Introduction
Type 2 diabetes (T2D) mellitus has been on the
increase in young individuals, and is correlated with the increase
in childhood obesity. A survey conducted in 2008 showed that the
prevalence of T2D increased from 9 to 23% over the span of 8 years,
between 2000 and 2008 (1). Rates of
T2D are also dependent on ethnicity as the greatest increase in
childhood T2D was predominantly in non-white ethnic groups. The
incidence of T2D in individuals of European, African-American and
American-Indian ethnicity was 3, 15.7 and 49.4 out of 100,000
individuals aged 10–19 years, respectively. In addition, more
females were diagnosed with T2D than males (2). Type 1 diabetes (T1D) mellitus, similar
to T2D, is also on the increase worldwide. The rates of T1D are the
highest in Scandinavian countries, the UK, the USA, Canada and
Australia. In Finland, the incidence of T1D is 57/100,000 children
while the incidence of T1D in other western countries is
approximately 20/100,000 children. Asian and African countries have
lower rates of T1D. Despite the already high rates in European
countries, the incidence of T1D is actually on the increase across
Europe, at 3.9% annually in children. Asian and African countries
have lower rates of T1D. Despite the already high rates in European
countries, the incidence of T1D is on the increase across Europe,
~3.9% year in children (3). In
addition, T1D has increased in the US at a rate of 21% in the 8
years between 2001 and 2009. In the present review, we provide the
current guidelines on treating T1D and T2D mellitus (4).
Pathology
Under non-pathological conditions, an increase in
blood glucose triggers a secretion of insulin from the β cells of
the pancreas. The hormone glucagon is secreted by the α cells of
the pancreas, which activates gluconeogenesis, thereby preventing
hypoglycemia during times when no food is being digested. T2D
mellitus develops when there is a discrepancy in glucose metabolism
and storage. The pathology behind T2D involves a decrease in
peripheral tissue sensitivity to insulin as well as decreased
insulin secretion from the β cells. When the tissues become less
sensitive to insulin, the β cells start to increase insulin
secretion. When the β cells start to overcompensate in this manner,
it leads to overburdening of work on the β cells. Overworked β
cells start to reduce their functions, which leads to an eventual
decrease in insulin secretion. This leads to the phenomenon of
glucose intolerance, which impairs the secretion of glucagon. When
glucagon no longer fulfills its regular function, the glycemic
state of a patient becomes exacerbated and leads to an aggravation
of diabetic pathology (5,6).
Childhood obesity and increased body fat are
correlated with insulin resistance. The mechanism behind the way
obesity affects T2D is well known. Obesity rates have increased
considerably over the past 40 years. In 1980, the childhood obesity
rates were 6.5%, which increased to 16.9% in 2008 (7). The percentage of overweight and obese
children and adolescents, aged 2–19 years, is ~33%. Overweight or
obese children are more at risk for developing T2D as well as other
diseases including cardiovascular disease. Due to the fact that
overweight or obese children are more at risk for other diseases
and complications, it is imperative to prevent a serious disease
such as T2D (8).
T1D is a disorder that affects genetically
susceptible individuals, but may also occur in individuals with no
genetic predisposition. T1D is an autoimmune disorder, leading to a
loss of pancreatic β cells. T1D occurs due to the presence of
autoantibodies that destroy proteins that form a β cell. The most
common antibody in patients with T1D is directed towards a β-cell
enzyme, glutamic acid decarboxylase. Patients with T1D may also
have anti-insulin antibodies. As the number of β cells decreases,
insulin concentration concurrently decreases until there is no
longer insulin available to regulate glucose levels. However,
hyperglycemia does not develop until 80–90% of the cells are
destroyed. If the condition goes untreated, diabetic ketosis may
develop. One of the ways that T1D is activated in individuals that
are genetically susceptible is through viral infection, which may
stimulate the production of antibodies towards the virus, which
contains similar antigens to β cells. The incidence of T1D is
increased in patients with other autoimmune diseases such as Graves
and Addison disease (7,9).
