Introduction
Renal cell carcinoma (RCC) is a frequently observed
malignant neoplasm in the urinary system and is the 6th leading
cause of cancer deaths in Western countries. Each year, ~200,000
patients are diagnosed with the malignancy, resulting in ~100,000
deaths, and its incidence has increased in recent years (1,2). There
is no specific tumor marker for RCC and RCC is resistant to
chemotherapy and radiotherapy; thus, surgical resection remains the
most effective treatment for localized primary RCC. However, ~30%
of RCC cases develop into metastatic disease following nephrectomy,
and the median survival period is 13 months (3,4).
Metastasis remains a significant challenge for urologists and novel
prophylactic/therapeutic agents against metastasis are required
(5).
Tumor metastasis is a complex process and an
important step involved in the process is the degradation of the
basement membrane (BM) and extracellular matrix (ECM), the latter
being the mechanical barrier that serves to prevent cell invasion
in tissues (6). Matrix
metalloproteinases (MMPs) are a family of neutral proteinases that
digest the primary BM and ECM components that are overexpressed in
almost all human cancers (7,8). Among the members of the MMP family,
MMP-2 (72 kDa type IV collagenase gelatinase A) and MMP-9 (92 kDa
type IV collagenase gelatinase B) have the ability to degrade
collagen, a major component of the basement membrane, and are
strongly associated with tumor invasion and metastasis (9). It is thus indicated that the inhibition
of MMPs, in particular MMP-2 and MMP-9, may be a therapeutic
strategy.
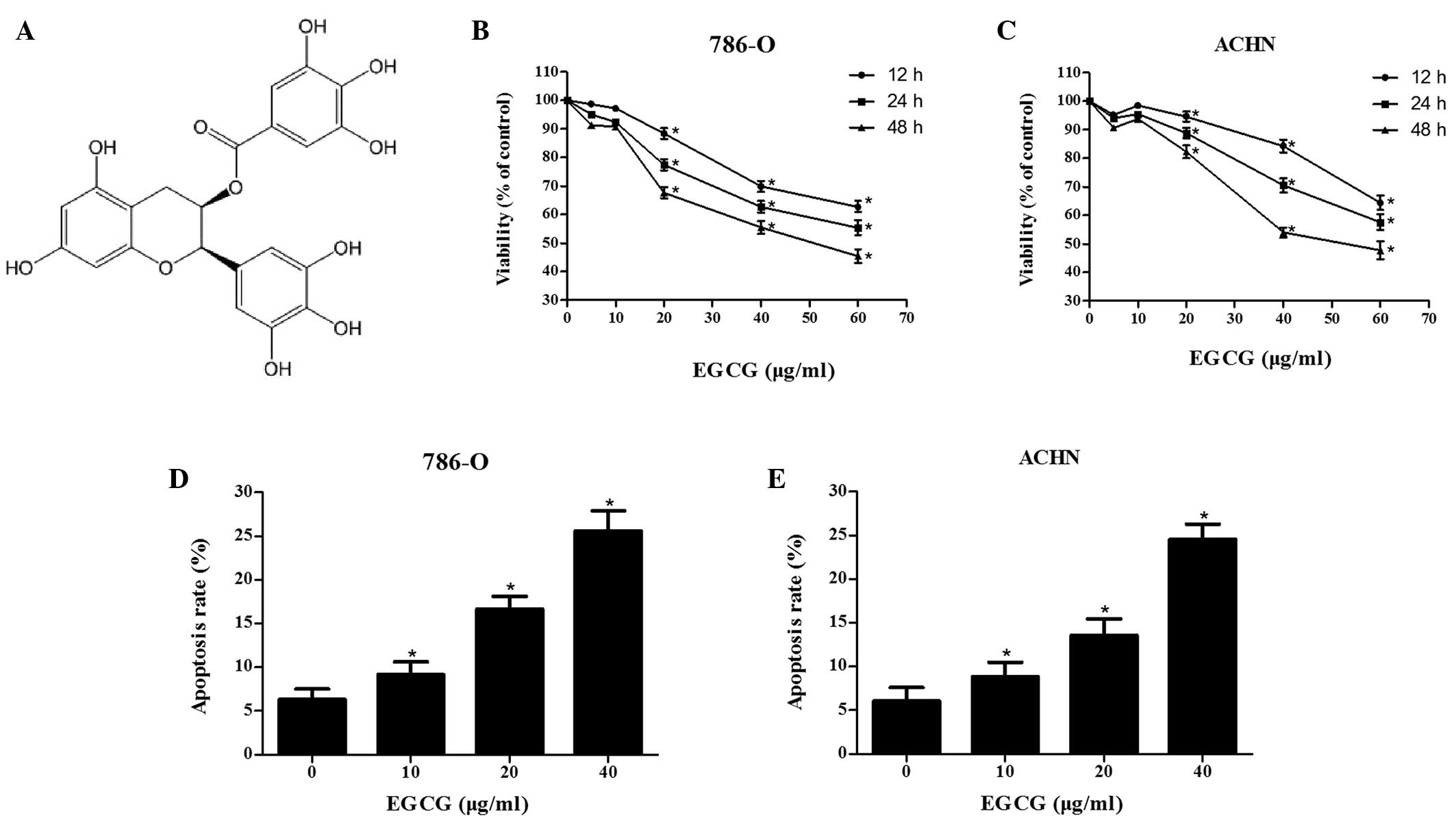
Epigallocatechin-3-gallate (EGCG; chemical structure
displayed in Fig. 1A) is a stable
water-soluble flavonoid that is the most abundant catechin in green
tea (10). A previous study
suggested that consumption of green tea may reduce the risk of
cancers including those of the stomach, colon, lung, liver, rectum,
breast and pancreas (11). EGCG
exerts its anticancer effects via the modulation of processes of
cellular differentiation, proliferation, apoptosis, angiogenesis
and metastasis (12). The inhibitory
effect of EGCG on MMP-2 and MMP-9 expression levels has been
reported in a number of cancer cell lines (13). In addition, EGCG may inhibit the
metastasis of hypopharyngeal, prostate and pancreatic cancers by
downregulating MMPs (14–16). However, the effect of EGCG on RCC
cells has yet to be elucidated.
The aim of the present study was to evaluate the
effect of EGCG on RCC cell migration and invasion in vitro.
MTT assays and flow cytometry were performed to evaluate the effect
of EGCG on RCC cell viability and wound-healing, and transwell
