Introduction
The clinical symptoms that accompany vasoactive
intestinal peptideoma (VIPoma) most commonly include watery
diarrhea, hypokalemia and achlorhydria (or metabolic acidosis);
this collection of symptoms is also known as WDHA syndrome. WDHA
syndrome was first described by Verner and Morrison in 1958
(1), and has been assumed to be
caused by the hypersecretion of vasoactive intestinal polypeptide
(VIP) (2). In adults, this tumor is
most commonly found in the pancreas, with 80% of the tumors
occurring in the body and tail of pancreas and 20% occurring in the
pancreatic head (3). These tumors
are usually solitary and >3 cm in diameter. Between 50–60% of
pancreatic VIPomas have already developed metastases at the point
of diagnosis, primarily in the liver and lymph nodes (4). The usual methods of treating VIPoma are
surgical excision, peptide receptor radionuclide therapy,
streptozotocin-based chemotherapy, ablation, hepatic artery
embolization, liver transplantation and adjuvant therapy, depending
on the condition of patient. The median overall survival of
pancreatic endocrine tumors is 38 months, with localized, regional
and distant islet cell carcinoma survival durations of 124, 70 and
23 months, respectively (5). The
present study describes the case of a patient who presented with
chronic watery diarrhea and hypokalemia due to a tumor in the
pancreatic head, which was immunohistochemically confirmed to
contain immunoreactive VIP and diagnosed as a metastatic hepatic
lesion through computed tomography (CT). Written informed consent
for publication was obtained from the patient.
Case report
A 65-year-old male, presenting with a six-month
history of profuse watery diarrhea, anorexia, vomiting, a 5-kg
weight loss and extreme weakness, was admitted to the First
Hospital of Dandong (Liaoning, China), in 2011. The patient had ≥10
watery bowel movements per day without blood or mucus. The symptoms
were not relieved following oral levofloxacin administration.
Physical examination revealed a dehydrated appearance and
generalized weakness. The patient's blood pressure was 85/55 mmHg
and his heart rate was 92 beats/min. Assessment of the breath
sounds revealed rough lung breath sounds with occasional wheezing;
however, cardiac auscultation was normal. No tenderness was present
in the liver, kidney or other areas of the abdomen. Active bowel
sounds were found with abdominal auscultation. The patient had a
previous history of bronchial asthma for 20 years and was diagnosed
with hyperthyroidism 10 years previously. This hyperthyroidism was
subsequently cured. The rest of the patient's history, and that of
his family, was not noteworthy.
Laboratory examination revealed that all the
biochemical tests, including the hemoglobin level, white blood cell
count, urinalysis, and renal and liver function, were normal.
Microbiological and parasitological examinations of the feces
yielded no positive findings. The blood cell dissemination was 39
mmol/l. Plasma sodium, chloride, phosphate, calcium, urine amylase
and fasting blood glucose levels were normal; however, marked
hypokalemia (2.26 mmol/l) was noted. The level of the tumor marker
carcinoembryonic antigen (CEA) was 3.63 ng/ml. The majority of the
indices of thyroid function were within the normal ranges, with the
exception of the levels of free thyroxine (18.15 pmol/l), which
were a little higher. A bilateral adrenal CT scan, colonoscopy,
double-contrast enteroclysis and an abdominal ultrasound were
performed, but no meaningful findings were obtained. Gastroscopy
showed chronic superficial gastritis. The electrocardiograph showed
prominent U waves consistent with hypokalemia. An abdominal CT scan
showed a mass with a 6-cm diameter in the pancreatic head.
Based on these results, the patient was diagnosed
with hypokalemia and diarrhea. Fluid infusions were initiated and
the patient was administered 6 g potassium per day and oral
anti-inflammatory drugs; however, the hypokalemia and diarrhea
reoccurred following the cessation of the drug administration. The
chronic diarrhea and persistent hypokalemia, and the presence of a
mass in the pancreas, suggested that a vasodilatory intestinal
peptide-secreting tumor could not be excluded.
