersen TE, Heegaard CW and Rasmussen JT: Inhibitory
effects of human and bovine milk constituents on rotavirus
infections. J Dairy Sci. 87:4088–4096. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bu HF, Zuo XL, Wang X, Ensslin MA, Koti V,
Hsueh W, Raymond AS, Shur BD and Tan XD: Milk fat globule-EGF
factor 8/lactadherin plays a curcial role in maintenance and repair
of murine intestinal epithelium. J Clin invest. 117:3673–3683.
2007.PubMed/NCBI
|
|
6
|
Kusunoki R, Ishihara S, Aziz M, Oka A,
Tada Y and Kinoshita Y: Role of milk fat globule-epidermal growth
factor 8 in intestinal inflammation. Digestion. 85:103–107. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ciarlet M and Estes MK: Interactions
between rotavirus and gastrointestinal cells. Curr Opin Microbiol.
4:435–441. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kanno T, Koyanagi N, Katoku Y, Yonekubo A,
Yajima T, Kuwata T, Kitagawa H and Harada E: Simplified preparation
of a refined milk formula comparable to rat's milk: Influence of
the formula on development of the gut and brain in artificially
reared rat pups. J Pediatr Gastroenterol Nutr. 24:242–252. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lu H, Zhu J, Zang Y, Ze Y and Qin J:
Cloning, high level expression of human paraoxonase-3 in Sf9 cells
and pharmacological characterization of its product. Biochem
Pharmacol. 70:1019–1025. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kaito MI, Ishida S, Tanaka H, Horiike S,
Fujita N, Adachi Y, Kohara M, Konishi M and Watanabe S: Morphology
of hepatitis C and hepatitis B virus particles as detected by
immunogold electron microscopy. Med Mol Morph. 39:63–71. 2006.
View Article : Google Scholar
|
|
11
|
Boshuizen JA, Reimerink JH, van Korteland
Male AM, van Ham VJ, Koopmans MP, Büller HA, Dekker J and Einerhand
AW: Changes in small intestinal homeostasis, morphology and gene
expression during rotavirus infection of infant mice. J Virol.
77:13005–13016. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li JY, Lu Y, Hu S, Sun D and Yao YM:
Preventive effect of glutamine on intestinal barrier dysfunction
induced by severe trauma. World J Gastroenterol. 8:168–171. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yeung T, Gilbert GE, Shi J, Silvius J,
Kapus A and Grinstein S: Membrane phosphatidylserine regulates
surface charge and protein localization. Science. 319:210–213.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang HM, Gao J, Sheng HY, Zhou Y, Chen TX,
Zhu JX and He ZJ: Effect of lactadherin on IL-2, IL-4 and INF-γ
secreted by intestinal tract of newborn rats with rotavirus
infection. Guo Ji Er Ke Xue Za Zhi. 36:328–330. 2009.(In
Chinese).
|
|
15
|
Yang LY, He ZJ and Zhu JX: The effect of
lactadherin in human milk on protecting diarrhea induced by
rotavirus infection. Guo Ji Er Ke Xue Za Zhi. 32:174–176. 2005.(In
Chinese).
|
|
16
|
Zhou YJ, Gao J, Yang HM, Yuan XL, Chen TX
and He ZJ: The role of the lactadherin in promoting intestinal DCs
development in vivo and vitro. Clin Dev Immunol. 2010:3575412010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dong HT: Research of lactadherin's
function. Guo Ji Er Ke Xue Za Zhi. 37:515–517. 2010.(In
Chinese).
|
|
18
|
Li WD, Jia L, Ou Y, Huang YX and Jiang SM:
Surveillance of intra-abdominal pressure and intestinal barrier
function in a rat model of acute necrotizing pancreatitis and its
potential early therapeutic window. PLoS One. 8:e789752013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shimizu K, Ogura H, Goto M, Asahara T,
Nomoto K, Morotomi M, Yoshiya K, Matsushima A, Sumi Y, Kuwagata Y,
et al: Altered gut flora and environment in patients with severe
SIRS. J Trauma. 60:126–133. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vella A and Farrugia G: D-lactate
acidosis: Pathologic consequence of saprophytism. Mayo Clin Proc.
73:451–456. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Moghaddam HS, Moghaddam HN, Kermanshahi H,
Houssavi AH and Raji A: The effect of vitamin A on Mucin2 gene
expression, histological and performance of broiler chicken. Global
Veterinaria. 5:168–174. 2010.
|
|
22
|
Boshuizen JA, Reimerink JH, van Korteland
Male AM, van Ham VJ, Bouma J, Gerwig GJ, Koopmans MP, Büller HA,
Dekker J and Einerhand AW: Homeostasis and function of goblet cells
during rotavirus infection in mice. Virology. 337:210–221. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Deplancke B and Gaskins HR: Microbial
modulation of innate defense: goblet cells and the intestinal mucus
layer. Am J Clin Nutr. 73:1131S–1141S. 2001.PubMed/NCBI
|
|
24
|
Faure M, Moënnoz D, Montigon F, Mettraux
C, Mercier S, Schiffrin EJ, Obled C, Breuillé D and Boza J: Mucin
production and composition is altered in dextran sulfate
sodium-induced colitis in rats. Dig Dis Sci. 48:1366–1373. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sharma R, Young C and Neu J: Molecular
modulation of intestinal epithelial barrier: Contribution of
microbiota. J Biomed Biotechnol. 2010:3058792010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Groschwitz KR and Hogan SP: Intestinal
barrier function: Molecular regulation and disease pathogenesis. J
Allergy Clin Immunol. 124:3–20. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Anthony T Blikslager: Mucosal epithelial
barrier repair to maintain pig health. Livestock Science.
133:194–199. 2010. View Article : Google Scholar
|
|
28
|
Zhou L: Progress in the pathogenesis of
rotavirus-associated disease. Guo Ji Er Ke Xue Za Zhi. 37:232–234.
2010.(In Chinese).
|
|
29
|
Colbère-Garapin F, Martin-Latil S, Blondel
B, Mousson L, Pelletier I, Autret A, François A, Niborski V,
Grompone G, Catonnet G and van de Moer A: Prevention and treatment
of enteric viral infections: Possible benefits of probiotic
bacteria. Microbes Infect. 9:1623–1631. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ehehalt R, Braun A, Karner M, Füllekrug J
and Stremmel W: Phosphatidylcholine as a constituent in the colonic
mucosal barrier: Physiological and clinical relevance. Biochim
Biophys Acta. 1801:983–993. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Irvine EJ and Marshall JK: Increased
intestinal permeability precedes the onset of Crohn's disease in a
subject with familial risk. Gastroenterology. 119:1740–1744. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Poritz LS, Harris LR III, Kelly AA and
Koltun WA: Increase in the tight junction protein claudin-1 in
intestinal inflammation. Dig Dis Sci. 56:2802–2809. 2011.
View Article : Google Scholar : PubMed/NCBI
|