invasion assays were employed to examine the effect of EGCG on RCC
cells. Finally, the effect of EGCG on metastasis-related MMP-2 and
MMP-9 expression levels was evaluated using gelatin zymography and
western blot analysis.
Materials and methods
Cell culture
The RCC cell lines 786–0 and ACHN were purchased
from Cell Bank of Type Culture Collection of Chinese Academy of
Sciences (Shanghai, China), were cultured in RPMI-1640
(Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) and 1% antibiotics (100,000 U/l penicillin and 100 mg/l
streptomycin; Thermo Fisher Scientific, Inc.). Cells were cultured
at 37°C in a humidified atmosphere containing 5% CO2.
Cells of the exponential phase of growth were used in the
subsequent experiments.
Cell proliferation assay
The effect of EGCG on RCC cell proliferation was
measured by MTT assay. Cell lines 786-O and ACHN were plated into
96-well plates at a density of 2×104 cells/well in RPMI
1640 culture medium and incubated at 37°C for 24 h. Subsequent to
treatment with EGCG (Sigma-Aldrich) at various concentrations (0,
5, 10, 20, 40 and 60 µg/ml) for 12, 24 and 48 h, the cells were
incubated with 20 µl MTT (5 mg/ml; Sigma-Aldrich) at 37°C for 4 h.
Following this, the medium was removed and 150 µl dimethyl
sulfoxide (Sigma-Aldrich) was added to each well for 10 min at room
temperature to solubilize the crystals. Finally, cell proliferation
was determined by absorbance measurements at 490 nm on a Model 680
microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Cell viability was expressed as a percentage of the absorbance
obtained for the control group. A minimum of three independent
experiments were performed.
Flow cytometry
The proapoptotic effect of EGCG on RCC cells was
evaluated using an FITC Annexin V Apoptosis detection kit (BD
Pharmingen, San Diego, CA, USA) and quantified using flow
cytometry. Briefly, cells (1×106) were plated into
six-well plates and incubated overnight at 37°C, then treated with
EGCG at 0, 10, 20 or 40 µg/ml for 24 h. Following centrifugation
(326 × g) for 5 min at room temperature, the harvested cells
(1×106) were washed twice with cold phosphate-buffered
saline (PBS) and immediately resuspended in the physiological
buffer (1X) provided within the kit. Cells were then maintained in
the dark for 15 min at room temperature with 5 µl of both propidium
iodide and fluorescein isothiocyanate conjugated annexin V, after
which the samples were analyzed immediately using a FACSCalibur
flow cytometer (BD Biosciences, San Jose, CA, USA). The results
were quantified using BD CellQuest 5.1 software (BD Biosciences).
Apoptosis rates were expressed as percentages of early and late
apoptosis cells. The experiments were repeated three times.