Diagnosis
Diagnosing diabetes in children is difficult as
there may be confusion between T1D and T2D. Historically, children
with diabetes were diagnosed with T1D. However, the rise in obesity
and the consequent rise of T2D in children have obscured the
diagnosis. Approximately 25% of children with T1D are overweight or
obese. There are guidelines to determine, and differentiate,
between the two diagnoses in children (Table I). T2D diagnosis in youth is normally
dependent on glucose concentrations and symptoms such as excess
urine, vision problems, excess thirst, weight loss and ketonuria. A
definite diagnosis of T2D is made when these symptoms are also
complemented with a >200 mg/dl random glucose concentration or a
>126 mg/dl fasting glucose concentration. Children with a high
body mass index (BMI), a family history of diabetes, antibodies
that denoted insulin resistance and other issues such as
hypertension are high-risk for T2D. The age at diagnosis for T2D in
the youth is generally at puberty; and rarely presents at <10
years. T2D usually presents with diabetic ketoacidosis in 5–20% of
the cases (10).
 | Table I.Features that characterize type 1 and
type 2 diabetes mellitus. |
Table I.
Features that characterize type 1 and
type 2 diabetes mellitus.
| Characteristics | T1D | T2D |
|---|
| Date of
presentation | 6 months-18
years | 10 years + |
| Length of
pathology | Sudden | Gradual |
| Ketoacidosis | Normal | Rare |
| Body weight | Normal | Obese (often) |
| Insulin levels | Few | Normal, greater or
fewer |
| Autoantibodies | Normal | Not present |
| Incidence | 5–10% of cases | 90–95% of cases |
|
| (USA) | (USA) |
The age at diagnosis for T1D is much lower than that
for T2D, at the age of 6 months to 18 years. The symptoms that
usually present with T1D range from weight loss, thyroid
autoimmunity and celiac disease. Approximately 25% of the time, T1D
cases presented with diabetic ketoacidosis. Similar to T2D, T1D
diagnosis is made through blood glucose tests. A fasting blood
glucose test can be performed, although they are often not
reliable. In that case, an oral glucose tolerance test may be
conducted. Such a test involves the patient ingesting a specific
glucose solution and then a glucose test is performed after 2 h.
Another test that can be performed is the glycated hemoglobin test
(A1C test) which analyzes blood glucose levels over the previous
few months as opposed to one particular time point. This test
assesses the percentage of hemoglobin, to which glucose is bound,
and a high percentage indicates diabetes. Once diabetes is
diagnosed, an antibody test would be administered, which could be
used differentiate between a T1D diagnosis and a T2D diagnosis
(10,11).
Primary treatment of type 2 diabetes
Children with T2D are almost always started on
metformin. Metformin is a drug that is classified as a biguanide.
This drug functions by reducing glucose production and by
activating glucose uptake in peripheral tissues. Metformin is
administered in children at a 500 mg dose, and is ingested at meal
times daily. The dose is increased by 500 mg each week until the
dose equals 2,000 mg. There are some adverse events associated with
the use of metformin including gastrointestinal problems (abdominal
pain and diarrhea), which may result in a patient not reaching
optimal dose of metformin. In some rare instances, lactic acidosis
and renal dysfunction can occur in individuals taking metformin
(12). Metformin reduces glycated
hemoglobin levels to 2% and also aids in weight loss. Clinical
trials have shown that the use of metformin is safe for T2D
pediatric patients. Another study found that in patients aged
10–17, metformin and 4 mg rosiglitazone was a superior therapy for
T2D than metformin alone (11,13).
Insulin treatment in type 2 diabetes
Insulin may also be used in treating childhood T2D.
Exogenous insulin helps maintain glucose homeostasis by aiding
muscle and adipose tissue uptake excess glucose and reduce glucose
production (14). Caveats to using
insulin include weight gain, hypoglycemia and increased insulin.
There are certain insulin types that are used for pediatric
patients, such as glulisine, detemir, glargine, hagedorn, aspart,
regular and neutral protamine (15).
