Introduction
Tourette syndrome (TS) is a chronic neurobehavioral
disorder with an unclear etiology and pathophysiology. The primary
symptoms of TS are vocal and motor tics, which may result in
lifelong impairment in certain individuals. In recent years, the
prevalence of TS has been increasing (1), and ~5% of TS patients present
life-threatening symptoms, which are defined as malignant TS
(2). These symptoms are difficult to
manage with conservative treatments and neurosurgical
procedures.
Recent studies have demonstrated that stem
cell-based therapy may be a potential treatment for numerous
neurological disorders (3,4). In 2008, our group transplanted neural
stem cells (NSCs) into TS rats and the therapeutic effects of NSCs
on the stereotypic behavior of TS rats was investigated (5). However, compared with NSCs, mesenchymal
stem cells (MSCs) are a better option for cell transplantation
therapy, since they are immunologically inert and easily
accessible. In addition, MSCs are able to rapidly expand in cell
culture and have been shown to present long-term survival and
integration with the host tissue. In a further study in 2010, our
group infused MSCs into the striatum of TS rats, revealing that a
fraction of MSCs differentiated into neurons and gliocytes
(6). Therefore, replacement of
neuronal cells by MSCs was hypothesized to contribute to the
functional improvement of TS rats. However, the differentiation
rate of MSCs observed in our previous study was lower than expected
(6). Therefore, assessing the
underlying mechanism through which MSCs act to alleviate the
symptoms of TS was difficult.
MSCs have also been found to improve the impaired
microenvironment induced by central nervous system disease, and to
regulate neurotransmitters and neurotrophic factors (7). Our study in 2013 reported that MSC
transplantation suppressed the dopamine system and decreased the
dopamine levels in the striatum of TS rats (8).
Neurotrophic factors involve in the endogenous
protective process of brain injury. Brain-derived neurotrophic
factor (BDNF) is one of the most important members of the
neurotrophin family and is able to mediate the neuronal growth and
differentiation, synapse formation and plasticity, and higher
cognitive functions (9). In the
present study, the effect of MSC transplantation on the BDNF levels
in TS rats was investigated and the possible underlying mechanisms
involved in the MSC transplantation were assessed.
Materials and methods
Animals
A total of 72 Wistar rats (age, 7 weeks; weight,
205–220 g), obtained from Vital River Laboratory Animal Technology
Co., Ltd. (Beijing, China), were acclimatized for 1 week prior to
the initiation of the experiments. The animals were housed in a
controlled environment, at a room temperature of 21±1°C and a 12-h
light/dark cycle (lights on between 7:00 and 19:00), and had free
access to food and water. All experimental procedures were
performed in accordance with the NIH Guide for the Care and Use of
Laboratory Animals.
MSC preparation and flow cytometric
analysis
The long bones of an additional 6 adult Wistar rats
(supplied by Shandong University) were used to isolate mononuclear
cells, using density gradient centrifugation. In brief, the rat
MSCs were isolated by ficoll density gradient centrifugation at 902
× g for 20 min. The mononuclear cells, located in the middle layer
(1–2 mm thickness), were removed by the pipette and centrifuged
twice in phosphate-buffered saline (PBS; Sigma-Aldrich, St. Louis,
MO, USA) solution at 157 × g for 5 min. Then, mononuclear cells
were further cultured with media supplemented with FBS (Gibco;
Thermo Fisher Scientific, Inc., Grand Island, NY, USA). Initially,
the bones were removed by dissection, and their distal and proximal
ends were removed to reveal the marrow cavity. The obtained bone
marrow MSCs were cultured in low-glucose Dulbecco's modified
Eagle's medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented
with 15% FBS, 100 U/ml penicillin, and 100 mg/ml streptomycin
(Gibco; Thermo Fisher Scientific, Inc.). Next, the cells were
incubated at 37°C in 5% CO2 and fresh culture medium was
added every 3–4 days. Upon reaching 80% confluence, trypsin
(Sigma-Aldrich) was used to harvest the adherent cells, which were
then passaged. Flow cytometry was used to assess 3×107
MSCs at the third passage (FACSCalibur; BD Biosciences, USA). The
remaining MSC samples (1×106 cells) were co-incubated
with medium and 10 mg/ml bromodeoxyuridine (BrdU; Sigma-Aldrich), a
thymidine analog and marker of newly synthesized DNA, for 24 h
prior to transplantation, in order to label the MSCs. The cells
were then harvested, resuspended in PBS at a density of
1×105 cells/µl and stored on ice until required for
grafting.
