Introduction
The emergence and growth of tumors are known to be
associated with tumor immunosurveillance and antitumor immune
responses (1). However, one of the
drawbacks of many therapeutic technologies for cancer patients is
the inadvertent induction of host immune responses (2). Thus, previous studies have focused on
immune-mediated protection against cancer in immunocompromised
patients with cancer and mouse models (3). Treatments for human cancers remain a
therapeutic challenge, and the identification and development of
novel agents to induce immune function is necessary.
Chitosan, a linear heteropolysaccharide composed of
β-(1,4)-linked D-glucosamine (GlcN) and
β-(1,4)-linked D-N-acetylglucosamine (GlcNAc),
can be derived from chitin (4),
which is a naturally occurring polysaccharide composed of GlcNAc
units (5). Chitosan can be used as a
biomaterial for tissue regeneration, and has antibacterial,
anti-inflammatory and drug delivery functions (6). Numerous studies have demonstrated that
chitosan may inhibit the growth of microbial organisms, such as
Porphyromonas gingivalis (7),
Actinobacillus actinomycetemcomitans, Streptococcus mutans
(8,9), Pseudomonas aeroginosa,
Staphylococcus aureus (10)
and Aggregatibacter actinomycetemcomitans (11).
In human astrocytoma cells, the secretion and
expression of the pro-inflammatory cytokines tumor necrosis factor
(TNF)-α and interleukin (IL)-6 has been shown to be markedly
inhibited following pretreatment with water-soluble chitosan
(9). It has also been reported that
chitosan-induced macrophages exhibit markedly downregulated
expression of pro-inflammatory markers, such as cluster of
differentiation CD86 and major histocompatibility complex II
(MHCII), and decrease the expression of pro-inflammatory cytokines,
specifically TNF-α; however, the anti-inflammatory markers IL-10
and TGF-β1 were found to be increased (12,13).
Despite the reports of several studies that chitosan
has an anti-inflammatory effect in vitro, knowledge
concerning the effect of chitosan on the immune responses of normal
mice is lacking. In the present study, the promoted immune
responses in BALB/c mice were evaluated in vivo.
Furthermore, the levels of certain enzymes, including glutamic
oxaloacetic transaminase (GOT), glutamic pyruvic transaminase (GPT)
and lactate dehydrogenase (LDH), were analyzed in BALB/c mice
following oral treatment with chitosan. The expression levels of
the white blood cell markers CD3, CD11b, CD19 and Mac-3 were also
investigated.
Materials and methods
Materials and reagents
Acetic acid was obtained from Sigma-Aldrich (St.
Louis, MO, USA). RPMI-1640 medium, fetal bovine serum, L-glutamine
and penicillin-streptomycin were purchased from Gibco (Thermo
Fisher Scientific, Inc., Waltham, MA, USA). Tissue culture plastic
wares and Tissue culture plastic wares and phycoerythrin
(PE)-conjugated anti-mouse-CD3 (cat. no. 553062), PE-conjugated
anti-mouse-CD19 (cat. no. 553786), FITC-conjugated anti-mouse-CD11b
(cat. no. 553310) and FITC-conjugated anti-mouse-Mac-3 (cat. no.
553322) were purchased from BD Pharmigen (San Diego, CA, USA).
Preparation of chitosan
Chitosan powder with a molecular weight of ~86,000
kDa (Koyo Chemical Co., Ltd., Sakaiminato, Japan) was obtained from
the National Taiwan University College of Medicine Animal Medicine
Center (Taipei, Taiwan). The doses of 5 and 20 mg/kg were
separately suspended in 0.2 ml acetic acid for 1 h at room
temperature prior to use (14).
Male BALB/c mice and chitosan
treatment
Forty male BALB/c mice aged 4 weeks and weighing
22–25 g, were obtained from the National Laboratory Animal Center
(Taipei, Taiwan). All mice were maintained at 25°C on 12 h
light/dark cycles in the animal center of China Medical University
(Taichung, Taiwan). All animal experiments were reviewed and
approved by the Institutional Animal Care and Use Committee of
China Medical University (approval ID, 103–215-B). All animal care
was in accordance with the institutional animal ethical guidelines
of the China Medical University (15). The 40 mice were randomly divided into
the following four groups (10 mice per group): Negative control
group, comprising untreated mice; positive control group, treated
with acetic acid; 5 mg/kg group, treated with 5 mg/kg chitosan in
acetic acid, and 20 mg/kg group, treated with 20 mg/kg chitosan in
acetic acid. Mice in all four groups were fed a normal diet.