Wound-healing assay
In vitro cell migration was assessed using a scratch
wound assay. Cell lines 786-O and ACHN were seeded into a 6-well
plate and cultured in complete medium to 80% confluency. Following
serum starvation for 24 h, the cell monolayers were carefully
wounded using a pipette tip and washed with PBS to remove floating
cells. Wounded monolayers were then incubated in media containing
various concentrations of EGCG at 37°C with 5% CO2 for
24 h. Cells migrating into the wound area were observed and counted
under an inverted microscope (SKZ1047; SKZ Industrial Co., Ltd.,
Shandong, China). Results were displayed as percentages of cells
migrated compared with those in the control group. All experiments
were performed in triplicate.
Transwell invasion assay
The inhibitory effect of EGCG on RCC cells invasion
in vitro was assessed using Transwell chambers (8 µm pore
size; EMD Millipore, Billerica, MA, USA) with membranes coated with
100 µl (1 mg/ml) matrigel (BD Biosciences). Cell lines 786-O and
ACHN were placed in serum-free-RPMI-1640 medium for 24 h. Following
trypsinization (Sigma-Adrich), cells were washed with PBS and
resuspended in serum-free medium. Subsequently, cell suspensions
(2×105 cells/ml) were added to the upper chambers
containing EGCG dissolved in the medium at various concentrations,
and RPMI-1640 containing 10% FBS was placed in the lower chambers.
Following incubation for 24 h in a humidified atmosphere containing
5% CO2 at 37°C, non-invasive cells on the upper surface
were removed with a cotton swab. The invasive cells on the lower
chamber were fixed with 75% ethanol and then stained with 0.5%
crystal violet (Beijing Chemical Works, Beijing, China). For each
membrane, images of three different fields were captured. Results
are presented as images of invading cells. All experiments were
performed in triplicate.
Gelatin zymography
The activity of MMP-2 and MMP-9 treated with various
concentrations of EGCG was evaluated using gelatin zymography.
Briefly, following treatment with EGCG in serum-free RPMI-1640
medium for 24 h, the conditioned medium was obtained and the
supernatant collected by centrifugation at 4°C and 447.2 × g for 10
min. The samples were loaded and separated by electrophoresis on
10% sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis
(Sigma-Aldrich) with 1 mg/ml gelatin at 100 V for 2 h at 4°C.
Following this, the gels were washed twice in 2.5% Triton X-100
(Sigma-Aldrich) for 30 min at room temperature to remove SDS, and
incubated overnight in zymography developing buffer containing 50
mM Tris-HCl and 10 mM CaCl2 (pH 7.5; Sigma-Aldrich) at
37°C. Subsequent to incubation, gels were stained with 0.5%
Coomassie Blue for 30 min at room temperature and stained with 30%
methanol and 10% glacial acetic acid (all Beijing Chemical Works,
Beijing, China). The gelatinase activity of MMP-2 and MMP-9 was
visualized as clear bands against the dark blue background, and
band density was measured using Quantity One 4.6.3 software
(Bio-Rad Laboratories, Inc.). Results were expressed by the
percentage of the density to the control bands. A minimum of three
independent experiments were conducted with individual protein
samples.
Western blot analysis
Western blotting was performed to determine the
protein expression levels of MMP-2 and MMP-9. Following treatment
with EGCG for 24 h, cell lines 786-O and ACHN were harvested and
lysed in radioimmunoprecipitation assay buffer (50 mM Tris/HCl, pH
7.4; 150 mM NaCl; 1% NP-40; 0.1% SDS) containing a protease
inhibitor cocktail (both Sigma-Aldrich) for 30 min on ice.
Following this, the lysates were collected and centrifuged for 20
min at 25,155 × g at 4°C. Protein concentration was evaluated with
the Protein Quantification Assay kit (K3000-BCA; Shanghai Shenergy
Biocolor BioScience & Technology Co., Ltd., Shanghai, China).
Proteins (50 µg) were separated on 10% SDS gels and transferred
onto polyvinylidene fluoride membranes (GE Healthcare, Chalfont,
UK). The membranes were blocked with 5% skimmed milk on a shaking
table for 2 h, washed three times with PBS for 5 min and incubated
with the following primary mouse anti-human monoclonal
immunoglobulin G1 antibodies: Anti-MMP-2 (sc-53630),
anti-MMP-9 (sc-13520) and anti-β-actin (sc-130301; all 1:600; all
Santa Cruz Biotechnology, Inc., Dallas, TX, USA) overnight at 4°C.