Experimental treatment of 0.1 mg octreotide twice
per day by intravenous infusion was subsequently carried out. The
patient showed significant relief from the diarrhea and nausea
following the first day of octreotide treatment. One week later,
the nausea and other discomfort were also reduced significantly,
and the octreotide was then terminated. The condition reoccurred
following the suspension of the octreotide; however,
re-administration of the octreotide still produced positive
results. The effectiveness of the octreotide on the diarrhea made
the diagnosis of a VIPoma highly likely. A blood sample was
therefore sent to the Chinese Medical University Affiliated
Hospital (Shenyang, China) to measure the VIP concentration. The
diagnosis was confirmed by the high level of VIP (>600 pg/ml).
18F-fluorodeoxyglucose-positron emission tomography
(PET) showed abnormal uptake at the same location as the pancreatic
tumor revealed by CT. The maximum standardized uptake value of the
lesion was 13.0 Hounsfield units (Fig.
1). The combination of the symptoms, response to octreotide and
findings of the imaging studies prompted the decision to perform an
exploratory laparotomy.
Multiple nodules in the liver were found during the
surgery, which were considered to be hepatic metastasis. The
pancreatic tumor, which measured 5.0×4.0×4.0 cm, exhibited
infiltration into the transverse mesocolon. The mass and the
superior mesenteric vein invasion could not be separated. The
intraoperative pathological biopsy diagnosis was VIPoma with liver