Chitosan in acetic acid was administered by gavage every 2 days for
a total of 24 days (12 times), during which the body weight was
recorded. Upon termination of the treatment, mice from each group
were weighed and sacrificed with CO2, as previously
described (15).
Immunofluorescence staining for
surface markers
Upon termination of the treatment, all mice were
individually weighed and blood samples, as well as the liver and
spleen of the mice were individually collected. The collected
spleens were used for the isolation of splenocytes and measurement
of natural killer (NK) cell activity, as previously described
(15). A blood sample of 1 ml from
each mouse was lysed to destroy the red blood cells using 1X BD
Pharm Lyse™ lysing buffer (BD Biosciences, Franklin Lakes, NJ, USA)
according to the manufacturer's protocol, and leukocytes were
collected as previously described (15). Phycoerythrin (PE)-labeled anti-mouse
CD3, PE-labeled anti-mouse CD19, fluorescein isothiocyanate
(FITC)-labeled anti-mouse CD11b and FITC-labeled anti-mouse Mac-3
antibodies (all dilution 1:40) were used to stain the isolated
leukocytes for 30 min, and then all samples were washed with
phosphate-buffered saline (PBS). After this, all samples were
analyzed using flow cytometry (BD FACSCalibur; BD Biosciences) to
measure the percentages of white blood cell markers, as previously
described (15).
Measurements of the phagocytic
activity of macrophages
Macrophages were isolated from the peripheral blood
mononuclear cells (PBMCs) and peritoneum of each mouse as
previously described (15) and were
placed in plates containing 50 µl target E. coli-FITC
according to PHAGOTEST® kit manufacturer's instructions (ORPEGEN
Pharma GmbH, Heidelberg, Germany). All samples were individually
mixed, then examined for phagocytosis using flow cytometery.
Quantification of phagocytosis was performed using CellQuest
software (version 5.1; BD Biosciences) as previously described
(15).
Measurements of NK cell cytotoxic
activity
Splenocytes were isolated from each spleen as
previously described (15) and were
placed in 96-well plates (1×105 cells/well) with 1 ml
RPMI-1640 medium. Target YAC-1 cells (2.5×107 cells;
Food Industry Research and Development Institute, Hsinchu, Taiwan)
and PKH-67/Diluent C buffer (Sigma-Aldrich) were individually added
to the cell-containing wells, according to the manufacturer's
protocol. The samples were mixed thoroughly for 2 min at 25°C and 2
ml PBS was added to each well for 1 min together with 4 ml medium.
The mix was then incubated for 10 min. Following incubation, all
samples were centrifuged for 2 min at 290 × g rpm (25°C). NK cell
cytotoxic activity was measured using flow cytometry as previously
described (15).
Measurements of T- and B-cell
proliferation
Isolated splenocytes (1×105 cells/well)
from each mouse were placed in 96-well plates containing 100 µl
RPMI-1640 medium. Following stimulation by incubation with
concanavalin A (Con A; 0.5 µg/ml; Sigma-Aldrich) for 3 days, T-cell
proliferation was measured. In addition, B-cell proliferation was
measured following stimulation with lipopolysaccharide (LPS, 1
µg/ml; Sigma-Aldrich) for 5 days. Cell proliferation was measured
using CellTiter 96 AQueous One Solution Cell Proliferation Assay
kit (Promega Corporation, Madison, WI, USA) as previously described
(15).
Measurement of blood levels of GOT,
GPT and LDH in BALB/c mice following exposure to chitosan
Following treatment, blood samples were collected
from all mice in order to measure the levels of GOT, GTP and LDH
using quantitative kits. The kits were liquiUV (aspartate
aminotransferase) for GOT, liquiUV (alanine aminotransferase) for
GPT and liquiUV (lactate dehydrogenase) for LDH, respectively,
which were purchased from HUMAN Gesellschaft für Biochemica und
Diagnostica mbH (Wiesbaden, Germany), and were used as previously
described (16,17).