Serum of TS patients
A previous study revealed that a high concentration
of antibasal ganglia antibody (ABGA) is present in the sera of TS
patients, which may result in impairments of the striatum, as well
as stereotypic behavior (10). In
the present study, serum samples from 8 TS patients (male, 4;
female, 4; age range, 8–13 years; mean age, 10.3 years) were
obtained from the serum bank of the Yuhuangding Hospital of Qingdao
University (Yantai, China). At the time of blood collection, none
of the subjects were taking psychostimulants. Serum samples were
collected according to a protocol approved by the Institutional
Review Board and subsequent to obtaining written informed consent
from all the patients. Enzyme-linked immunosorbent assay (ELISA)
was performed, as previously described (11), to determine the optical density of
the ABGA in the TS patients selected for microinfusion, which was
found to be 0.23±0.07 U/l. The patient serum was obtained and
injected into rats in order to increase their ABGA concentration
and establish a TS model.
Animal preparation and in vivo
surgery
In the present study, an autoimmune TS rat model was
established, as previously described (10). The 72 Wistar rats were randomly
allocated to the control (sham surgery group, microinfused with
normal sera; n=24) and two experimental groups, including the
TS+vehicle and TS+MSC groups (n=24 each). MSCs were co-cultured
with BrdU for 24 h for labeling prior to grafting. Normal serum
normal blood samples were obtained from the serum library.
Rats were deeply anesthetized with chloral hydrate
(400 mg/kg, intraperitoneal injection) and placed in a stereotaxic
apparatus (Stoelting Co., Wood Dale, IL, USA), with the incisor bar
set at 3.5 mm below the interaural line. Through a surgical aseptic
technique, the skull was exposed and holes were drilled in
appropriate locations, following which a 28-gauge guide cannula was
implanted into the bilateral striata. The cannula was placed at the
following coordinates: At 2.0 mm anterior-posterior from bregma,
4.0 mm medial-lateral and −7.0 mm dorsoventral from the skull. The
rats were provided with appropriate postsurgical care, with a diet
supplemented with egg yolk and fresh fruit, in order to maintain
their body weight.
The animals were allowed to recover for 1 week to
reestablish the integrity of the blood-brain barrier. Following
recovery, Alzet osmotic mini-pumps (Durect Corporation, Palo Alto,
CA, USA) filled with PBS were connected to each cannula using a
polyethylene tube that was loaded with 50 µl undiluted TS or
control serum, under sterile conditions. The serum was infused into
the rats at a rate of 0.5 µl/h for 72 h, after which the rats were
sedated with chloral hydrate (100 mg/kg, intraperitoneal injection)
and placed in the stereotaxic apparatus. The skull was exposed
through an incision along the midline and the osmotic mini-pump was
removed. In the TS+MSC group, 5 µl/site MSC suspension
(105 cells/µl) was bilaterally injected into the
sera-infusion sites, one on each side of the rat striatum. After 5
min, the needle was slowly removed and a surgical suture was used
to close the wound. Each grafted animal received a total of
106 MSCs, with 5×105 MSCs injected into each
side of the striatum. For sham grafting, rats in the TS+vehicle
group were subjected to the same grafting procedure, but received a
vehicle infusion of an equal volume of PBS, rather than MSC
suspension. Subsequently, the TS rats were intramuscularly
administered 65,000 units of sodium penicillin (Hebei
Chengshengtang Animal Pharmaceutical Co., Ltd., Hebei, China) and
were maintained on a thermal pad until awakened, after which they
were returned to their cages. The animals underwent behavioral
assessment tests and were then sacrificed for BDNF detection at 1,
7, 14 and 28 days after transplantation, with 6 rats from each
group sacrificed at the different time points.
Assessment of stereotypy
Following the completion of the light cycle, audio
and video recordings of the rat movements were obtained for 30 min.
Stereotypies were recorded based on previously reported
instructions (12,13), and these included head shaking,
self-gnawing, biting (which was identified by wood chips, teeth
touching the cage, vacuous chewing or biting other objects
excluding the rats' body), episodic utterances, grooming, paw
shaking, taffy pulling (forepaw to the mouth and face), licking and
rearing. Grooming behavior was recorded according to the number of
minutes for which grooming occurred. Episodic utterance was
determined as repeated medium-pitched sounds of short duration. The
aforementioned stereotypic movements were recorded at 1, 7, 14 and
28 days after transplantation and a total score was determined for
each rat based on the sum of the observed stereotypic movements.