Following washing three times with PBS with Tween 20 on a shaking
table for 5 min, membranes were incubated with secondary goat
anti-mouse antibodies (sc-395763; 1:300; Santa Cruz Biotechnology,
Inc.) for 2 h at room temperature. Finally, blots were scanned with
red and green light at intensities of 3.5 and 8.0, respectively, to
reveal the expression levels of MMP-2 and MMP-9. Protein
quantification was conducted with BCA working media
(Sigma-Aldrich). Protein expression levels were normalized against
those of β-actin. All experiments were conducted in triplicate.
Statistical analysis
All values are expressed as the mean ± standard
error of the mean. Each value is the mean of at least three
separate experiments in each group. Data from in vitro
experiments between the treatment groups were analyzed using
one-way analysis of variance. A paired Student's t test was
performed to analyze the results for statistical significance, when
only two conditions were compared. P<0.05 was considered to
indicate a statistically significant difference.
Results
EGCG inhibits proliferation and
induces apoptosis in RCC cells
An MTT assay was performed to evaluate the effect of
EGCG on the viability of RCC cells. EGCG treatment at various
concentrations (0, 5, 10, 20, 40 and 60 µg/ml) resulted in dose-
and time-dependent anti-proliferative effect on 786-O and ACHN cell
lines (Fig. 1B and C). EGCG-induced
apoptosis was then measured using flow cytometry. The data reveals
higher apoptosis rates in EGCG treatment groups compared with the
control group (Fig. 1D and E). The
aforementioned results demonstrated that EGCG was able to
significantly inhibit the proliferation of RCC cells by inducing
apoptosis (P<0.05).
EGCG inhibits the migration and
invasion of RCC cells
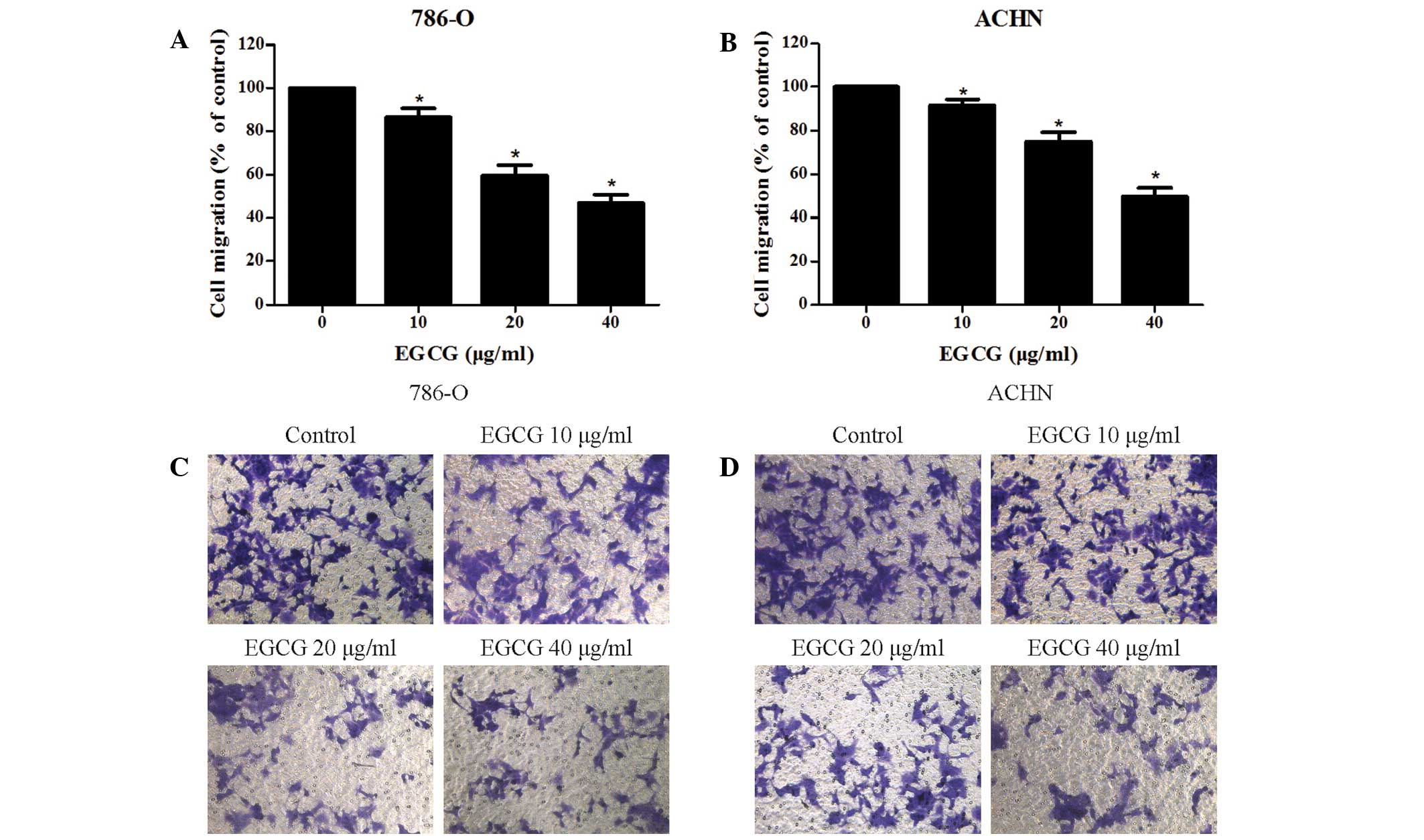
The effect of EGCG on RCC cells migration and
invasion was evaluated using wound-healing and Transwell invasion
assays, respectively. The present results indicate that EGCG is
able to effectively suppress the migration of RCC cells (Fig. 2A and B), and the inhibitory effect of
EGCG on RCC cell invasion was also indicated (Fig. 2C and D).