However, the most successful treatment is the administration of a
single dose of long-acting insulin at bedtime. Although the
clinical guidelines state that insulin should be used as a
first-line treatment in T2D pediatric patients that are ketotic,
some clinicians choose not to due to the potentially undesirable
effects. Administration of insulin early in the disease
pathogenesis is good practice as it leads to a normalization of
glucose (11,16).
Alternative drug treatments in childhood
type 2 diabetes
Another class of drugs that is useful in the
decrease of glucose concentrations is thiazolidinediones. They
function by increasing insulin sensitivity in areas of the body
including liver, muscles and adipose tissue. These drugs also
decrease glucose synthesis of the liver. However,
thiazolidinediones are not approved for use in children. In adults,
thiazolidinediones decrease glycated hemoglobin to 1% (17). Some of the drugs in this class
include rosiglitazone and pioglitazone, which have been studied in
clinical trials. Another class of drugs that can be used to treat
T2D but are not approved for use in children is meglitinides. Two
drugs included in this class are repaglinide and nateglinide, which
function by stimulating insulin from the pancreas. Complications
that can result from meglitinides include hypoglycemia, upper
respiratory tract infection, diarrhea and headaches (18).
Sulfonylureas can also be used to treat diabetes and
function by activating the β cells to secrete insulin. Although
sulfonylureas are not generally used for the pediatric population,
they have been known to be safe for children (19). The specific types of sulfonylureas
that have been studied in children are glimepiride and glipizide.
The caveats of sulfonylureas are usually weight gain and
hypoglycemia. Clinical studies using glimepride in children showed
it was equally effective at treating T2D as metformin.
Sulfonylureas in adults also reduced glycated hemoglobin levels to
1.25% in adults (20). The use of
sulfonylureas in other conditions such as maturity-onset diabetes
of the young has been investigated. Long-term use of sulfonylureas
enhanced insulin levels by approximately 68% (11,21).
α-glucosidase inhibitors such as acarbose and
miglitol can be used to treat T2D by reducing the absorption of
carbohydrates in the distal small intestine, which reduces serum
glucose. While α-glucosidase inhibitors can decrease glycated
hemoglobin levels, their use in children has not been sufficiently
investigated. Common complications from these drugs are diarrhea
and abdominal cramps. In a double-blind randomized trial, it was
found that acarbose may be useful for the paediatric population
with T2D, although at a significant cost because of
gastrointestinal side effects. Glucagon-like peptide-1 (GLP-1)
agonists are another class of T2D drugs. GLP-1 is a hormone
released from the gut in response to digestion of meals and
functions in insulin biosynthesis. Similar to other T2D drugs,
these agonists have yet to be approved for pediatric patients.
Exenatide is a GLP-1 agonist, which has been studied in the youth
population for its effect on T2D. Previously, 5 mcg exenatide was
administered twice a day in obese youth aged 12–19 years, which led
to a significant decrease in BMI (21,22).
Treatment of type 1 diabetes
Unlike children diagnosed with T2D, children with
T1D need insulin treatment to survive. Oral insulin is not an
option for patients with diabetes as gastric enzymes impede oral
insulin (23).
There are many types of insulin that are available
for the treatment of T1D. Rapid-acting insulin, such as lispro and
aspart, begins functioning within 15 min post-injection. Another
type of insulin is short-acting insulin, which begins working
approximately 30 min post-injection and reaches peak performance
within 2–4 h. Long-acting insulin is another type of insulin, which
does not have a peak but provides use for 20–25 h. Some examples of
long-acting insulin include glargine and detemire.
Intermediate-acting insulin is another type of insulin that starts
to function at 30 min to 1 h and reaches its peak at 4–6 h. An
example of this type of insulin is NPH insulin. When prescribing
insulin, doctors take into account a child's age and his/her needs
and therefore, may prescribe a mixture of these insulin types.
Insulin is delivered using a fine needle and syringe or pen, which
looks similar to an ink pen but is instead filled with insulin.
