Introduction
Soy sauce (SS) is a traditional fermented Asian food
product consisting of soybeans and salt. Previous studies have
demonstrated that SS contains antioxidants (1–4) and
exhibits high antioxidant activity in vitro and in
vivo (1,5,6),
anti-allergic properties (7),
aspirin-like, anti-platelet activity (8), and anti-carcinogenic (2) and anti-microbial activities (9). Furthermore, it has been suggested that
SS is able to inhibit serum lipid peroxidation and may exert
antioxidant effects that are ~10x more effective than red wine, and
~150x more effective than vitamins E and C (1). Therefore, it has been suggested that SS
may have a role in the prevention of various diseases (8,10–13). In
spite of the numerous beneficial pharmacological effects of SS,
commercially available SS has one shortcoming: It contains a large
amount of common salt, which has been shown to raise blood pressure
and increase the risk of cardiovascular diseases when consumed in a
quantity that exceeds the daily recommended amount (11,14,15). One
solution is to reduce the quantity of salt in SS; however, this may
negatively affect the taste of the product. An alternative solution
may be to replace the common salt with a healthier salt; bamboo
salt (BS) is considered to be a good candidate for this.
BS is processed by repeatedly (≤9x) roasting
sun-dried salt (SDS) within a bamboo trunk, sealed by yellow soil,
at a temperature >1,000°C. BS becomes purple following these
roasting procedures. The roasting process is performed within a
furnace and is fueled by pinewood and pine resin. Throughout the
roasting procedure, >70 essential minerals and micronutrients
from bamboo, yellow soil, pinewood and pine resin are amalgamated
into the BS via chemical and physical changes (16). BS has a higher concentration of iron,
silicon and potassium minerals, as compared with common salt
(17). Furthermore, BS is known to
have a high medical efficacy for in vitro anti-cancer
(18), anti-apoptosis (19) and anti-inflammatory activities
(20). In addition, BS exerts
cytoprotective effects and reduces susceptibility to diverse
diseases, including viral infections, dental plaque, diabetes,
cardiovascular diseases, and cancer and inflammatory disorders
(16,18,20–24).
Bamboo salt soy sauce (BSSS), which contains BS
instead of common salt, is produced from fermented small black
beans and brine alongside dissolved BS, and is regarded as a
healthy and medicinal food in Asia. As both BS and SS have
demonstrated cytoprotective roles via antioxidative effects, the
present study hypothesized that BSSS may exert greater
cytoprotective effects, as compared with regular SS. To the best of
our knowledge, the present study is the first to examine the
cytoprotective effects of BSSS using a hydrogen peroxide
(H2O2)-induced neuronal cell death rat
model.
Oxidative stress has been widely implicated in
neuronal cell death, which in turn has been considered a pathogenic
mechanism underlying neurodegenerative disorders (25,26). The
production of reactive oxygen species (ROS), and their
detoxification, form part of normal physiological processes
(27); however, at high
concentrations, ROS may promote neuronal dysfunction and cell
death. Numerous forms of ROS cause damage to essential cellular
components, including lipids, proteins and DNA (28). Furthermore, ROS are able to initiate
cell death via necrosis or apoptosis. Therefore, ROS may contribute
to neuronal toxicity and be associated with acute and chronic
neuropathological conditions. H2O2 is
commonly used as an exogenous source of ROS. Neuronal cells exposed
to H2O2 may undergo cell death, with mild
oxidative stress causing apoptosis, and severe oxidative stress
triggering necrosis (29).
Substantial evidence has indicated etiological links between the
generation of H2O2 and neurodegenerative
diseases (30). Therefore, an
H2O2-induced cytotoxicity model is considered
suitable for the study of neurodegeneration induced by oxidative
stress (31,32).
The present study evaluated the neuroprotective
effects of BSSS, including its ability to reduce levels of
oxidative stress, enhance survival signaling, and inhibit death
signals, in a H2O2-induced rat neuronal cell
death model, as compared with two controls: Traditional soy sauce
(TRSS) and brewed soy sauce (BRSS). Furthermore, the interactions
of salt and minerals in BSSS were analyzed by X-Ray diffraction
(XRD), and the mineral compounds were assessed via an inductively
coupled plasma-atomic emission spectrometer (ICP-AES), and by ion
chromatography.
Materials and methods
Preparation of BSSS, TRSS and
BRSS
BSSS was prepared by combining the standard
procedure for SS production (33)
with a special process involving BS instead of common salt
(34). The process for making SS was
as follows: Small black beans, which were purchased from a local
market in Korea, were cleaned, soaked and cooked for 2 h at
atmospheric pressure. Subsequently, small black beans were boiled
at 100°C, crushed in water at 80°C and molded into a brick shape,
following which they were dried for 2 days in the air, suspended by
rice straw and fermented for 30–60 days under natural environmental
conditions, in order to produce fermented meju. The meju was brined
with a ratio of meju:BS:water, 18.4:14.6:67.0. This meju-brine
mixture was ripened for 2 months, after which it was separated into
liquid and solid phases. The liquid phase was filtered and boiled
to produce the SS (33). The TRSS,
consisting of large soybeans (Dea-du), and SDS, was purchased from
Sinanmade Co. Ltd. (Paju, Republic of Korea). The BRSS used was
‘Chungjungwon Yangjo Soy Sauce’, consisting of soybeans and
purified salt (PS), was produced in Paju, Korea and purchased from
the internet market TMON (http://www.ticketmonster.co.kr/home). The BSSS ‘HAIWON
Jukyeom’ was produced ni Gangwon-do (Republic of Korea). BSSS, TRSS
and BRSS were filtered through a 0.45 mm filter and maintained at
4°C, after which they were diluted with culture medium to various
concentrations (0.001, 0.01, 0.1, 1 and 10%).
Reagents
Neurobasal media (NBM) and B27 supplement were
purchased from Gibco Life Technologies (Carlsbad, CA, USA).
H2O2, a protein protease inhibitor cocktail,
trypan blue solution, insulin, DNase I and LY294002, were obtained
from Sigma-Aldrich (St. Louis, MO, USA). Prior to use, these were
dissolved in distilled water and further diluted with culture
medium to the desired concentrations.
Primary cultures and treatment of rat
cortical neurons
All of the procedures for the care and use of the
rats were performed in accordance with the guidelines of the
Institutional Animal Care and Use Committee (IACUC) of Hanyang
University (Seoul, South Korea). These guidelines follow
international guidelines on animal welfare, as well as local and
national regulations. Furthermore, the instructions for procedures
were approved by the IACUC of Hanyang University
(HY-IACUC-12-062A). Every effort was made in order to minimize the
number of rats used and their suffering. All of the rats were used
only once and none of the experiments were carried out on human
materials. The cortical neurons were obtained from the cerebral
cortices of fetal Sprague-Dawley rats (16 days gestation; Orient
Bio Inc., Gyeonggi, Republic of Korea), following sacrifice in a
chamber by 5% CO2 inhalation. Primary cultures were
generated in vitro and were suspended in NBM, supplemented
with B27 at 37°C, in an atmosphere containing 5% CO2.
Two days following plating, non-neuronal cells were removed via the
addition of 5 µM cytosine arabinoside (Sigma-Aldrich) for 24 h.
Only mature cultures (7 days in vitro) were used for
experiments. The cultures consisted of ~80% primary cortical
neurons (35).
In order to examine the effects of BSSS on neuronal
cell viability, cortical neurons were pretreated with various
concentrations of BSSS (0, 0.001, 0.01, 0.1, 1 and 10%) for 24 h,
after which they were washed repeatedly with phosphate-buffered
saline (PBS; Gibco Life Technologies). Subsequently, the cortical
neuronal cells were exposed to H2O2 (0, 25,
50, 100, 150 or 200 µm) for 30 min, and cell viability was
evaluated using Cell Counting kit-8 assays (CCK-8; Dojindo
Molecular Technologies, Inc., Kumamoto, Japan) at
2.5×106 cells/cm2, as described previously
(35). To compare the effects of
different types of soy sauce (BSSS, TRSS and BRSS) on neuronal
viability, cortical neurons were pretreated with various
concentrations of soy sauce (0, 0.001, 0.01, 0.1, 1 and 10%) for 24
h after being washed repeatedly with PBS. In addition, LY294002, a
PI3K inhibitor, was purchased from Sigma-Aldrich to directly block
PI3K. Cortical neurons were treated with 10 µM LY294002 as a
co-treatment with BSSS for 24 h.
Terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL) staining
Cells (2.5×106 cells/cm2) were
fixed with 4% paraformaldehyde (Sigma-Aldrich) in PBS for 1 h at
room temperature. Apoptotic cell death and the inhibition of DNA
fragmentation were assessed via TUNEL staining, according to the
manufacturer's instructions (Roche Diagnostics Corporation,
Indianapolis, IN, USA). Nuclei were counterstained with
4,6-diamidino-2-phenylindole (Sigma-Aldrich). The percentage of
TUNEL-positive cells (2.5×106 cells/cm2)was
determined according to the total number of cells (36).
Measurement of ROS
The cell-permeable, non-fluorescent compound,
H2DCF-DA (Invitrogen Life Technologies, Carlsbad, CA, USA), was
used to measure the intracellular concentration of ROS. H2DCF-DA
was dissolved in dimethylsulfoxide (Sigma-Aldrich), and diluted
with PBS to a final concentration of 10 µM, according to the
manufacturer's instructions. Subsequently, 10 µM H2DCF-DA was
added, and the cells were incubated for 40 min at 37°C, after which
the cells were returned to pre-warmed growth medium and incubated
for a further 10 min at 37°C. Subsequently, cells were harvested
with trypsin (Gibco Life Technologies) and washed once with PBS in
preparation for fluorescence intensity determination using flow
cytometry (BD FACSCanto; BD Biosciences, San Jose, CA, USA) and the
data acquisition program FACSDIVA software (BD Biosciences).
Western blot analysis
Following all treatments, the cells were harvested,
washed twice with PBS and lysed with radioimmunoprecipitation
buffer (Sigma-Aldrich), supplemented with phosphatase inhibitor
(Sigma-Aldrich). The whole cell lysates were centrifuged at 18,000
× g for 20 min at 4°C and the supernatant was collected. To obtain
subcellular fractions, the Qproteome Cell Compartment kit (Qiagen
Sciences, Inc., Germantown, MD, USA) was used. Protein
concentrations were determined using a Bio-Rad Protein Assay kit
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). Equal amounts (40
µg) of protein were separated by 10% SDS-PAGE (Bio-Rad
Laboratories, Inc.) and transferred to nitrocellulose membranes (GE
Healthcare Life Sciences, Little Chalfont, UK). The membranes were
blocked with 5% skimmed milk and then incubated with specific
primary antibodies against phosphorylated (phospho)-AKT (Ser473)
(1:1,000; 9271; Cell Signaling Technology, Inc., Danvers, MA, USA),
AKT (1:1,000; 9272; Cell Signaling Technology, Inc.),
phospho-glycogen synthase kinase (GSK)-3β (Ser9) (1:1,000;
sc-11757; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), GSK-3β
(1:1,000; sc-9166; Santa Cruz Biotechnology, Inc.),
apoptosis-induced factor (AIF; 1:500; 4642; Cell Signaling
Technology, Inc.), cytochrome c (1:500; sc-514435; Santa
Cruz Biotechnology, Inc.), caspase-9 (1:1,000; 9502; Cell Signaling
Technology, Inc.), cleaved poly (ADP-ribose) polymerase (PARP;
1:1,000; sc-56196; Santa Cruz Biotechnology, Inc.), B-cell
lymphoma-2-associated X protein (BAX; 1:1,000; sc-20067; Santa Cruz
Biotechnology, Inc.), PAR (1:500; 4335-MC-100; Trevigen) and
caspase-3 (1:1,000; 9662; Cell Signaling Technology, Inc.) at 4°C
overnight. The membranes were washed with Tris-buffered saline
containing 0.05% Tween-20 (Gibco Life Technologies), and then
further incubated with horseradish peroxidase (HRP)-conjugated
anti-rabbit (RPN4301) or anti-mouse (NXA931) secondary antibodies
(GE Healthcare Bio-Sciences, Pittsburgh, PA, USA) for 2 h at room
temperature. The blots were visualized using enhanced
chemiluminescence detection (GE Healthcare Bio-Sciences). The
western blot results were quantified using an image analyzer
(Quantity One-4,2,0; Bio-Rad Laboratories, Inc.). The membranes
were also probed with anti-β-actin antibody (1:2,000; sc-47778;
Santa Cruz Biotechnology, Inc.), which served as an internal
control (35).
Mineral analysis of SS
Element analysis of the minerals in BSSS, TRSS and
BRSS samples, was carried out using an ICP-AES (iCAP 6000; Thermo
Fisher Scientific, Inc., Cambridge, UK) and analysis of CI content
was performed using ion chromatography (Metrohm AG, Herisau,
Switzerland). The ion chromatography (Metrohm MIC 7 Advanced,
Metrohm AG) was used with a column, Metrosep assup 7 250/4 and a
conductivity detector. The eluent was 3.6 mmol/L Na2CO3 and the
flow rate was 0.7 mL/min.
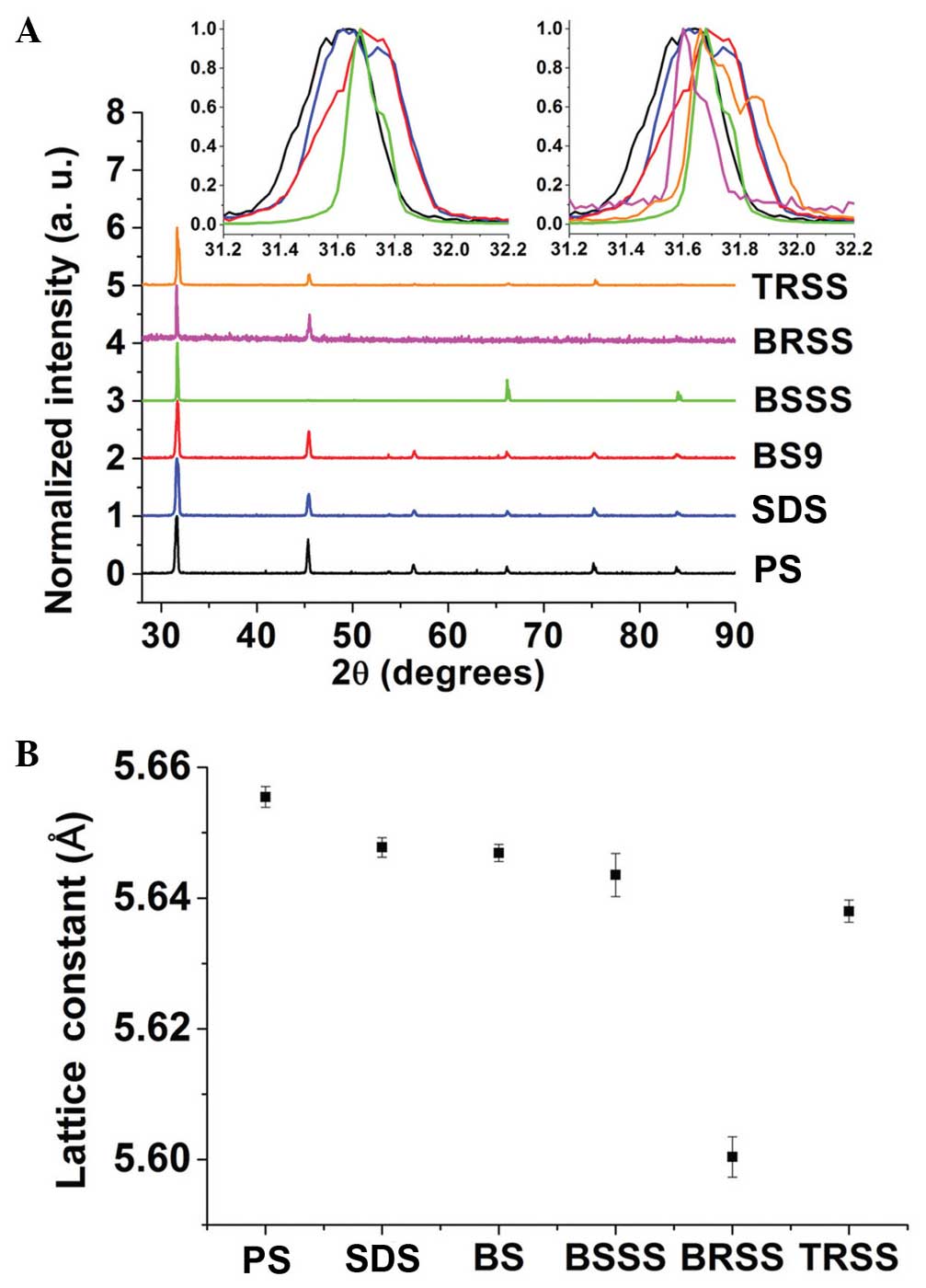
XRD analysis
XRD analyses were performed for BSSS, BRSS and TRSS
with BS, SDS and PS controls. Shimadzu (X2) (Shimadzu Corporation,
Kyoto, Japan), with a 1.0×10 mm copper X-ray tube and vertical type
goniometer (185 mm), was used for the XRD analysis. All of the
samples were scanned from 10–90°. BSSS, BRSS and TRSS were dried
overnight and heated for 30 min on the 50°C hot plate.