EGCG downregulates MMP-2 and MMP-9
activity and expression levels in RCC cells
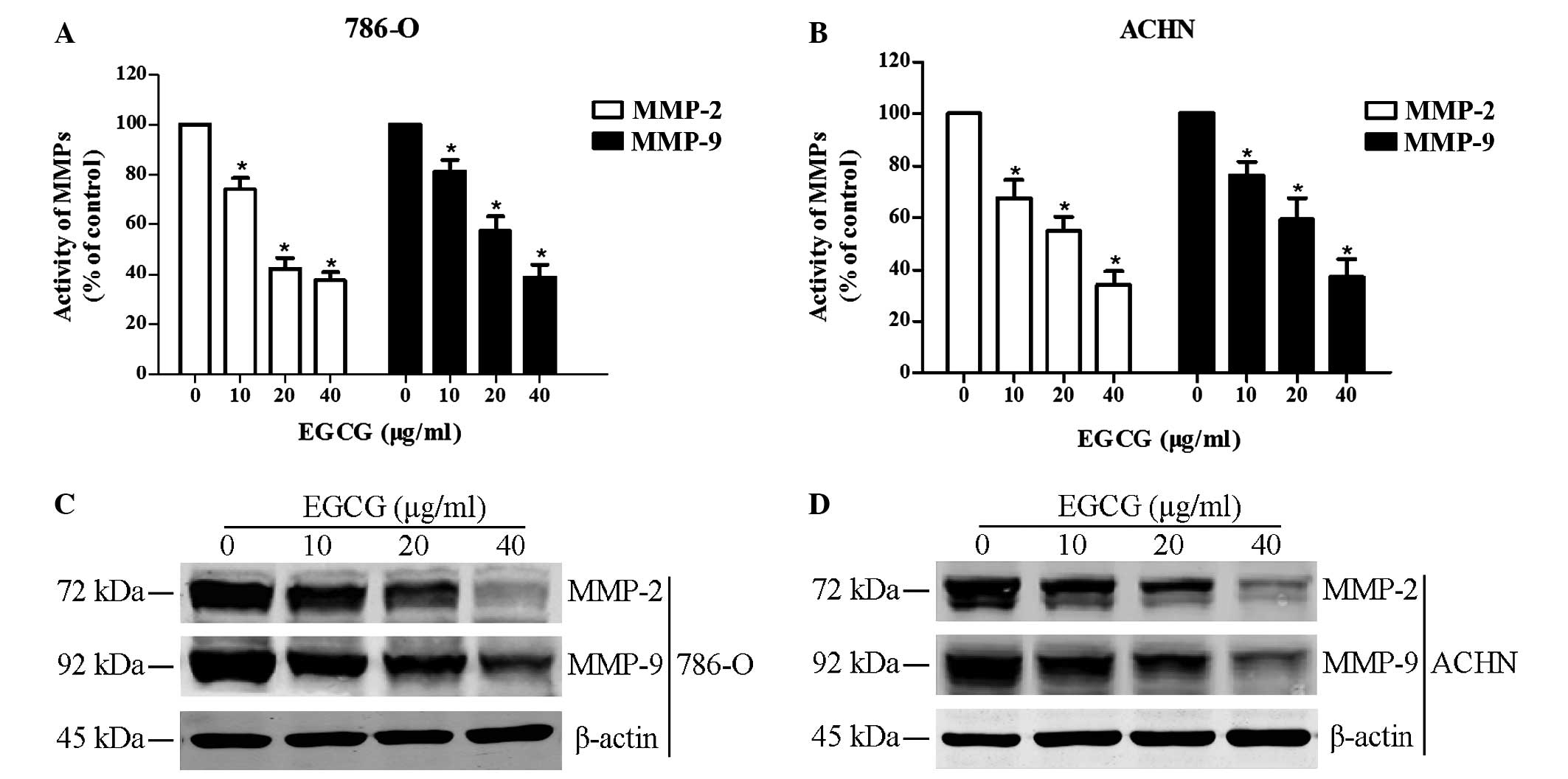
Gelatin zymography and western blot analysis were
performed to analyze the effect of EGCG on the activity and protein
expression levels of MMP-2 and MMP-9 following treatment with EGCG.