Many children can also use an insulin pump, which is generally the
size of a cell phone. A tube connects the insulin to a catheter
that is inserted in the abdomen. The pump can be adjusted to
deliver the appropriate amount of insulin depending on glucose
level (24).
Insulin is used in the majority of cases of children
with T1D. However, clinical trials have been conducted to determine
the effectiveness of other drugs for treatment. In pediatric
patients with T1D, 4 mg glimepiride was administered to 40 children
in a randomized clinical trial. However, the investigators found no
difference between the groups with regard to weight, blood
pressure, insulin dose, serum glucose, hypoglycemia and serum
lipids. The drug was found to be safe for the patients, although
further research is essential to determine whether a higher dose
may actually have a beneficial effect. Exenatide, a GLP-1 agonist,
was investigated as an adjunctive treatment to insulin in children
with T1D (25). Eight adolescents,
aged 13–22 years, were examined and administered two doses of
exenatide or insulin. Exenatide was found to decrease hyperglycemia
but did not suppress glucose. The investigators of that study
concluded that exenatide may be used as an effective adjunctive
treatment to insulin for the treatment of T1D.
Another class of drugs is amylin analogs, an example
of which is pramlintide. Amylin is a hormone that is known to
inhibit glucagon secretion, cause a delay in gastric emptying and
increase satiety. In pediatric patients with T1D, pramlintide was
studied at a dose of 15–30 mcg. Pramlintide was shown to reduce
glucose levels with minimal complications. Previous findings showed
that when given to youth with T1D, pramlintide reduced glycated
hemoglobin, BMI and total insulin dose. Thus, amylin analog
pramlintide is a promising drug that can be used in the treatment
of diabetes (11,26).
Lifestyle modifications
In children with T1D and T2D, lifestyle
modifications are an important part of the treatment. Lifestyle
modifications include ≥1 h of vigorous physical activity and
maintaining a healthy BMI (18.5–24.9). In children with T2D,
lifestyle modifications are paramount and a cornerstone for
treatment of the disease (27).
However, only 10% of children achieve their lifestyle modification
aims. There are many reasons for this lack of success, including
peer pressure for unhealthy eating and that many patients do not
understand the importance of a diet. The prescribed amount of
physical activity a day is ≥60 min. In addition, other lifestyle
modifications include decreasing screen-time such as using a
television or computer, to <2 h/day (28).
Nutritional change is another necessary lifestyle
modification that is advised to children with T1D and T2D. Normal
dietary recommendations involve consumption of regular meals and
healthy snacks, decreased portion sizes, consumption of
calorie-free beverages such as water, and an increase in the
consumption of fruits and vegetables. However, there is no
‘diabetes diet’. A good diet should include foods that are high in
nutrients and low in fat and calories, and consumption of fewer
animal products and sweets. High-fat foods are especially of poor
quality because fat slows digestion leading to a spike in blood
sugar levels several hours after meal consumption (27–29).
Investigational treatments
Besides the abovementioned clinical trials for drugs
that are being investigated for their treatment potential for
diabetes, other treatments have been considered. The first of these
treatments is pancreas transplant, which, if successful, lead to a
child no longer having to inject insulin. However, these
transplants are not always successful and constitute a high-risk
factor. In addition, with a pancreas transplant, the patient always
require immune-suppressing drugs to preven organ rejection. Due to
the issues with pancreas transplant, it is limited to individuals
with kidney failure. The second treatment is islet cell
transplantation, which provides new β cells from a donor pancreas.
New techniques and improved drugs are now in use to prevent islet
cell rejection. However, this transplant requires
immune-suppressing medication. In the case of T1D patients, the
body may occasionally destroy transplanted islet cells, just as it
did the original cells (30–31).