Subsequently, the dried samples were cut using a laser blade
cutter, after which the samples underwent XRD analysis. The final
particles were less fine than the salt controls, although the sizes
of the particles were <1 mm, which is sufficient to see the
overall trend in the XRD analysis, even if they were less randomly
distributed than those of the salt controls. The salt samples, PS,
SDS and BS were ground down in order to make them finer. For
quantitative XRD analysis, the software, DIFFRAC.SUITE TOPAZ
(Bruker AXS GmbH, Karlsruhe, Germany) was used, and lattice
parameters were measured.
Statistical analysis
All statistical analyses were performed using SPSS
software, version 17.0 (SPSS, Inc., Chicago, IL, USA). Data are
presented as the mean ± standard error of the mean of ≥5
independent experiments. Statistical comparisons between the
various treatment groups were performed using Tukey's test
following one-way analysis of variance. P<0.05 was considered to
indicate a statistically significant difference.
Results
Determining the optimal toxic dose of
H2O2 for assessing neuronal cell
viability
To determine the optimal toxic dose of
H2O2 for assessing neuronal cell viability,
rat cortical neurons were treated with 0, 25, 50, 100, 150 or 200
µm H2O2 for 30 min, and the viability of
these cells was measured using CCK8 assays. As demonstrated in
Fig. 1A, cell viability was
gradually reduced in a concentration-dependent manner. Cell
viability was 82.9±1.45% at 25 µM, 71.0±1.32% at 50 µM, 65.4±1.09%
at 100 µM, 49.4±1.45% at 150 µM and 36.5±2.25% at 200 µM, as
compared with the non-treated controls (P<0.01). Based on these
data, 100 µM was selected as the optimal toxic dose of
H2O2, as ~65% viability is usually deemed
appropriate for the study of H2O2-induced
neuronal toxicity.
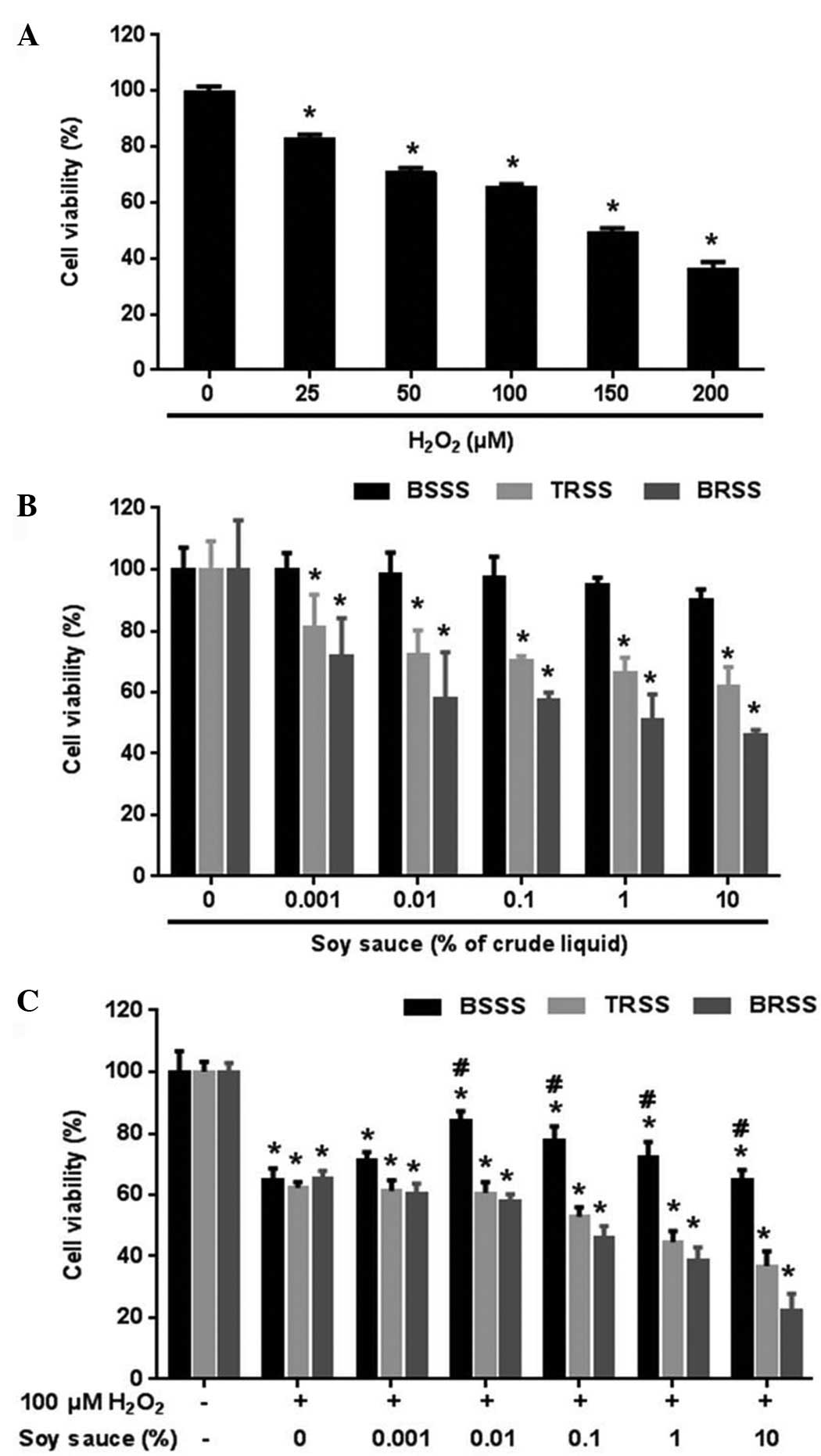 | Figure 1.Bamboo salt soy sauce (BSSS)
protected rat cortical neuronal cells from hydrogen peroxide
(H2O2)-induced cell death. (A) To determine
the optimal toxic dose of H2O2 for assessing
neuronal cell viability, rat cortical neurons were treated with
various concentrations of H2O2 (0, 25, 50,
100, 150, and 200 µM) for 30 min. (B) Comparisons between the
various soy sauces in rat cortical neurons. Rat cortical neuronal
cells were treated with various concentrations of BSSS, traditional
soy sauce (TRSS), or brewed soy sauce (BRSS; 0, 0.001, 0.01, 0.1,
1, and 10%) for 24 h. (C) To estimate the neuroprotective effects
of the various soy sauces against 100 µM
H2O2-mediated cell death, rat cortical
neuronal cells were pretreated with various concentrations of BSSS,
TRSS, and BRSS (0, 0.001, 0.01, 0.1, 1, and 10%) for 24 h, followed
by 100 µM H2O2 treatment for 30 min. Data are
presented as the mean (% of control) ± standard error of the mean
from ≥5 independent experiments. *P<0.05 vs. the non-treated
group; #P<0.05 vs. the group treated with 100 µM
H2O2 only. |
Determining the optimal concentration
of BSSS for the 100 µM H2O2-induced
neurotoxicity model
To compare the effects of various types of soy sauce
(BSSS, TRSS, or BRSS) on neuronal cell viability, rat cortical
neuronal cells were treated with various concentrations of soy
sauce (0, non-treated group; 0.001, 0.01, 0.1, 1 and 10%) for 24 h
and viability was measured. Unlike TRSS and BRSS, BSSS had no
detrimental effect on neuronal cell viability at 0, 0.001, 0.01,
0.1, 1 or 10% crude liquid (Fig.