Statistical analysis
The data from three independent experiments were
expressed as the mean ± standard error. Statistical comparison
between the chitosan and control groups was performed using the
Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Chitosan affects the body, liver and
spleen weights of normal BALB/c mice
Representative images of the mice in the four
groups, and animal body, liver and spleen weights are presented in
Fig. 1. These results indicate that
chitosan did not significantly affect the appearance of the animals
(Fig. 1A) or the body (Fig. 1B), liver (Fig. 1C) or spleen (Fig. 1D) weights when compared with those of
the vehicle-treated group.
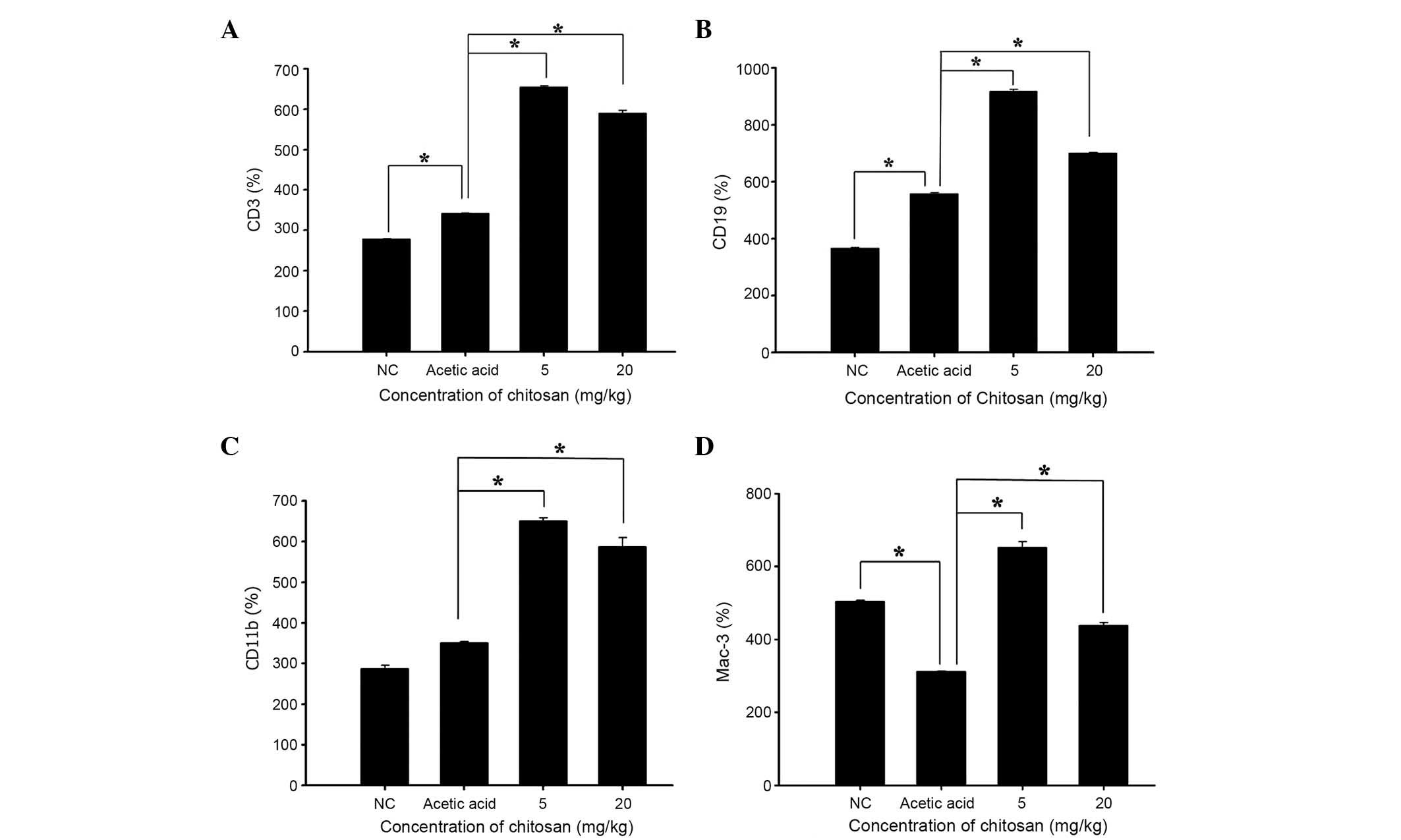
Chitosan affects cell markers of white
blood cells in normal BALB/c mice
Blood samples were collected from each mouse and the
levels of CD3, CD19, CD11b and Mac-3 cell markers were measured.