metastasis. The tumor was excised from the pancreas.
The VIP level returned to the normal range following
surgery. Oral potassium supplementation, octreotide and oral
prednisolone were still required for symptom relief. During the
one-and-a-half years after the surgery, the frequency of the
diarrhea episodes was reduced to approximately four times per
day.
The aforementioned symptoms became apparent once
more one-and-a-half years after the surgery. Abdominal CT indicated
that the pancreas exhibited a lack of uniform density, while the
liver was normal in size and shape. The left and right hepatic
lobes showed multiple sizes of low-density nodules with CT values
between 17 and 55 HU, which were considered to be liver metastasis.
The abdominal lymph nodes were normal. Magnetic resonance imaging
(MRI) confirmed the observations of the CT. Under general
anesthesia, pancreatoduodenectomy, superior mesenteric vein partial
resection and reconstruction, partial resection of the liver
metastatic nodules (four nodules with a diameter >0.5 cm in the
left outer lobe, two in the left inner lobe, three in the right
anterior lobe and one in the right posterior lobe) and liver
surface metastasis nodule cauterization were performed
successively. The pathological diagnosis was pancreatic mixed
ductal-endocrine cancer. The lymph node biopsy showed cancer
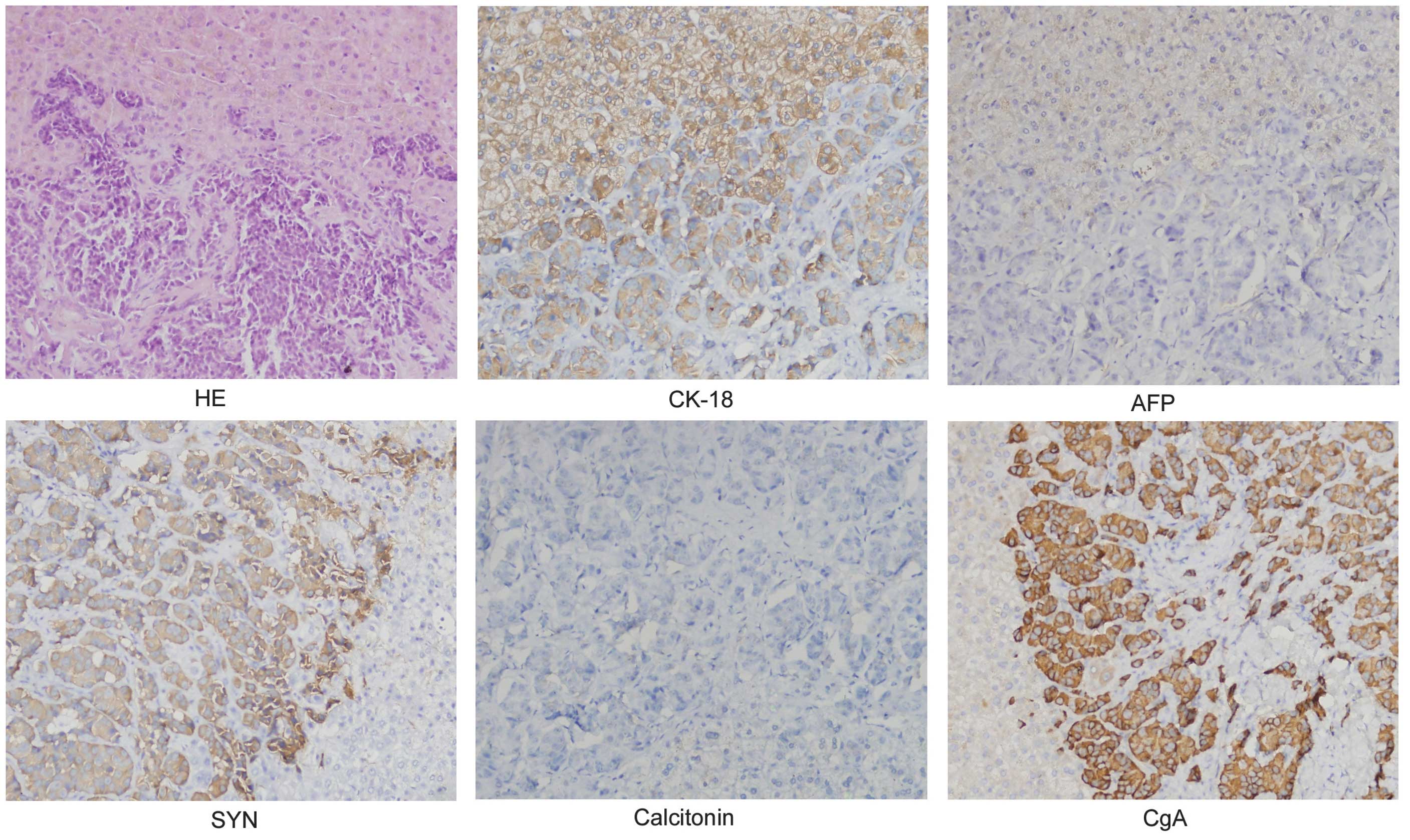
metastasis. The results of the immunohistochemistry were as follows
(Fig. 2): α-fetoprotein (AFP) (−),
calcitonin (−), chromogranin A (CgA) (+), cytokeratin (CK) 18 (+)