1B). Conversely, TRSS and BRSS had cytotoxic effects that led
to cell apoptosis and neuronal cell damage when consumed in excess,
whereas BSSS did not exert cytotoxic activity within the same
concentration range.
To determine the effect of soy sauce (BSSS, TRSS, or
BRSS) on H2O2-induced neuronal toxicity, rat
cortical neuronal cells were pretreated with various concentrations
of soy sauce for 24 h, after which they were treated with 100 µM
H2O2 for 30 min, and cell viability was
determined. Only pretreatment of neuronal cells with BSSS increased
cell viability at the 0.001–1% concentration, as compared with the
100 µM H2O2 treatment group; however, cell
viability did not increase above the 1% concentration, as compared
with the 100 µM H2O2 treatment group
(65.2±3.45% in 100 µM H2O2 treatment group;
71.4±2.47% at 0.001% BSSS; 84.3±2.89% at 0.01% BSSS; 77.8±4.38% at
0.1% BSSS; and 72.3±4.91% at 1% BSSS) (P<0.01; Fig. 1C). Based on the viability data, 0.01
and 0.1% BSSS concentrations were associated with maximal cell
viability, and were selected as the optimal concentrations for all
subsequent experiments (Fig.
1C).
BSSS pretreatment protects cortical
neurons from H2O2-induced apoptosis
To analyze the rate of apoptosis, TUNEL analysis was
performed (Fig. 2). Briefly,
cultured neuronal cells were pretreated with BSSS for 24 h, after
which they were treated with 100 µM H2O2 for
30 min. As a control, cultured neuronal cells were stimulated with
100 µM H2O2 for 30 min, without BSSS
pretreatment. TUNEL staining demonstrated that 61.3±3.21% neurons
underwent apoptosis following incubation with 100 µM
H2O2 for 30 min, whereas pretreatment of
neurons with 0.01 and 0.1% BSSS significantly decreased
H2O2-mediated apoptosis by ~40% (36.3±2.31%
at 0.01% and 38.6±1.52% at 0.1%; P<0.05). These results suggest
that BSSS was able to inhibit H2O2-mediated
neuronal cell apoptosis and DNA fragmentation.
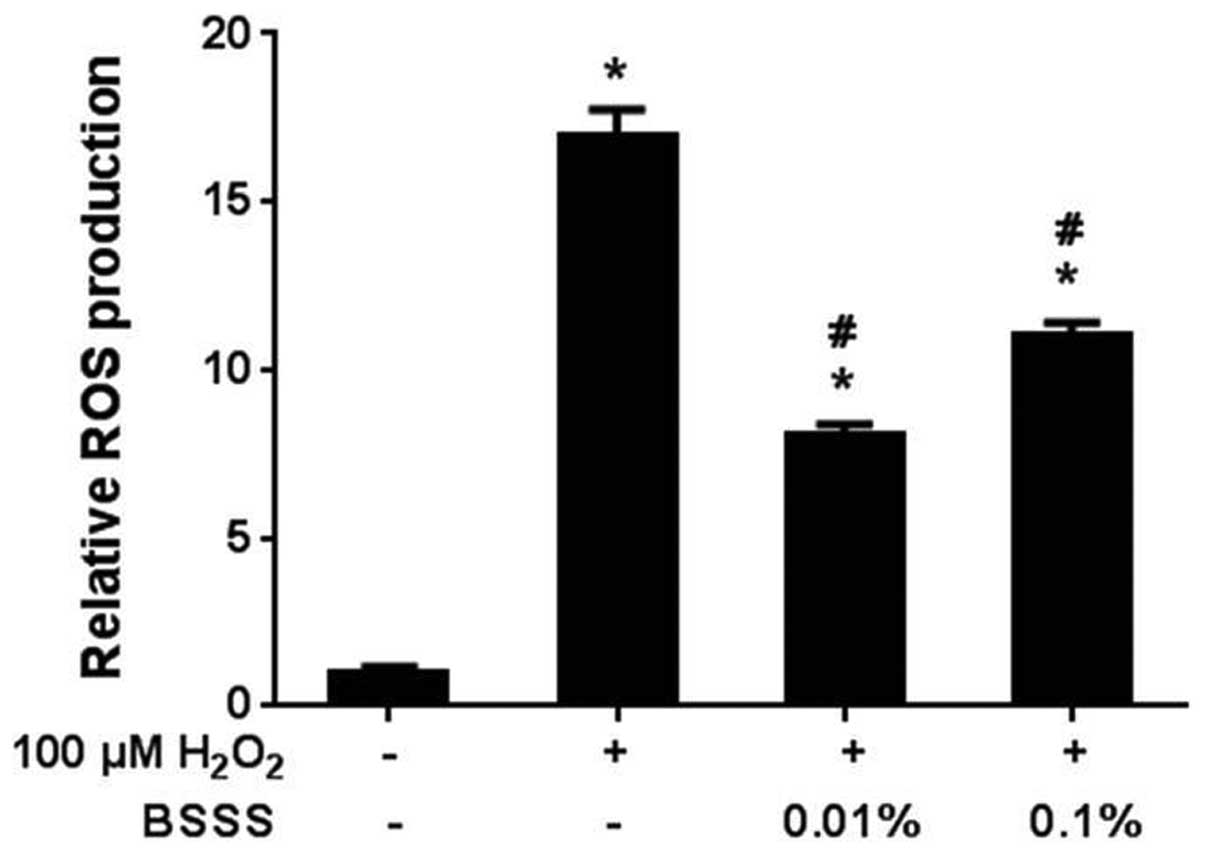
Anti-oxidative effects of BSSS on
H2O2-induced neurotoxicity
To assess H2O2-dependent free
radical production in rat cortical neuronal cells, the H2DCF-DA
method was used to measure the levels of ROS in neurons treated
with 100 µM H2O2 for 30 min. Free radical
production significantly increased in the
H2O2-treated cells (16.9±0.81; P<0.05;
Fig. 3), although not in the BSSS
pretreated cells. Pretreatment with BSSS for 24 h decreased the
free radical production following H2O2
treatment (8.1±0.31% at 0.01% and 10.6±0.4% at 0.1%; P<0.05).
These results indicate that BSSS may have antioxidative effects on
H2O2-mediated ROS generation.