The results presented in Fig. 2
indicate that chitosan promoted CD3 (Fig. 2A), CD19 (Fig. 2B), CD11b (Fig. 2C) and Mac-3 (Fig. 2D) expression at both doses, when
compared with the acetic acid-treated group.
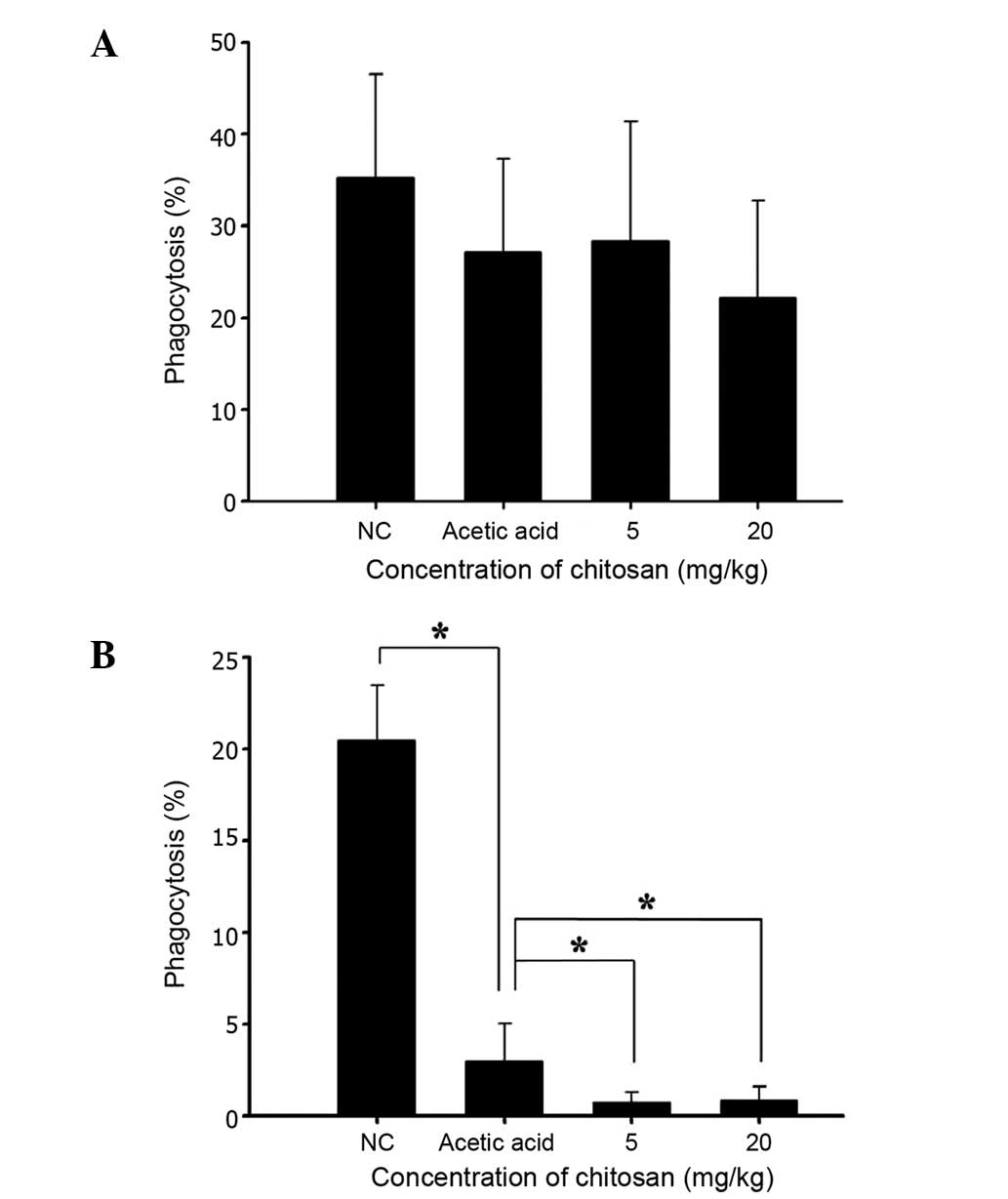
Chitosan affects the phagocytic
activity of macrophages from the PBMCs and peritoneal cavity of
normal BALB/c mice
Following treatment, cells were isolated from the
PBMCs and peritoneal cavity of each animal, in order to measure the
percentage of phagocytosis and results are shown in Fig. 3A and B, respectively. Neither of the
two doses of chitosan (5 and 20 mg/kg) was found to significantly
affect phagocytosis by macrophages from PMBCs (Fig. 3A); however, both doses were found to
decrease phagocytosis by macrophages from the peritoneal cavity
(Fig. 3B).