Results indicated that EGCG significantly reduced the activity and
expression of MMP-2 and MMP-9 (P<0.05; Fig. 3). The present results suggested that
decreasing MMP-2 and MMP-9 activity and expression levels may be a
mechanism by which EGCG's exerts its anti-cancer effects on RCC
cell lines.
Discussion
RCC is the most commonly observed carcinoma in
adults and its incidence has gradually increased in the last two
decades (17). RCC is the third most
common urological cancer and also has the highest mortality rate
(18). Metastasis is the greatest
challenge in the clinical management of patients with RCC (19) with approximately one third of
patients eventually developing metastases, predominately to bone,
lung and brain (20), despite having
previously undergone a successful nephrectomy. Previous studies
revealed that MMP-2 and MMP-9 are associated with metastatic and
invasive behavior in a number of cancer types (21,22).
Therefore, MMP-2 and MMP-9 are potential targets for the treatment
of RCC, and novel agents or phytochemical compounds are attracting
increasing attention.
Natural plant extracts have historically been
regarded as potential therapeutic agents and several plant extracts
have demonstrated anticancer effects. Ma et al demonstrated
that curcumin was able to inhibit pancreatic cancer cell growth and
invasion through upregulation of miR-7 (23), whilst Lee et al (24) demonstrated the anti-metastatic effect
of resveratrol against 4T1 mouse breast cancer cells by decreasing
MMP-9 activity. Furthermore, Kim et al (25) reported that quercetin was able to
induce apoptosis in HT-29 colon cancer cells via the adenosine
monophosphate-activated protein kinase signaling pathway. In recent
years plant polyphenols, in particular EGCG (the major catechin
found in green tea), have received increased attention. The
anticancer effects of EGCG have been investigated in several in
vitro studies. Luo et al (26) reported that EGCG inhibited the cell
growth and proliferation of MCF-7 breast cancer cells by
downregulating hypoxia-inducible factor-1α and vascular endothelial
growth factor protein expression levels. Sakamoto et al
(27) revealed that EGCG suppresses
the proliferation and angiogenesis in lung cancer A549 cells and
Lee et al (28) reported that
EGCG induces human laryngeal epidermoid carcinoma Hep2 cell
apoptosis by upregulating apoptosis-inducing factor and
endonuclease G. However, the effects of EGCG on RCC cells have yet
to be elucidated.
In the present study, it was initially demonstrated
that EGCG effectively inhibits the migration and invasion of human
RCC cells by downregulating MMP-2 and MMP-9. As demonstrated by MTT
assay and flow cytometry, EGCG was able to inhibit proliferation
and promote apoptosis of RCC cells in a dose- and time-dependent
manner. In addition, wound-healing assays revealed that EGCG
markedly decreases RCC cell migration and a Transwell invasion
assessment indicated that EGCG significantly reduces the invasion
ability of RCC cells. As MMP-2 and MMP-9 have previously been
demonstrated to promote invasion and metastasis in malignant tumors
(29,30), we hypothesized that EGCG would
inhibit the migration and invasion of RCC cells by downregulating
MMP-2 and MMP-9 expression levels. Gelatin zymography and western
blot analysis were performed, respectively. As hypothesized, EGCG
decreased MMP-2 and MMP-9 activity in RCC cells and MMP-2 and MMP-9
expression levels subsequent to EGCG treatment was strongly
suppressed. Thus, the present results suggest that EGCG is able to
inhibit the proliferation, migration and invasion of RCC cells and
the underlying mechanisms are associated with the suppression of
the activity and expression levels of MMP-2 and MMP-9.