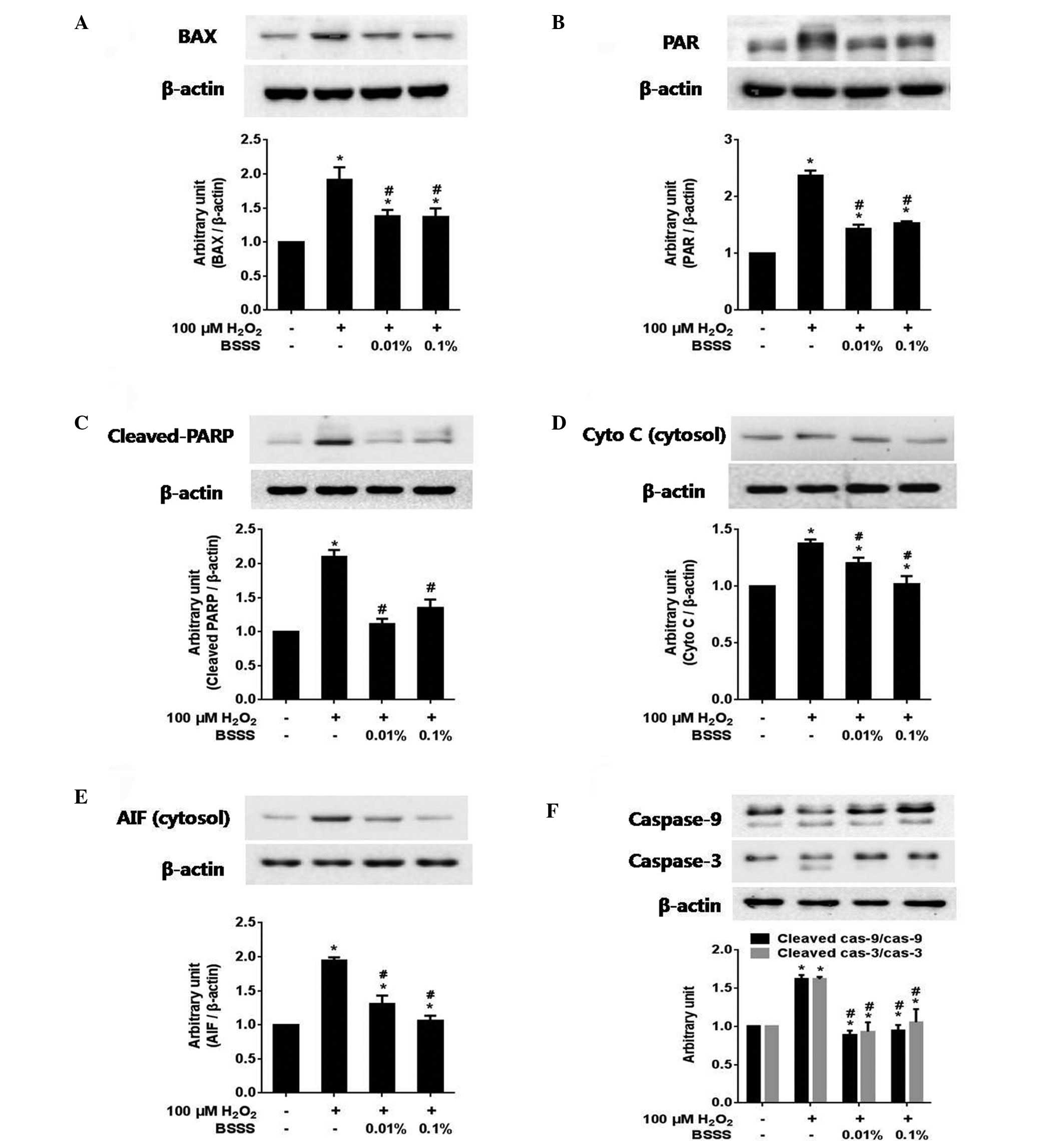
BSSS inhibits
H2O2-mediated neuronal cell death by
regulating intracellular signaling protein expression
To confirm the effects of BSSS on intracellular
signaling pathways, the expression levels of BAX, poly (ADP-ribose)
(PAR), PARP, cleaved PARP, cytosolic cytochrome c, cytosolic
AIF, caspase-9 (total/cleaved), caspase-3 (total/cleaved), Akt
(total/phosphorylated) and GSK3-β (total/phosphorylated), were
measured. The immunoreactivities (IRs) of BAX (Fig. 4A), PAR (Fig. 4B), cleaved PARP (Fig. 4C), cytosolic cytochrome c
(Fig. 4D), cytosolic AIF (Fig. 4E) and cleaved caspase-9/cleaved
caspase-3 (Fig. 4F) were
significantly decreased following pretreatment with BSSS, as
compared with following 100 µM H2O2 treatment
alone (P<0.05; Fig. 4). These
results suggest that BSSS may exert anti-apoptotic effects that
resist H2O2-induced cytotoxic damage,
including inhibiting BAX and PAR activities, and decreasing the
levels of cleaved PARP, cytosolic cytochrome c, cytosolic
AIF, cleaved caspase-9 and cleaved caspase-3.
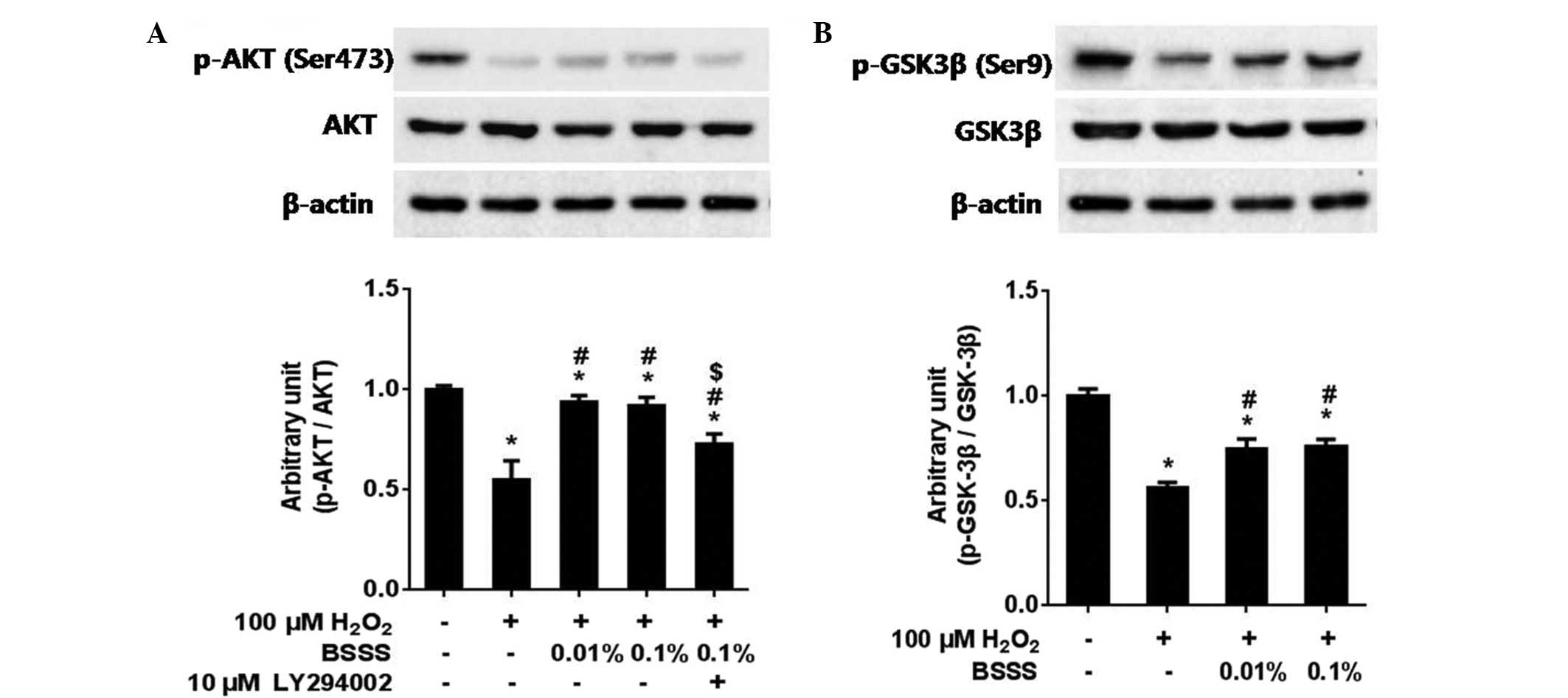
Pretreatment with BSSS significantly increased the
IRs of phospho-Akt (Ser473) and phospho-GSK-3β (Ser9) (Fig. 5A and B). In addition, whether the
neuroprotective effects of BSSS were associated with the
phosphatidylinositol 3-kinase (PI3K)/Akt pathway, was examined by
co-administering 10 µM LY294002, a PI3K inhibitor, with BSSS for 24
h. As compared with the BSSS treatment group, the IR ratio of
phospho-Akt decreased in the 10 µM LY294002 pretreated group (only
H2O2 treated group, 0.55±0.09; combined
H2O2 with 0.01% BSSS pretreated group,
0.93±0.03; combined H2O2 with 0.1% BSSS
pretreated group, 0.91±0.04; and combined
H2O2 with 0.1% BSSS and 10 µM LY294002
pretreated group, 0.72±0.05; P<0.05; Fig. 5A). These results suggest that
BSSS-mediated neuroprotective effects were partially prohibited by
the presence of the PI3K inhibitor (LY294002), thus indicating that
the neuroprotective effects of BSSS were at least partially
mediated via the PI3K/Akt signaling pathway.
Mineral analysis
In an effort to elucidate the mechanism by which
BSSS enhanced neuronal cell viability and inhibited
H2O2-mediated cell apoptosis, the mineral
content of BSSS, as compared with TRSS and BRSS, was analyzed. SS
contains indispensable minerals, including K, Ca, Mg, S, Fe, P, Rb,
Mo, V, Au, Pt, Ge and Se (Table I).
BSSS was shown to contain higher levels of potassium, as compared
with TRSS and BRSS. Furthermore, BSSS had unique elements,
including Mo, V, Au, and Se, in higher quantities than either TRSS
or BRSS. These results suggest that the unique mineral content of
BSSS may contribute to its neuroprotective activity.
 | Table I.Mineral contents of various soy
sauces, analyzed with an inductively coupled plasma-atomic emission
spectrometer. |
Table I.