Chitosan affects the cytotoxic
activity of NK cells from normal BALB/c mice
YAC-1 cells were used as targets for isolated
splenocytes and were examined using flow cytometry. The results
(Fig. 4) indicated that chitosan did
not significantly affect the cytotoxic activity of NK cells at an
effector to target ratio of 50:1; however, at the dose of 20 mg/kg
chitosan and an effector to target ratio of 25:1 led to a
significant reduction in the cytotoxic activity of the NK cells
when compared with that in the acetic acid-treated group
(P<0.05; Fig. 4).
Chitosan affects T- and B-cell
proliferation in normal BALB/c mice
Isolated splenocytes were assayed for T- and B-cell
proliferation using flow cytometry and the results are presented in
Fig. 5. The two chitosan doses (5
and 20 mg/kg) notably decreased T cell proliferation when compared
with the acetic acid-treated group (Fig.
5A), but did not significantly affect B-cell proliferation
(Fig. 5B).
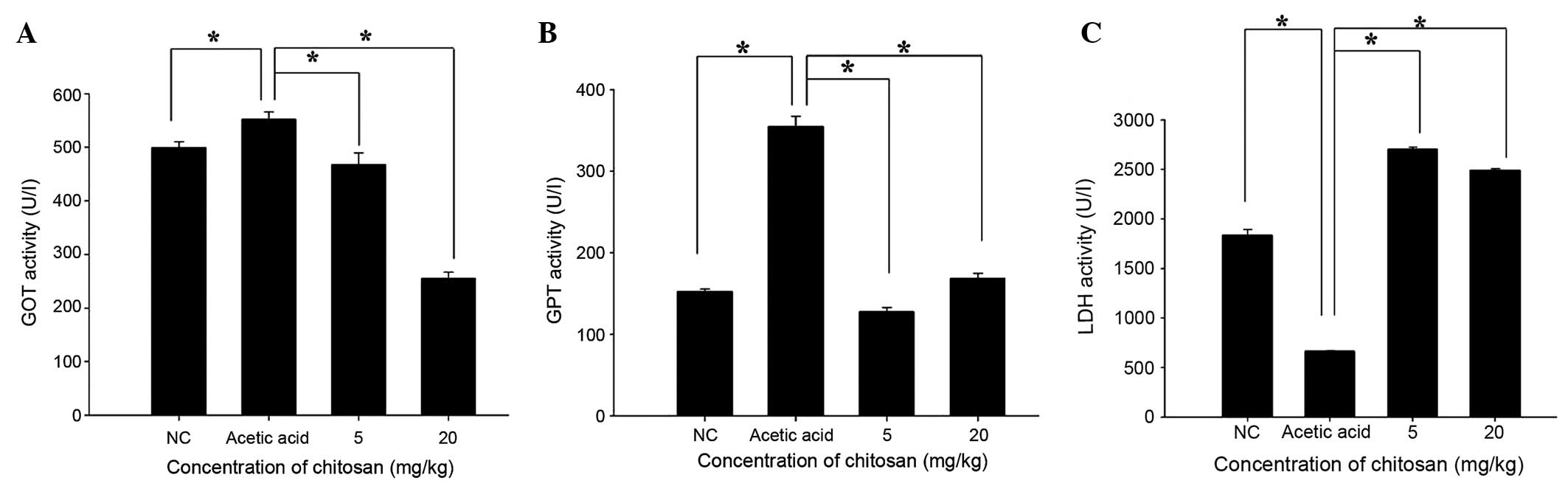
Chitosan affects the activity of blood
enzymes GOT, GPT and LDH in BALB/c mice
Following treatment, the mice were sacrificed and
blood samples were collected from each mouse in order to measure
the activity of GOT, GTP and LDH (Fig.