and synaptophysin (+). The patient was transferred to the intensive
care unit following the surgery. Rehydration, anti-inflammatory
agents and gastric acid and trypsin inhibitors were administered,
and nutritional support was carried out. Persistent low levels of
potassium and intermittent atrial fibrillation were noted following
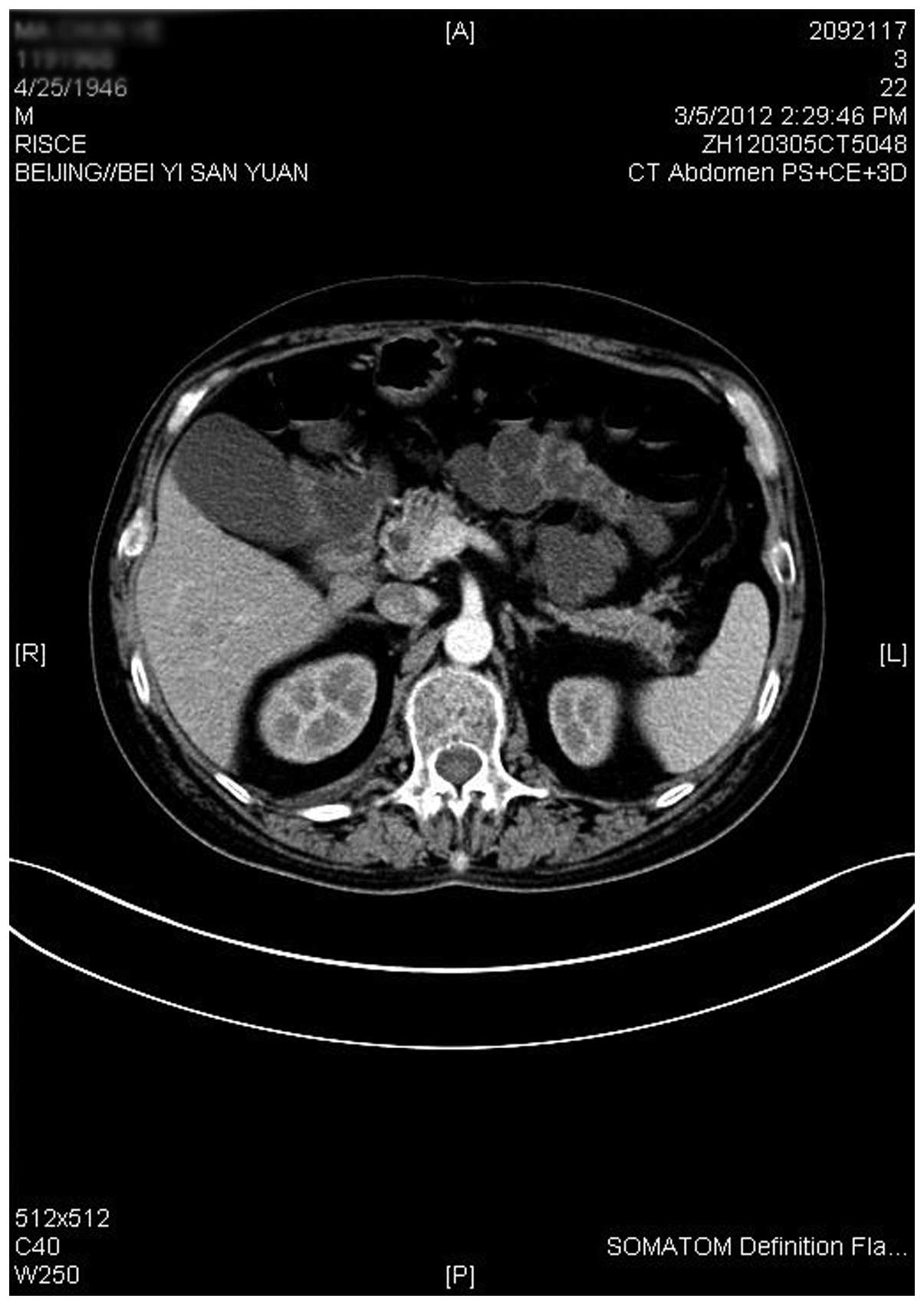
the surgery. The left hepatic abscess was found through abdominal
CT examination (Fig 3). Subsequent
to cardioversion, potassium supplementation and anti-infection
therapy, the condition of the patient gradually stabilized. A
follow-up at 18 months showed that the patient is still alive and
has achieved partial control of his symptoms.
Discussion
Pancreatic VIP-secreting tumors are rare islet cell
tumors associated with secretory diarrhea. The incidence of this
type of neoplasm is estimated to be one per 10,000,000 individuals
in the general population annually (2). A total of 90% of the VIPomas in adults
originate from the pancreas (3). VIP
is a 28-amino acid polypeptide with high homology in structure to
secretin. Following the binding of VIP to receptors on the
intestinal epithelial cells, adenylate cyclase and cyclic adenosine
monophosphate production is activated. This leads to the secretion
of water and electrolytes into the intestinal lumen (1). Patients typically present with chronic
diarrhea and are diagnosed late due to the slow-growing nature of
the tumor. It is difficult to find VIPomas when they are small.