Mineral contents of various soy
sauces, analyzed with an inductively coupled plasma-atomic emission
spectrometer.
| Mineral | BSSS | TRSS | BRSS |
|---|
| K | 4,300 | 2650 | 3,160 |
| Ca | 119 | 83.3 | 277 |
| Mg | 740 | 1,290 | 533 |
| Fe | 19.12 | 125 | 17.1 |
| S | 657 | 997 | 330 |
| P | 218 | 75.8 | 1,230 |
| Rb | 6.81 | 2.08 | 5.55 |
| Mo | 9.30 | ≤0.1 | ≤0.1 |
| V | 0.0109 | <0.0001 | <0.0001 |
| Au | 0.024 | ≤0.0001 | ≤0.0001 |
| Pt | ≤0.001 | ≤0.001 | ≤0.0001 |
| Ge | <0.002 | <0.002 | <0.002 |
| Se | 0.0106 | 0.0024 | ≤0.0001 |
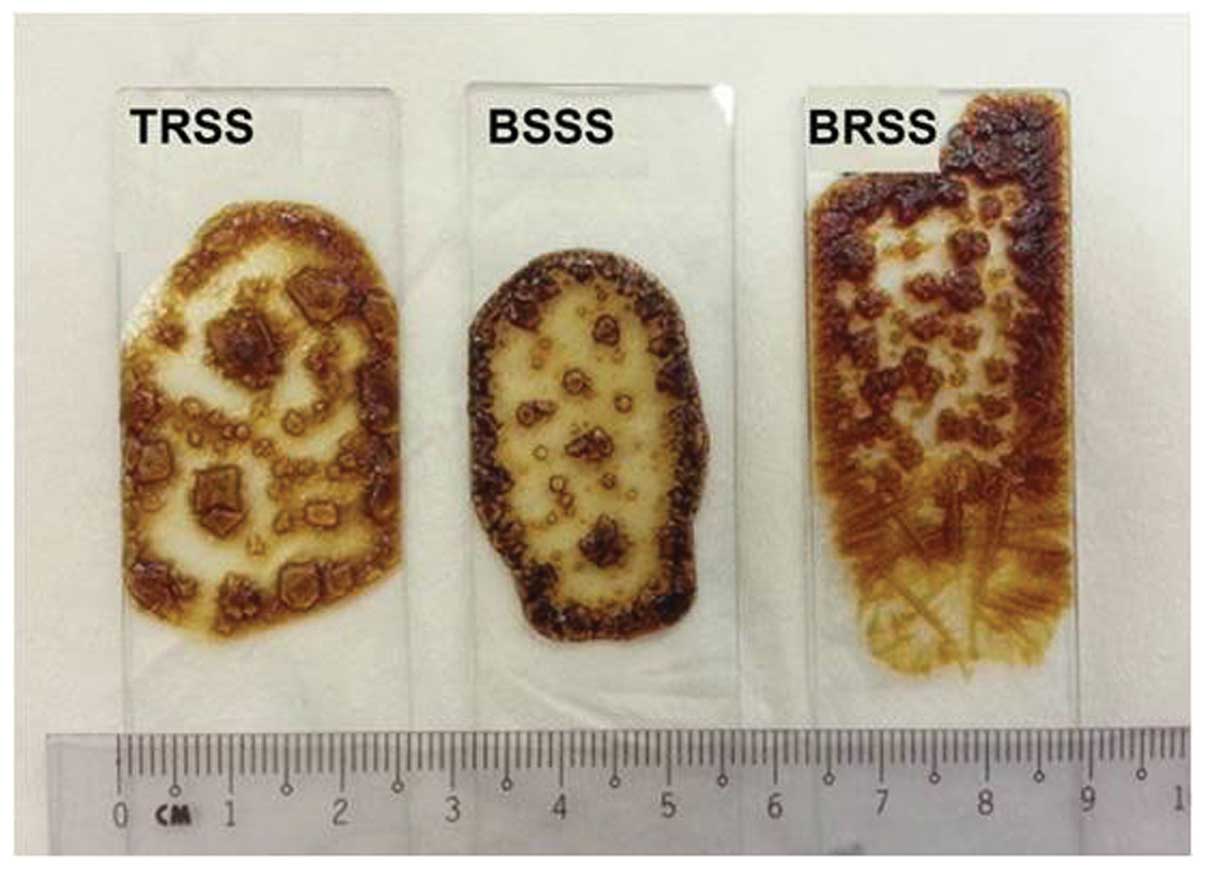
XRD analysis
In the present study, XRD analyses were performed in
order to evaluate the salt and mineral interactions occurring in
the BSSS and to assess whether they resembled those formed in BS
alone. In addition, XRD was used to determine whether the
interactions in BS could be distinguished from those in SDS or
PS.
During the preparation of dried XRD samples, it was
observed that the BSSS crystals were more regular, as compared with
those formed by TRSS, and were clearer than those formed by BRSS
(Fig. 6). This may be due to the
well-homogenized distribution of the minerals within the BS used to
produce the BSSS.
The main peaks of the XRD analysis output
corresponded to PS peaks, although there were slight shifts of
angles (Fig. 7A) and lattice
constants (Fig. 7B), as compared
with the PS control. PS peaks coincided with those noted by
Cherginets et al (37). These
results of the analysis demonstrated that the major PS structures
were retained in all samples. However, the slight shifts of angles
and lattice constants showed that certain minerals were replaced or
amalgamated together in PS structures. Notably, BSSS had a similar
shift of peak (inserts of Fig. 7A)
and lattice constant (Fig. 7B), as
compared with the BS. BS had a greater peak shift from the PS peak,
as compared with SDS. Therefore, XRD analysis demonstrated that
BSSS could retain not only minerals from BS but also the same
amalgamation of minerals with salt to produce near-identical
crystals, which are important for retaining the benefits of BS.
Discussion
In spite of the numerous reported pharmacological
benefits of SS, commercial SS contains common salt, which has been
associated with raised blood pressure, and an increased risk of
cardiovascular diseases and stroke when consumed at higher levels
than the daily recommended amount. In order to overcome this
shortcoming of SS, BSSS was prepared by replacing common salt with
BS during the manufacturing process. In the present study, BSSS
exhibited pharmacological efficacy without the side effects of
common salt, as well as retaining the desired salty taste of SS
(8,10–13).
The present study hypothesized that BSSS may limit
the side effects of SS, which includes high levels of common salt,
and would increase the potential pharmacological efficacy of SS.
Among the numerous advantages of SS and BS, the present study
focused on the reports that SS contains high levels of antioxidants
(1–4), and exhibits a high total antioxidant
activity (1,5), as well as the anti-apoptotic effects
reported for BS (19). Therefore, it
was hypothesized that BSSS may have a superior protective efficacy
against oxidative stress, as compared with conventional SS, due to
the replacement of common salt with BS.
The present study aimed to evaluate whether BSSS had
unique neuroprotective effects in the prevention of
H2O2-induced neuronal cell death, and to
demonstrate its underlying protective mechanisms, particularly
focusing on the PI3K/Akt mediated signaling pathway. Initially, the
optimal H2O2 concentration for studying
H2O2-induced cortical neuronal cell toxicity,
providing ~65% cell viability, was deduced as 100 µM
H2O2. In addition, it was determined that
BSSS did not have direct toxic effects on cell viability at any
concentration, from 0.001 to 10%, as compared with TRSS and BRSS.
Furthermore, it was demonstrated that only BSSS pretreatment
exerted cytoprotective effects against 100 µM
H2O2-induced neuronal cell death, and reduced
apoptotic cell damage and H2O2-induced ROS
production. The results of the present study suggested that BSSS
had protective efficacy against H2O2-induced
oxidative stress, suppressed cell dysfunction in cortical neuronal
cells and induced antioxidative effects. A previous study supported
the involvement of BSSS in the inhibition of ROS production, and
demonstrated its antioxidative activities (38).
In order to understand the protective mechanism
underlying the BSSS-mediated prevention of oxidative stress, the
present study particularly focused on the PI3K/Akt pathway. The
PI3K/Akt pathway has been demonstrated to have an important role in
cell survival (39). Phospho-Akt
directly affects GSK-3β activity via phosphorylation at Ser9, and
GSK-3β activation via phospho-Akt inhibition may induce the
mitochondrial cell death pathway, which is associated with
cytochrome c release from the mitochondria and activation of
caspase-3 (40). Numerous studies
have demonstrated that H2O2-induced neuronal
cell death is associated with the PI3K/Akt pathway (41,42); and
this prompted the present study to hypothesize that BSSS-mediated
Akt activation may be associated with the protective effects of
BSSS against H2O2-induced cytotoxicity.