6). Chitosan significantly decreased GOT and GPT activity when
compared with that in the acetic acid-treated group (P<0.05;
Fig 6A and B). However, GPT activity
in the 20 mg/kg chitosan group was slightly higher than that in
normal mice. Furthermore, chitosan significantly increased LDH
activity when compared with that in the acetic acid-treated group
(P<0.05).
Discussion
Although numerous studies have shown that chitosan
is able to inhibit the growth of microbial organisms (7–11), it
has also been shown to cause significant downregulation of the
expression of pro-inflammatory markers CD86 and MHCII on
macrophages, decrease the expression of the pro-inflammatory
cytokine TNF-α and increase that of the anti-inflammatory cytokines
IL-10 and TGF-β1 (12,13). In addition, our earlier study has
shown that the hypolipidemic effect of chitosan is partly
attributed to its suppression of intestinal lipid absorption and
hepatic acyl-coenzyme A: cholesterol acyltransferase-2 expression
(18). Furthermore, chitosan has
also been found to slow down the rate of tumor growth without
inhibiting tumor formation (14);
however, no detailed analysis of the immune responses in
chitosan-treated animals, including mice, has been reported.
In the present study, normal BALB/c mice were
randomly divided into four groups. The negative control group
received a normal diet, the positive control group received a
normal diet and acetic acid, and two treatment groups received a
normal diet and the oral administration of 5 or 20 mg/kg chitosan
in acetic acid. During the treatment, chitosan and/or acetic acid
was administered, and the animals were weighed, every 2 days. At
the end of the treatment period, blood samples were collected from
all mice for cell marker analysis and measurement of phagocytosis,
and splenocytes were isolated in order to examining NK cell
activities and T- and B-cell proliferation.
To the best of our knowledge, this is the first
study evaluating the effect of chitosan on immune responses in
normal mice in vivo. The present results showed the
following: i) chitosan did not significantly affect the appearance
(Fig. 1A) or body (Fig. 1B), liver (Fig. 1C) and spleen (Fig. 1D) weights of the mice when compared
with the acetic acid group; ii) chitosan increased CD3 (T cell;
Fig. 2A), CD19 (B cell; Fig. 2B), CD11b (monocyte; Fig. 2C) and Mac-3 (macrophage; Fig. 2D) markers when compared with the
acetic acid group; iii) chitosan treatment did not significantly
increase the phagocytic activity of macrophages in PBMCs (Fig. 3A) but significantly decreased it in
the peritoneal cavity (Fig. 3B); iv)
chitosan at 20 mg/kg significantly decreased the cytotoxic effect
of NK cells compared with that in the acetic acid group (Fig. 4); v) 20 mg/kg chitosan treatment
significantly decreased T cell proliferation (Fig. 5A) compared with that in the acetic
acid group, but B cell proliferation was not significantly affected
by treatment with 5 and 20 mg/kg doses (Fig. 5B), and vi) chitosan decreased GOT and
GPT activity compared with that in the acetic acid group, with GPT
activity in the 20 mg/kg group being slightly higher than the
levels in normal mice (Fig. 6B).
Chitosan significantly increased LDH levels when compared with
those in the acetic acid-treated group (Fig. 6C).
Chitosan promoted the cell markers CD3 (T cell),
CD19 (B cell), CD11b (monocytes) and Mac-3 (macrophages) when
compared with the acetic acid-treated mice. A previous study has
reported that these four cell types play an important role in
immune responses, particularly against the invasion of foreign
antigens (19). Other studies have
shown that macrophages play an important role in innate immunity
(20,21). Despite reports suggesting the
involvement of chitosan in inflammatory responses, reliable data in
the literature regarding the effects of chitosan on immune
responses in normal mice in vivo are lacking. The aim of the
present study, therefore, was to investigate the effects of
chitosan on the immune responses of normal BALB/c mice in
vivo.
A notable observation of the present study is that
chitosan did not significantly affect the phagocytic activity of
macrophages in PBMCs (Fig. 3A), but
significantly decreased this activity in the peritoneal cavity
(Fig. 3B); thus, the effects of
chitosan on the Mac-3 marker and macrophage function require
further study. It has been suggested that chitosan may exert an
anti-inflammatory activity in astrocytoma cells (11) and macrophages (12,13).