The present study did not; however, determine how
EGCG impacts the activity and expression levels of MMP-2 and MMP-9
in RCC cells. As previously reported in the literature, EGCG was
able to induce G2/M arrest in CL1–5 lung cancer cells via c-Jun
N-terminal kinase JNK signaling (31). In addition, ECGG was able to regulate
the focal adhesion kinase/extracellular regulated kinase/nuclear
factor-κB and activator protein (AP)-1 axis in a human breast
cancer cell line (32). Furthermore,
EGCG was able to suppress mitogen-activated protein kinase and AP-1
activation in human gastric AGS cells (33). Future studies are required to
elucidate the underlying process and mechanism regarding the
effects of EGCG on MMP-2 and MMP-9.
In conclusion, the present study proposed that EGCG
would exert anticancer effects on RCC cells and the present
findings demonstrated that EGCG was able to inhibit the migration
and invasion of RCC cells by downregulating MMP-2 and MMP-9, which
suggested EGCG may be a potential therapeutic or adjuvant strategy
for the treatment of patients with RCC. However, clinical trials
are required in the future to determine safety and efficacy.
Acknowledgements
The present study was partially supported by grants
from the National Natural Science Foundation of China (grant nos.
81000311 and 81270831).
References
|
1
|
Xue YJ, Xiao RH, Long DZ, Zou XF, Wang XN,
Zhang GX, Yuan YH, Wu GQ, Yang J, Wu YT, et al: Overexpression of
FoxM1 is associated with tumor progression in patients with clear
cell renal cell carcinoma. J Transl Med. 10:2002012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mancuso A and Sternberg CN: New treatments
for metastatic kidney cancer. Can J Urol. 12(Suppl 1): S66–S70;
discussion 105. 2005.
|
|
3
|
Zisman A, Pantuck AJ, Wieder J, Chao DH,
Dorey F, Said JW, deKernion JB, Figlin RA and Belldegrun AS: Risk
group assessment and clinical outcome algorithm to predict the
natural history of patients with surgically resected renal cell
carcinoma. J Clin Oncol. 20:4559–4566. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cohen HT and McGovern FJ: Renal-cell
carcinoma. N Engl J Med. 353:2477–2490. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chambers AF, MacDonald IC, Schmidt EE,
Morris VL and Groom AC: Clinical targets for anti-metastasis
therapy. Adv Cancer Res. 79:91–121. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bracken CP, Gregory PA, Khew-Goodall Y and
Goodall GJ: The role of microRNAs in metastasis and
epithelial-mesenchymal transition. Cell Mol Life Sci. 66:1682–1699.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cakarovski K, Leung JY, Restall C,
Carin-Carlson A, Yang E, Perlmutter P, Anderson R, Medcalf R and
Dear AE: Novel inhibitors of urokinase-type plasminogen activator
and matrix metalloproteinase expression in metastatic cancer cell
lines. Int J Cancer. 110:610–616. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Toda D, Ota T, Tsukuda K, Watanabe K,
Fujiyama T, Murakami M, Naito M and Shimizu N: Gefitinib decreases
the synthesis of matrix metalloproteinase and the adhesion to
extracellular matrix proteins of colon cancer cells. Anticancer
Res. 26:129–134. 2006.PubMed/NCBI
|
|
9
|
Nelson AR, Fingleton B, Rothenberg ML and
Matrisian LM: Matrix metalloproteinases: Biologic activity and
clinical implications. J Clin Oncol. 18:1135–1149. 2000.PubMed/NCBI
|
|
10
|
Stoner GD and Mukhtar H: Polyphenols as
cancer chemopreventive agents. J Cell Biochem Suppl. 22:169–180.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Katiyar S and Mukhtar H: Tea in
chemoprevention of cancer. Int J Oncol. 8:221–238. 1996.PubMed/NCBI
|
|
12
|
Chen D, Wan SB, Yang H, Yuan J, Chan TH
and Dou QP: EGCG, green tea polyphenols and their synthetic analogs
and prodrugs for human cancer prevention and treatment. Adv Clin
Chem. 53:155–177. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Roomi MW, Monterrey JC, Kalinovsky T, Rath
M and Niedzwiecki A: Comparative effects of EGCG, green tea and a
nutrient mixture on the patterns of MMP-2 and MMP-9 expression in
cancer cell lines. Oncol Rep. 24:747–757. 2010.PubMed/NCBI
|
|
14
|
Lim YC, Park HY, Hwang HS, Kang SU, Pyun
JH, Lee MH, Choi EC and Kim CH: (−)-Epigallocatechin-3-gallate
(EGCG) inhibits HGF-induced invasion and metastasisin
hypopharyngeal carcinoma cells. Cancer Lett. 271:140–152. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Siddiqui IA, Malik A, Adhami VM, Asim M,