Symptomatic pancreatic VIPomas are usually >3 cm in diameter. At
the time of presentation, >70% of patients have developed
metastases (3).
The diagnosis of VIP-secreting tumors includes
clinical symptoms and laboratory assays. The major clinical
symptoms of VIP-secreting tumors include severe watery diarrhea,
hypokalemia and acid-free or low levels of gastric acid secretion,
which is referred to as WDHA syndrome or pancreatic cholera
(1). A high volume of diarrhea is
universal, and hypokalemia occurs in 70–100% of patients (2). Other symptoms include severe
dehydration, gallbladder enlargement, intestinal pseudo-infarction,
high blood sugar and high blood calcium levels. The patient in the
present case report had watery diarrhea >10 times per day and
exhibited persistent low blood potassium and gastric acid secretion
levels, hypovolemic shock and gallbladder enlargement, all of which
were diagnostically consistent with the disease. Laboratory assays
in the diagnosis of the disease mainly include serum VIP,
pancreatic polypeptide and CgA (2).
In the present case, only the concentration of VIP was measured due
to the limitations of the laboratory conditions. The plasma VIP
concentration was found to measure >600 ng/l, while the accepted
standard for plasma the VIP concentration in patients with
VIP-secreting tumors is ≥200 ng/l (2). In addition to laboratory testing, CT
and MRI have a sensitivity of 80–85%, and functional PET imaging
has a sensitivity of ~97% (6). These
imaging techniques are also used to determine whether metastases
are present. VIP-secreting tumors are typically located in the
pancreatic body and tail, and more than half of the clinical cases
have a complete capsule (3). In the
current case, the patient showed lesions in the pancreatic head, as
detected by CT.
Considerable advances have been made in the
management of VIPoma. The first line of treatment is surgical
excision for patients with benign and non-metastatic disease;
however, there is no accepted standard management for patients with
metastatic disease. Surgery for tumor clearance, peptide receptor
radionuclide therapy (6),
streptozotocin-based chemotherapy (7), ablation (8), hepatic artery embolization (9), liver transplantation,
sunitinib/everolimus (10) and
adjuvant therapy, such as octreotide, interferon-α and
glucocorticoids, are suggested, depending on the condition of
patients. In the present case, the patient underwent surgeries to
remove tumors on the pancreas and liver, respectively. The diarrhea
improved following surgery and adjuvant treatment, and its
frequency was reduced to four times a day as compared with 10 times
preoperatively. The somatosatin analogue, octreotide, was
administrated for symptomatic relief. Long-term application of the
drug, however, may lead to drug resistance and the necessity for an
increase in dosage (11,12). Prednisone and indomethacin are also
considered to be effective in alleviating the clinical symptoms;
therefore, octreotide, at a dosage of 100 mg daily, combined with
20 mg prednisone daily were administered to the patient in this
study to control the symptom of diarrhea and arrest the liver
metastasis.
In conclusion, VIPoma is rare, and the disease has
typically already metastasized at presentation. Considering VIPoma
in patients with chronic diarrhea and hypokalemia would aid the
early diagnosis of this disease. In total, 40% of patients with
non-metastatic disease are likely to be cured by a complete
resection of the tumor (13).
Palliative surgery is indicated in advanced disease, followed by
somatostatin analogue therapy. Somatostatin analogues improve
hormone-mediated symptoms and reduce tumor volume; however,
long-term application of octreotide is likely to lead to drug
resistance and inhibit the secretion of insulin, growth hormone and
glucagon, which is problematic. The use of corticosteroids has
achieved successful responses in several cases (14,15). The
combined application of corticosteroids with octreotide may reduce
the required dosage of octreotide, avoid the side-effects and ease
the financial burden of the patient.