Furthermore, it has been reported that chitosan exerts
anti-inflammatory activity by modulating prostaglandin E synthase 2
levels through the c-Jun N-terminal kinase pathway, which has been
suggested to be useful in the prevention or treatment of
periodontal inflammation (22).
Treatment with 20 mg/kg chitosan significantly decreased the
cytotoxic effect of NK cells from normal mice. Compared with the
acetic acid-treated group, chitosan did not significantly affect
B-cell proliferation following LPS stimulation (Fig. 5B) but both doses of chitosan
decreased T-cell proliferation following Con A stimulation
(Fig. 5A). Further investigations
are necessary to investigate this. It has been suggested that the
great variability observed in chitosan samples, such as degrees of
deacetylation, molecular weight, viscosity, and pKa may affect its
properties (4).
Chitosan decreased the levels of GOT and GPT
compared with those in the acetic acid-treated group; although the
GPT level in the 20 mg/kg group was slightly higher than the level
in normal mice (Fig. 6B). High
levels of GPT and GOT activity in the serum have been recognized to
be a reflection of hepatic cell destruction (23). The results of the present study
indicate that chitosan may have a protective effect against hepatic
cell death following exposure to acetic acid. Chitosan
significantly increased LDH levels when compared with those in the
acetic acid-treated group. Abnormal hepatic transaminase and LDH
levels have been suggested to be associated with liver injury in
patients with abdominal trauma (24). Acetic acid treatment in mice may lead
to liver injury; on the basis of the increased levels of LDH in the
blood observed in the present study following treatment with acetic
acid and chitosan, it appears that chitosan may have a protective
effect.
In conclusion, these findings suggest that chitosan
modulates immune responses by increasing T-cell, B-cell, monocyte
and macrophage cell markers in normal mice in vivo.
Furthermore, comparisons between mice treated with acetic acid and
chitosan, or chitosan alone indicate that chitosan treatment may
protect against liver injury in vivo.
Acknowledgements
This study was supported by from China Medical
University (Taichung, Taiwan; grant no. CMU102-ASIA-20) and Cheng
Hsin General Hospital (Taipei, Taiwan; grant no. 103-01).
References
|
1
|
Jones LM, Broz ML, Ranger JJ, Ozcelik J,
Ahn R, Zuo D, Ursini-Siegel J, Hallett M, Krummel M and Muller WJ:
Stat3 establishes an immunosuppressive microenvironment during the
early stages of breast carcinogenesis to promote tumor growth and
metastasis. Cancer Res. 30–Dec;2015.(Epub ahead of print).
|
|
2
|
Vinay DS, Ryan EP, Pawelec G, Talib WH,
Stagg J, Elkord E, Lichtor T, Decker WK, Whelan RL, Kumara HM, et
al: Immune evasion in cancer: Mechanistic basis and therapeutic
strategies. Semin Cancer Biol. (Suppl 35): S185–S198. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vesely MD, Kershaw MH, Schreiber RD and
Smyth MJ: Natural innate and adaptive immunity to cancer. Annu Rev
Immunol. 29:235–271. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Domard A: A perspective on 30 years
research on chitin and chitosan. Carbohydr Polym. 84:696–703. 2011.
View Article : Google Scholar
|
|
5
|
Chung MJ, Park JK and Park YI:
Anti-inflammatory effects of low-molecular weight chitosan
oligosaccharides in IgE-antigen complex-stimulated RBL-2H3 cells
and asthma model mice. Int Immunopharmacol. 12:453–459. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Francesko A and Tzanov T: Chitin, chitosan
and derivatives for wound healing and tissue engineering. Adv
Biochem Eng Biotechnol. 125:1–27. 2011.PubMed/NCBI
|
|
7
|
Ikinci G, Senel S, Akincibay H, Kaş S,
Erciş S, Wilson CG and Hincal AA: Effect of chitosan on a
periodontal pathogen Porphyromonas gingivalis. Int J Pharm.