Conclusion
Diabetes has surfaced as a serious problem in
children in recent few decades. There are guidelines in place to
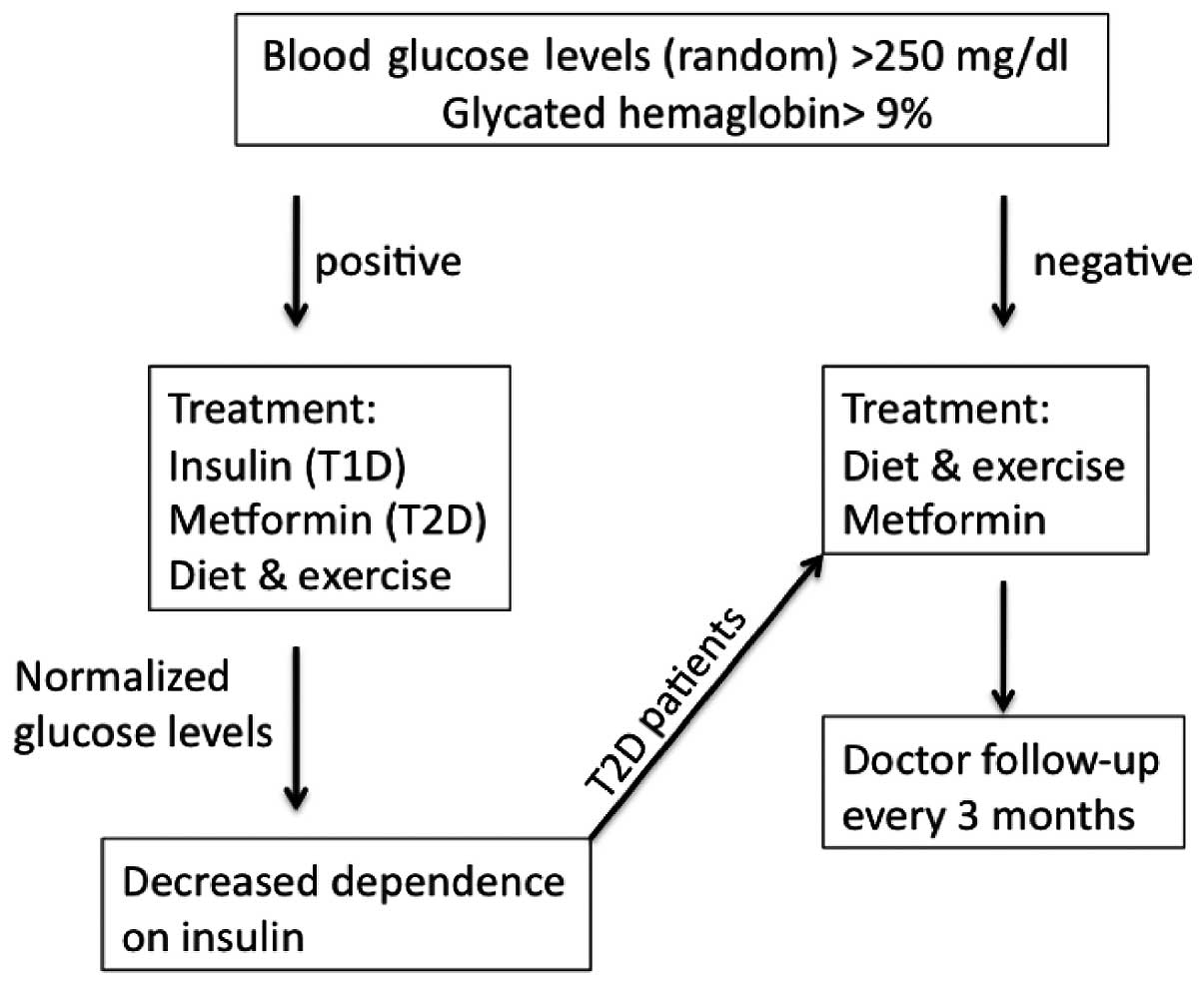
diagnose and treat children with T1D and T2D (Fig. 1). T1D mellitus is an autoimmune
disease that occurs in children that may be genetically
susceptible. Children with T1D develop antibodies to β-cell
components, which leads to an eventual decrease in insulin
production. Children with T2D develop the problem for a number of
reasons, one of which is an increase in peripheral fat deposits
that desensitize tissues to insulin and decrease insulin
production. The primary way to treat T1D is to administer insulin
while metformin is used to treat T2D. Other classes of drugs, such
as thiazolidiniones, sulfonylureas, meglitinides, α-glucosidase
inhibitors, GLP-1 agonists and amylin analogs, have emerged as
potential alternatives to insulin and metformin. However, these
drugs have not been studied in children and therefore, are not
currently in use for the pediatric population. Lifestyle
modifications are also extremely important for proper management of
the two types of diabetes. Lifestyle modifications include physical
activity and nutrition management. Due to the increasing prevalence
of T1D and T2D in children, these new drugs and transplants need to
be further investigated to determine their safety and effect on
children.