Hafeez BB, Sarfaraz S and Mukhtar H: Green tea polyphenol EGCG
sensitizes human prostate carcinoma LNCaP cells to TRAIL-mediated
apoptosis and synergistically inhibits biomarkers associated with
angiogenesis and metastasis. Oncogene. 27:2055–2063. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shankar S, Ganapathy S, Hingorani SR and
Srivastava RK: EGCG inhibits growth, invasion, angiogenesis and
metastasis of pancreatic cancer. Front Biosci. 13:440–452. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Volpe A and Patard JJ: Prognostic factors
in renal cell carcinoma. World J Urol. 28:319–327. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qiao HP, Gao WS, Huo JX and Yang ZS: Long
non-coding RNA GAS5 functions as a tumor suppressor in renal cell
carcinoma. Asian Pac J Cancer Prev. 14:1077–1082. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang L, Xul B, Chen S, Lu K, Liu C, Wang
Y, Zhao Y, Zhang X, Liu D and Chen M: The complex roles of
microRNAs in the metastasis of renal cell carcinoma. J Nanosci
Nanotechnol. 13:3195–3203. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Janzen NK, Kim HL, Figlin RA and
Belldegrun AS: Surveillance after radical or partial nephrectomy
for localized renal cell carcinoma and management of recurrent
disease. Urol Clin North Am. 30:843–852. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Waas ET, Wobbes T, Lomme RM, DeGroot J,
Ruers T and Hendriks T: Matrix metalloproteinase 2 and 9 activity
in patients with colorectal cancer liver metastasis. Br J Surg.
90:1556–1564. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang L, Shi J, Feng J, Klocker H, Lee C
and Zhang J: Type IV collagenase (matrix metalloproteinase-2 and
−9) in prostate cancer. Prostate Cancer Prostatic Dis. 7:327–332.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ma J, Fang B, Zeng F, Pang H, Zhang J, Shi
Y, Wu X, Cheng L, Ma C, Xia J and Wang Z: Curcumin inhibits cell
growth and invasion through up-regulation of miR-7 in pancreatic
cancer cells. Toxicol Lett. 231:82–91. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee HS, Ha AW and Kim WK: Effect of
resveratrol on the metastasis of 4T1 mouse reast cancer cells in
vitro and in vivo. Nutr Res Pract. 6:294–300. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim HJ, Kim SK, Kim BS, Lee SH, Park YS,
Park BK, Kim SJ, Kim J, Choi C and Kim JS: Apoptotic effect of
quercetin on HT-29 colon cancer cells via the AMPK signaling
pathway. J Agric Food Chem. 58:8643–8650. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Luo HQ, Xu M, Zhong WT, Cui ZY, Liu FM,
Zhou KY and Li XY: EGCG decreases the expression of HIF-1α and VEGF
and cell growth in MCF-7 breast cancer cells. J BUON. 19:435–439.
2014.PubMed/NCBI
|
|
27
|
Sakamoto Y, Terashita N, Muraguchi T,
Fukusato T and Kubota S: Effects of epigallocatechin-3-gallate
(EGCG) on A549 lung cancer tumor growth and angiogenesis. Biosci
Biotechnol Biochem. 77:1799–1803. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee JH, Jeong YJ, Lee SW, Kim D, Oh SJ,
Lim HS, Oh HK, Kim SH, Kim WJ and Jung JY: EGCG induces apoptosis
in human laryngeal epidermoid carcinoma Hep2 cells via mitochondria
with the release of apoptosis-inducing factor and endonuclease G.
Cancer Lett. 290:68–75. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chambers AF and Matrisian LM: Changing
views on the role of matrix metalloprotenases in metastasis. J Natl
Cancer Inst. 89:1260–1270. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kleiner DE and Stetler-Stevenson WG:
Matrix metalloproteinases and metastasis. Cancer Chemother
Pharmacol. 43(Suppl): S42–S51. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Deng YT and Lin JK: EGCG inhibits the
invasion of highly invasive CL1-5 lung cancer cells through
suppressing MMP-2 expression via JNK signaling and induces G2/M
arrest. J Agric Food Chem. 59:13318–13327. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sen T, Dutta A and Chatterjee A:
Epigallocatechin-3-gallate (EGCG) downregulates gelatinase-B
(MMP-9) by involvement of FAK/ERK/NFkappaB and AP-1 in the human
breast cancer cell line MDA-MB-231. Anticancer Drugs. 21:632–644.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim HS, Kim MH, Jeong M, Hwang YS, Lim SH,
Shin BA, Ahn BW and Jung YD: EGCG blocks tumor promoter-induced
MMP-9 expression via suppression of MAPK and AP-1 activation in
human gastric AGS cells. Anticancer Res. 24:747–753.
2004.PubMed/NCBI
|