In order to validate this hypothesis, western
blotting was used to demonstrate the ability of BSSS to attenuate
cell death-related signals, and enhance survival signals through
the PI3K-Akt pathway. BSSS was able to increase the levels of the
extrinsic growth factors, Akt and GSK3β, which generate an
anti-apoptotic response and promote cell survival through their
ability to promote phosphorylation and inactivate apoptotic factors
(43). Conversely, BSSS was able to
downregulate components of the intrinsic pathway, decreasing the
levels of apoptosis signaling molecules, including BAX, caspase-9,
caspase-3, cytochrome c, PAR, cleaved PARP, and AIF
(44). Furthermore, the present
study demonstrated that the protective effects of BSSS were
attenuated following treatment of the cells with LY294002, a PI3K
inhibitor. The results of the present study supported the
hypothesis that activation of the PI3K/Akt pathway may be
associated with the protective effects of BSSS.
In an effort to elucidate why BSSS enhanced neuronal
cell viability and inhibited apoptosis, the mineral contents of
BSSS, TRSS, and BRSS, were analyzed. BSSS contains 39 categories of
minerals indispensable for human functioning, including K, Ca, Mg,
S, Fe, P, Rb, Mo, V, Au, Pt, Ge and Se. Among them, the levels of
K, Ca, P, Rb, Mo, V, Au, and Se in BSSS, were higher, as compared
with those of TRSS and BRSS. These various mineral ions have
crucial roles in cellular functions, including cell proliferation,
energy metabolism, protein and DNA synthesis, cytoskeleton
activation, and ROS scavenging activities (19). In particular, at high concentrations
in BSSS, the additional potassium may have antioxidant activities
by inhibiting ROS over-production in salt-sensitive hypertension,
and thereby preventing cardiovascular damage (45). Furthermore, intracellular potassium
may influence the efficacy and polarity of synaptic transmission in
neurons (46). Selenium has been
shown to protect against glutamate toxicity, hypoxia and ischemic
brain damage, and has been associated with mitochondrial function
(47). Vanadium is known for its
antioxidant activity, supposedly forming well-defined complexes
with antioxidants, including glutathione or superoxide dismutase
(48,49). In addition, the formation of vanadium
complexes on triglycerides may confer a positive antioxidant effect
by inhibiting lipid peroxidation, which prevents the production of
ROS (50). Molybdenum deficiency
results in neurological damage in humans, which is most apparent in
untreatable seizures and various brain dysmorphisms (51). The results of the present study
suggested that a combination of various beneficial mineral ions in
BSSS may act synergistically in the neuronal cell to protect
against H2O2-induced oxidative stress.
The present study demonstrated that BSSS, which was
produced using BS instead of common salt, was non-toxic to rat
neuronal cells when administered at a concentration up to 10%, and
may have potential neuroprotective effects, including the
prevention of apoptosis through inhibition of cell toxicity caused
by H2O2-induced oxidative stress. The
findings of the present study clearly distinguish BSSS from
conventional SS products, including TRSS and BRSS, which were shown
to be toxic at high concentrations and were unable to confer
protection against oxidative stress. Further in vitro and
in vivo studies are required, in order to confirm and
understand the antioxidant activity of BSSS.
In conclusion, the present study demonstrated that
the neuroprotective effects of BSSS against
H2O2-induced oxidative stress conditions in a
rat cortical neuronal cell model were related not only to
anti-apoptotic and ROS-scavenging activities, but also to the
activation of the PI3K/AKT pathway, which was verified using the
PI3K inhibitor, LY294002. Conversely, the general SS products, TRSS
and BRSS, did not demonstrate such neuroprotective activities.
Therefore, considering the only difference between BSSS and SS was
the use of BS instead of common salt in the production process, it
may be hypothesized that it was the unique mineral composition of
BSSS that contributed to the neuroprotective effect of BSSS on
H2O2-induced oxidative stress.
Following the results of the present study, future
endeavors should include identifying the active ingredients of BSSS
and studying the neuroprotective effects of BSSS in vivo.
Future studies may contribute to the prevention and treatment of
brain diseases or aging processes, including Alzheimer's disease,
which is closely associated with neuronal cell death (52).
Acknowledgements
This study was supported by Hanyang University
Research Fund (Dr Seung Hyun Kim) and Nano·Material Technology
Development Program (No. 2012M3A7B4035286) through the National
Research Foundation funded by the Ministry of Science, ICT and
Future Planning.
References
|
1
|
Long LH, Kwee DC and Halliwell B: The
antioxidant activities of seasonings used in Asian cooking.
Powerful antioxidant activity of dark soy sauce revealed using the
ABTS assay. Free Radic Res. 32:181–186. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kataoka S, Liu W, Albright K, Storkson J
and Pariza M: Inhibition of benzo[a]pyrene-induced mouse
forestomach neoplasia and reduction of H2O2
concentration in human polymorphonuclear leucocytes by flavour
components of Japanese-style fermented soy sauce. Food Chem
Toxicol. 35:449–457. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Esaki H, Kawakishi S, Morimitsu Y and
Osawa T: New potent antioxidative o-dihydroxyisoflavones in
fermented Japanese soybean products. Biosci Biotechnol Biochem.
63:1637–1639. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ando M, Harada K, Kitao S, Kobayashi M and
Tamura Y: Relationship between peroxyl radical scavenging
capability measured by the chemiluminescence method and an
aminocarbonyl reaction product in soy sauce. Int J Mol Med.
12:923–928. 2003.PubMed/NCBI
|
|
5
|
Wang H, Jenner AM, Lee CY, Shui G, Tang
SY, Whiteman M, Wenk MR and Halliwell B: The identification of
antioxidants in dark soy sauce. Free Radic Res. 41:479–488. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee CY, Isaac HB, Wang H, Huang SH, Long
LH, Jenner AM, Kelly RP and Halliwell B: Cautions in the use of
biomarkers of oxidative damage; the vascular and antioxidant
effects of dark soy sauce in humans. Biochem Biophys Res Commun.
344:906–911. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kobayashi M, Matsushita H, Shioya I, Nagai
M, Tsukiyama R, Saito M, Sugita T, Sugimura T and Yamamoto K:
Quality of life improvement with soy sauce ingredients, Shoyu
polysaccharides, in perennial allergic rhinitis: A double-blind
placebo-controlled clinical study. Int J Mol Med. 14:885–889.
2004.PubMed/NCBI
|
|
8
|
Tsuchiya H, Sato M and Watanabe I:
Antiplatelet activity of soy sauce as functional seasoning. J Agric
Food Chem. 47:4167–4174. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kinoshita E and Saito M: Novel histamine
measurement by HPLC analysis used to assay histidine decarboxylase
inhibitory activity of shoyuflavones from soy sauce. Biosci
Biotechnol Biochem. 62:1488–1491. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stiefelhagen P: Allergy or histamine
intolerance? Cheilitis caused by soy sauce. MMW Fortschr Med.
154:312012.(In German). PubMed/NCBI
|
|
11
|
Carlberg DJ, Borek HA, Syverud SA and
Holstege CP: Survival of acute hypernatremia due to massive soy
sauce ingestion. J Emerg Med. 45:228–231. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oguri M, Okano K, Ieki H, Kitagawa M,
Tadokoro O, Sano Y, Oishi K, Hirooka H and Kumagai H: Feed intake,
digestibility, nitrogen utilization, ruminal condition and blood
metabolites in wethers fed ground bamboo pellets cultured with
white-rot fungus (Ceriporiopsis subvermispora) and mixed
with soybean curd residue and soy sauce cake. Anim Sci J.
84:650–655. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ito A, Watanabe H and Basaran N: Effects
of soy products in reducing risk of spontaneous and neutron-induced
liver-tumors in mice. Int J Oncol. 2:773–776. 1993.PubMed/NCBI
|
|
14
|
Kearney PM, Whelton M, Reynolds K, Muntner
P, Whelton PK and He J: Global burden of hypertension: Analysis of
worldwide data. Lancet. 365:217–223. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Furukawa S, Takaya A, Nakagawa T,
Sakaguchi I and Nishi K: Fatal hypernatremia due to drinking a
large quantity of shoyu (Japanese soy sauce). J Forensic Leg Med.