References
|
1
|
May AL, Kuklina EV and Yoon PW: Prevalence
of cardiovascular disease risk factors among US adolescents,
1999–2008. Pediatrics. 129:1035–1041. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McBean AM, Li S, Gilbertson DT and Collins
AJ: Differences in diabetes prevalence, incidence, and mortality
among the elderly of four racial/ethnic groups: Whites, blacks,
hispanics, and asians. Diabetes Care. 27:2317–2324. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Onkamo P, Väänänen S, Karvonen M and
Tuomilehto J: Worldwide increase in incidence of Type I diabetes -
the analysis of the data on published incidence trends.
Diabetologia. 42:1395–1403. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Patterson C, Guariguata L, Dahlquist G,
Soltész G, Ogle G and Silink M: Diabetes in the young - a global
view and worldwide estimates of numbers of children with type 1
diabetes. Diabetes Res Clin Pract. 103:161–175. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Klöppel G, Löhr M, Habich K, Oberholzer M
and Heitz PU: Islet pathology and the pathogenesis of type 1 and
type 2 diabetes mellitus revisited. Surv Synth Pathol Res.
4:110–125. 1985.PubMed/NCBI
|
|
6
|
Zammitt NN and Frier BM: Hypoglycemia in
type 2 diabetes: Pathophysiology, frequency, and effects of
different treatment modalities. Diabetes Care. 28:2948–2961. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hannon TS, Rao G and Arslanian SA:
Childhood obesity and type 2 diabetes mellitus. Pediatrics.
116:473–480. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ogden CL, Flegal KM, Carroll MD and
Johnson CL: Prevalence and trends in overweight among US children
and adolescents, 1999–2000. JAMA. 288:1728–1732. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tuomilehto J, Zimmet P, Mackay IR, Koskela
P, Vidgren G, Toivanen L, Tuomilehto-Wolf E, Kohtamäki K, Stengård
J and Rowley MJ: Antibodies to glutamic acid decarboxylase as
predictors of insulin-dependent diabetes mellitus before clinical
onset of disease. Lancet. 343:1383–1385. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Association AD: American Diabetes
Association: Type 2 diabetes in children and adolescents.
Pediatrics. 105:671–680. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Scheen AJ: Clinical pharmacokinetics of
metformin. Clin Pharmacokinet. 30:359–371. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Aronoff SL, Berkowitz K, Shreiner B and
Want L: Glucose metabolism and regulation: beyond insulin and
glucagon. Diab Spectr. 17:183–190. 2004. View Article : Google Scholar
|
|
13
|
Miles HL and Acerini CL: Insulin analog
preparations and their use in children and adolescents with type 1
diabetes mellitus. Paediatr Drugs. 10:163–176. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Davidson MB, Castellanos M, Kain D and
Duran P: The effect of self monitoring of blood glucose
concentrations on glycated hemoglobin levels in diabetic patients
not taking insulin: a blinded, randomized trial. Am J Med.