235:121–127. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Choi BK, Kim KY, Yoo YJ, Oh SJ, Choi JH
and Kim CY: In vitro antimicrobial activity of a
chitooligosaccharide mixture against Actinobacillus
actinomycetemcomitans and Streptococcus mutans. Int J
Antimicrob Agents. 18:553–557. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sarasam AR, Brown P, Khajotia SS, Dmytryk
JJ and Madihally SV: Antibacterial activity of chitosan-based
matrices on oral pathogens. J Mater Sci Mater Med. 19:1083–1090.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chung YC, Wang HL, Chen YM and Li SL:
Effect of abiotic factors on the antibacterial activity of chitosan
against waterborne pathogens. Bioresour Technol. 88:179–184. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim MS, Sung MJ, Seo SB, Yoo SJ, Lim WK
and Kim HM: Water-soluble chitosan inhibits the production of
pro-inflammatory cytokine in human astrocytoma cells activated by
amyloid beta peptide and interleukin-1beta. Neurosci Lett.
321:105–109. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chou TC, Fu E and Shen EC: Chitosan
inhibits prostaglandin E2 formation and cyclooxygenase-2 induction
in lipopolysaccharide-treated RAW 264.7 macrophages. Biochem
Biophys Res Commun. 308:403–407. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yoon HJ, Moon ME, Park HS, Im SY and Kim
YH: Chitosan oligosaccharide (COS) inhibits LPS-induced
inflammatory effects in RAW 264.7 macrophage cells. Biochem Biophys
Res Commun. 358:954–959. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yeh MY, Wu MF, Shang HS, Chang JB, Shih
YL, Chen YL, Hung HF, Lu HF, Yeh C, Wood WG, et al: Effects of
chitosan on xenograft models of melanoma in C57BL/6 mice and
hepatoma formation in SCID mice. Anticancer Res. 33:4867–4873.
2013.PubMed/NCBI
|
|
15
|
Lu HF, Tung WL, Yang JS, Huang FM, Lee CS,
Huang YP, Liao WY, Chen YL and Chung JG: In vitro suppression of
growth of murine WEHI-3 leukemia cells and in vivo promotion of
phagocytosis in a leukemia mice model by indole-3-carbinol. J Agric
Food Chem. 60:7634–7643. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nagamatsu Y, Yamamoto J, Fukuda A, Ohta M,
Tsuda Y and Okada Y: Determination of leukocyte elastase
concentration in plasma and serum by a simple method using a
specific synthetic substrate. Haemostasis. 21:338–345.
1991.PubMed/NCBI
|
|
17
|
No authors listed: Recommendations of the
German Society for Clinical Chemistry. Standardization of methods
for the determination of enzyme activities in biological fluids. Z
Klin Chem Klin Biochem. 8:658–660. 1970.PubMed/NCBI
|
|
18
|
Wu CC, Lin SY, Chen CT, Chang YP, Huang
YS, Lii CK, Yu CC, Hsieh SL and Chung JG: Differential blood
lipid-lowering effects of alkylsulfonated chitosan of different
molecular weights in Syrian hamsters in vivo. Mol Med Rep.
5:688–694. 2012.PubMed/NCBI
|
|
19
|
Arpinati M and Curti A: Immunotherapy in
acute myeloid leukemia. Immunotherapy. 6:95–106. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gordon S, Plüddemann A and Mukhopadhyay S:
Sinusoidal immunity: Macrophages at the lymphohematopoietic
interface. Cold Spring Harb Perspect Biol. 7:a0163782015.
View Article : Google Scholar
|
|
21
|
Kim KH, Kim TS, Lee JG, Park JK, Yang M,
Kim JM, Jo EK and Yuk JM: Characterization of proinflammatory
responses and innate signaling activation in macrophages infected
with Mycobacterium scrofulaceum. Immune Netw. 14:307–320.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Arancibia R, Maturana C, Silva D, Tobar N,
Tapia C, Salazar JC, Martínez J and Smith PC: Effects of chitosan
particles in periodontal pathogens and gingival fibroblasts. J Dent
Res. 92:740–745. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yamashita T, Ohshima H, Asanuma T, Inukai
N, Miyoshi I, Kasai N, Kon Y, Watanabe T, Sato F and Kuwabara M:
The effects of alpha-phenyl-tert-butyl nitrone (PBN) on
copper-induced rat fulminant hepatitis with jaundice. Free Radic
Biol Med. 21:755–761. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bilgic I, Gelecek S, Akgun AE and Ozmen
MM: Predictive value of liver transaminases levels in abdominal
trauma. Am J Emerg Med. 32:705–708. 2014. View Article : Google Scholar : PubMed/NCBI
|