18:91–92. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim HY, Lee ES, Jeong JY, Choi JH, Choi
YS, Han DJ, Lee MA, Kim SY and Kim CJ: Effect of bamboo salt on the
physicochemical properties of meat emulsion systems. Meat Sci.
86:960–965. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Choi CH, Ha MO, Youn HJ, Jeong SS, Iijima
Y, Sohn W and Hong SJ: Effect of bamboo salt-NaF dentifrice on
enamel remineralization. Am J Dent. 25:9–12. 2012.PubMed/NCBI
|
|
18
|
Zhao X, Kim SY and Park KY: Bamboo salt
has in vitro anticancer activity in HCT-116 cells and exerts
anti-metastatic effects in vivo. J Med Food. 16:9–19. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jeong HJ, Kim JJ, Kim MH and Kim HM:
Specific blockage of caspase-1 activation by purple bamboo-salt
prevents apoptosis of auditory cell line, HEI-OC1. J Med Food.
14:53–61. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shin HY, Lee EH, Kim CY, Shin TY, Kim SD,
Song YS, Lee KN, Hong SH and Kim HM: Anti-inflammatory activity of
Korean folk medicine purple bamboo salt. Immunopharmacol
Immunotoxicol. 25:377–384. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hwang KM, Jung KO, Song CH and Park KY:
Increased antimutagenic and anticlastogenic effects of doenjang
(Korean fermented soybean paste) prepared with bamboo salt. J Med
Food. 11:717–722. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao X, Ju J, Kim HM and Park KY:
Antimutagenic activity and in vitro anticancer effects of bamboo
salt on HepG2 human hepatoma cells. J Environ Pathol Toxicol Oncol.
32:9–20. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao X, Deng X, Park KY, Qiu L and Pang L:
Purple bamboo salt has anticancer activity in TCA8113 cells in
vitro and preventive effects on buccal mucosa cancer in mice
in vivo. Exp Ther Med. 5:549–554. 2013.PubMed/NCBI
|
|
24
|
Shin HY, Na HJ, Moon PD, Shin T, Shin TY,
Kim SH, Hong SH and Kim HM: Inhibition of mast cell-dependent
immediate-type hypersensitivity reactions by purple bamboo salt. J
Ethnopharmacol. 91:153–157. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Emerit J, Edeas M and Bricaire F:
Neurodegenerative diseases and oxidative stress. Biomed
Pharmacother. 58:39–46. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gorman AM, McGowan A, O'Neill C and Cotter
T: Oxidative stress and apoptosis in neurodegeneration. J Neurol
Sci. 139(Suppl): 45–52. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Berlett BS and Stadtman ER: Protein
oxidation in aging, disease, and oxidative stress. J Biol Chem.
272:20313–20316. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Finkel T and Holbrook NJ: Oxidants,
oxidative stress and the biology of ageing. Nature. 408:239–247.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hampton MB and Orrenius S: Dual regulation
of caspase activity by hydrogen peroxide: Implications for
apoptosis. FEBS Lett. 414:552–556. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pomytkin IA: H2O2
signalling pathway: A possible bridge between insulin receptor and
mitochondria. Curr Neuropharmacol. 10:311–320. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fan B, Li GY, Li YP and Cui JZ:
Neuroprotective effect of epigallocatechin gallate on
oxidative-stress-injured retinal cells. Zhonghua Yi Xue Za Zhi.
88:1711–1714. 2008.(In Chinese). PubMed/NCBI
|
|
32
|
Nakajima Y, Inokuchi Y, Nishi M, Shimazawa
M, Otsubo K and Hara H: Coenzyme Q10 protects retinal cells against
oxidative stress in vitro and in vivo. Brain Res. 1226:226–233.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Golbitz P: Traditional soyfoods:
Processing and products. J Nutr. 125(3 Suppl): 570S–572S.
1995.PubMed/NCBI
|
|
34
|
Singhal P, Bal LM, Satya S, Sudhakar P and
Naik SN: Bamboo shoots: A novel source of nutrition and medicine.
Crit Rev Food Sci Nutr. 53:517–34. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Noh MY, Koh SH, Kim SM, Maurice T, Ku SK
and Kim SH: Neuroprotective effects of donepezil against
Aβ42-induced neuronal toxicity are mediated through not only
enhancing PP2A activity but also regulating GSK-3β and nAChRs
activity. J Neurochem. 127:562–574. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lee YJ, Park HH, Koh SH, Choi NY and Lee
KY: Amlodipine besylate and amlodipine camsylate prevent cortical
neuronal cell death induced by oxidative stress. J Neurochem.
119:1262–1270. 2013. View Article : Google Scholar
|
|
37
|
Cherginets VL, Baumer VN, Galkin SS,
Glushkova LV, Rebrova TP and Shtitelman ZV: Solubility of
Al2O3 in some chloride-fluoride melts. Inorg
Chem. 45:7367–7371. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jeong JH and Om AS: Specially-treated soy
sauces regulate antioxidant activity and ROS in human astrocyte
U373MG cells. Cancer Prev Res (Phila). 12:296–302. 2007.
|
|
39
|
Cantley LC: The phosphoinositide 3-kinase
pathway. Science. 296:1655–1657. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cantrell DA: Phosphoinositide 3-kinase
signalling pathways. J Cell Sci. 114:1439–1445. 2001.PubMed/NCBI
|
|
41
|
Yu XR, Jia GR, Gao GD, Wang SH, Han Y and
Cao W: Neuroprotection of insulin against oxidative stress-induced
apoptosis in cultured retinal neurons: Involvement of
phosphoinositide 3-kinase/Akt signal pathway. Acta Biochim Biophys
Sin (Shanghai). 38:241–248. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang Q, Huang WD, Lv XY and Yang YM:
Puerarin protects differentiated PC12 cells from
H2O2-induced apoptosis through the PI3K/Akt
signalling pathway. Cell Biol Int. 36:419–426. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Datta SR, Dudek H, Tao X, Masters S, Fu H,
Gotoh Y and Greenberg ME: Akt phosphorylation of BAD couples
survival signals to the cell-intrinsic death machinery. Cell.
91:231–241. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Benn SC and Woolf CJ: Adult neuron
survival strategies - slamming on the brakes. Nat Rev Neurosci.
5:686–700. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ando K, Matsui H, Fujita M and Fujita T:
Protective effect of dietary potassium against cardiovascular
damage in salt-sensitive hypertension: Possible role of its
antioxidant action. Curr Vasc Pharmacol. 8:59–63. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Boulenguez P, Liabeuf S, Bos R, Bras H,
Jean-Xavier C, Brocard C, Stil A, Darbon P, Cattaert D, Delpire E,
et al: Down-regulation of the potassium-chloride cotransporter KCC2
contributes to spasticity after spinal cord injury. Nat Med.
16:302–307. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mendelev N, Mehta SL, Idris H, Kumari S
and Li PA: Selenite stimulates mitochondrial biogenesis signaling
and enhances mitochondrial functional performance in murine
hippocampal neuronal cells. PLoS One. 7:e479102012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Macara IG, Kustin K and Cantley LC Jr:
Glutathione reduces cytoplasmic vanadate. Mechanism and
physiological implications. Biochim Biophys Acta. 629:95–106. 1980.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zwolak I and Zaporowska H: Preliminary
studies on the effect of zinc and selenium on vanadium-induced
cytotoxicity in vitro. Acta Biol Hung. 60:55–67. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Francik R, Krośniak M, Barlik M, Kudła A,
Gryboś R and Librowski T: Impact of vanadium complexes treatment on
the oxidative stress factors in wistar rats plasma. Bioinorg Chem
Appl. 2011:2063162011.PubMed/NCBI
|
|
51
|
Reiss J, Bonin M, Schwegler H, Sass JO,
Garattini E, Wagner S, Lee HJ, Engel W, Riess O and Schwarz G: The
pathogenesis of molybdenum cofactor deficiency, its delay by
maternal clearance, and its expression pattern in microarray
analysis. Mol Genet Metab. 85:12–20. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Niikura T, Tajima H and Kita Y: Neuronal
cell death in Alzheimer's disease and a neuroprotective factor,
humanin. Curr Neuropharmacol. 4:139–147. 2006. View Article : Google Scholar : PubMed/NCBI
|