Glossary
Abbreviations
Abbreviations:
|
WDHA
|
watery diarrhea associated with
hypokalemia and achlorhydria
|
|
VIP
|
vasoactive intestinal polypeptide
|
|
CT
|
computed tomography
|
|
MRI
|
magnetic resonance imaging
|
|
PET
|
positron emission tomography
|
References
|
1
|
Verner JV and Morrison AB: Islet cell
tumor and a syndrome of refractory watery diarrhea and hypokalemia.
Am J Med. 25:374–380. 1958. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ghaferi AA, Chojnacki KA, Long WD, Cameron
JL and Yeo CJ: Pancreatic VIPomas: subject review and one
institutional experience. J Gastrointest Surg. 12:382–393. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Perry RR and Vinik AI: Clinical review 72
diagnosis and management of functioning islet cell tumors. J Clin
Endocrinol Metab. 80:2273–2278. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Soga J and Yakuwa Y: VIPoma/diarrheogenic
syndrome: A statistical evaluation of 241 reported cases. J Exp
Clin Cancer Res. 17:389–400. 1998.PubMed/NCBI
|
|
5
|
Yao JC, Eisner MP, Leary C, Dagohoy C,
Phan A, Rashid A, Hassan M and Evans DB: Population-based study of
islet cell carcinoma. Ann Surg Oncol. 14:3492–3500. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Baum RP and Kulkarni HR: THERANOSTICS:
from molecular imaging using Ga-68 labeled tracers and PET/CT to
personalized radionuclide therapy - the Bad Berka experience.
Theranostics. 2:437–447. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Moertel CG, Lefkopoulo M, Lipsitz S, Hahn
RG and Klaassen D: Streptozocin-doxorubicin,
streptozocin-fluorouracil or chlorozotocin in the treatment of
advanced islet-cell carcinoma. N Engl J Med. 326:519–523. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Moug SJ, Leen E, Horgan PG and Imrie CW:
Radiofrequency ablation has a valuable therapeutic role in
metastatic VIPoma. Pancreatology. 6:155–159. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shaib W, Mitchell K and Saif MW:
Amelioration of symptoms and reduction of VIP levels after hepatic
artery chemoembolization in a patient with sandostatin resistant
VIPoma. Yale J Biol Med. 83:27–33. 2010.PubMed/NCBI
|
|
10
|
Strosberg JR, Cheema A and Kvols LK: A
review of systemic and liver-directed therapies for metastatic
neuroendocrine tumors of the gastroenteropancreatic tract. Cancer
Control. 18:127–137. 2011.PubMed/NCBI
|
|
11
|
Adam N, Lim SS, Ananda V and Chan SP:
VIPoma syndrome: challenges in management. Singapore Med J.
51:e129–e132. 2010.PubMed/NCBI
|
|
12
|
Yasunami Y, Funakoshi A, Ryu S, et al: In
vitro release of vasoactive intestinal polypeptide and pancreatic
polypeptide from human VIPoma cells and its inhibition by
somatostatin analogue (SMS 201–995). Surgery. 115:713–717.
1994.PubMed/NCBI
|
|
13
|
Arnold R, Frank M and Kajdan U: Management
of gastroenteropancreatic endocrine tumors: the place of
somatostatin analogues. Digestion. 55(Suppl 3): 107–113. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nguyen HN, Backes B, Lammert F, et al:
Long-term survival after diagnosis of hepatic metastatic VIPoma:
report of two cases with disparate courses and review of
therapeutic options. Dig Dis Sci. 44:1148–1155. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zimmermann K, Rapp T, Binder J, Frölich J
and Bode JC: Vipoma. 9-year observations using currently available
therapy methods. Dtsch Med Wochenschr. 111:298–301. 1986.(In
German). View Article : Google Scholar : PubMed/NCBI
|