118:422–425. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ludwig DS and Ebbeling CB: Type 2 diabetes
mellitus in children: primary care and public health
considerations. JAMA. 286:1427–1430. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Patel A, MacMahon S, Chalmers J, Billot L,
Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P and
Harrap S: Intensive blood glucose control and vascular outcomes in
patients with type 2 diabetes. N Engl J Med. 358:2560–2572. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Carino GP and Mathiowitz E: Oral insulin
delivery. Adv Drug Deliv Rev. 35:249–257. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gutniak M, Ørkov C, Holst JJ, Ahrén B and
Efendić S: Antidiabetogenic effect of glucagon-like peptide-1
(7–36) amide in normal subjects and patients with diabetes
mellitus. N Engl J Med. 326:1316–1322. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Diabetes Prevention Program Research
Group. Reduction in the incidence of type 2 diabetes with lifestyle
intervention or metformin. N Engl J Med. 346:393–403. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Goran MI, Bergman RN, Avila Q, Watkins M,
Ball GD, Shaibi GQ, Weigensberg MJ and Cruz ML: Impaired glucose
tolerance and reduced β-cell function in overweight Latino children
with a positive family history for type 2 diabetes. J Clin
Endocrinol Metab. 89:207–212. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jones KL, Arslanian S, Peterokova VA, Park
JS and Tomlinson MJ: Effect of metformin in pediatric patients with
type 2 diabetes: A randomized controlled trial. Diabetes Care.
25:89–94. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ehtisham S, Barrett TG and Shaw NJ: Type 2
diabetes mellitus in UK children - an emerging problem. Diabet Med.
17:867–871. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bandyopadhyay GK, Yu JG, Ofrecio J and
Olefsky JM: Increased malonyl-CoA levels in muscle from obese and
type 2 diabetic subjects lead to decreased fatty acid oxidation and
increased lipogenesis; thiazolidinedione treatment reverses these
defects. Diabetes. 55:2277–2285. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pearson ER, Flechtner I, Njølstad PR,
Malecki MT, Flanagan SE, Larkin B, Ashcroft FM, Klimes I, Codner E,
Iotova V, et al: Neonatal Diabetes International Collaborative
Group: Switching from insulin to oral sulfonylureas in patients
with diabetes due to Kir6.2 mutations. N Engl J Med. 355:467–477.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Krentz AJ and Bailey CJ: Oral antidiabetic
agents: Current role in type 2 diabetes mellitus. Drugs.
65:385–411. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Atkinson MA and Eisenbarth GS: Type 1
diabetes: New perspectives on disease pathogenesis and treatment.
Lancet. 358:221–229. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shapiro AM, Lakey JR, Ryan EA, Korbutt GS,
Toth E, Warnock GL, Kneteman NM and Rajotte RV: Islet
transplantation in seven patients with type 1 diabetes mellitus
using a glucocorticoid-free immunosuppressive regimen. N Engl J
Med. 343:230–238. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tuomilehto J, Lindström J, Eriksson JG,
Valle TT, Hämäläinen H, Ilanne-Parikka P, Keinänen-Kiukaanniemi S,
Laakso M, Louheranta A, Rastas M, et al: Finnish Diabetes
Prevention Study Group: Prevention of type 2 diabetes mellitus by
changes in lifestyle among subjects with impaired glucose
tolerance. N Engl J Med. 344:1343–1350. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sone H, Katagiri A, Ishibashi S, Abe R,
Saito Y, Murase T, Yamashita H, Yajima Y, Ito H, Ohashi Y, et al:
Effects of lifestyle modifications on patients with type 2
diabetes: the Japan Diabetes Complications Study (JDCS) study
design, baseline analysis and three year-interim report. Horm Metab
Res. 34:509–515. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fioretto P, Steffes MW, Sutherland DE,
Goetz FC and Mauer M: Reversal of lesions of diabetic nephropathy
after pancreas transplantation. N Engl J Med. 339:69–75. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Onge ES, Miller SA, Motycka C and DeBerry
A: A review of the treatment of type 2 diabetes in children. J
Pediatr Pharmacol Ther. 20:4–16. 2015.PubMed/NCBI
|