The recordings of the rats were reviewed and quantified by a
researcher who was trained to identify the aforementioned
stereotypies and was blinded to the graft and serum microinfusion
details of the rats.
BDFN detection using reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
RT-qPCR was performed to determine the mRNA
expression of BDNF in the striatum tissues isolated from the rats
at 1, 7, 14 and 28 days after transplantation. Total RNA was
extracted from the striatum according to the manufacturer
instructions of the TRIzol kit (Guangzhou Dongsheng Biotech Co.,
Ltd., Guangzhou, China). Subsequently, total RNA was reverse
transcribed into cDNA in a total reaction volume of 20 µl, using
PrimeScript™ RT reagent Kit (RR037A; Takara Bio, Inc., Otsu,
Japan). The annealed mixture had a volume of 15.5 µl, including 2
µg RNA, 1 µl 0.5 g/l oligo dT and 1 µl dNTPs, whereas the RT
reaction solution was comprised of 2 µl 10X RT buffer, 1 µl
dithiothreitol, 0.5 µl RNA inhibitors and 1 µl M-MLV reverse
transcriptase. The RT reaction was performed in a 37°C water bath
for 60 min, and then incubated at 70°C for 15 min.
Next, qPCR was performed in a total volume of 20 µl,
comprising 2 µl DNA template, 10 µl Platinum SYBR Green qPCR
SuperMix (2X; Thermo Fisher Scientific, Inc., Carlsbad, CA, USA),
0.4 µl downstream PCR primers (10 µΜ), 0.4 µl ROX Reference Dye
(50X; Thermo Fisher Scientific, Inc.) and 6.8 µl double-distilled
H2O. The primers used in qPCR were designed by Beijing
Zhongshan Golden Bridge Biotechnology Co., Ltd. (Beijing, China)
according to the GenBank sequences (MIM113505), as follows: BDNF
(466 bp), 5′-TCCCTGGCTGACACTTTTGAG-3′ (sense) and
5′-ATTGGGTAGTTCGGCATTGCG-3′ (antisense); β-actin (336 bp),
5′-GCAGAAGGAGATCACAGCCCT-3′ (sense) and 5′-GCTGATCCACATCTGCTGGAA-3′
(antisense). The reaction system was divided into a 96-well optical
plate and covered with a special optical film (Thermo Fisher
Scientific, Inc.). Applied Biosystems 7500 Real-Time PCR system
(Thermo Fisher Scientific, Inc.) was used for qPCR cycling, as
follows: 50°C for 2 min, 95°C for 2 min, then 45 cycles of 95°C for
15 sec and 60°C for 34 sec, followed by 95°C for 15 sec, 60°C for 1
min, and 95°C for 15 sec. The mRNA expression levels were
quantified using ABI Prism 7000 software (Thermo Fisher Scientific,
Inc.).
BDNF detection using sandwich
ELISA
BDNF ELISA kits were used to measure the BDNF
protein expression (RAB0026; Sigma-Aldrich). All procedures were
conducted according to standard guidelines provided by the
manufacturer. The plates included the following samples: i) Blank
wells, including a biotin-labeled anti-BDNF antibody and
streptavidin-biotin-horseradish peroxidase (HRP); ii) standard
wells, including 50 µl standard and 50 µl streptavidin-biotin-HRP;
iii) sample wells, including 40 µl sample, followed by addition of
10 µl anti-BDNF antibody and 50 µl streptavidin-biotin-HRP. The
plates were covered using a closure plate membrane, gently shaken,
incubated at 37°C for 60 min and then washed. Next, the plates were
carefully uncovered to discard the liquid, and then dried and
washed. The aforementioned procedure was repeated 5 times and then
the plates were dried with blotting paper to remove any unbound
enzyme-labeled antibody. Subsequently, 50 µl chromogenic reagent A
was added to each well, followed by 50 µl color reagent B, and the
plate were gently mixed, 37°C color for 10 min. To terminate the
reaction, 50 µl stop solution was added to terminate the reaction.
Reagents A and B and the stop solution were included in the ELISA
kit (Sigma-Aldrich). After 10 min, the absorbance of each well in
terms of the optical density (OD) was measured at 450 nm, setting
the OD of the blank well to zero. Each plates concentration was
corrected by means of its standard curves dilution factor. A
CCL-2600C microplate reader (Guangzhou Cancare Medical Trading Co.,
Ltd., Guangzhou, China) was used to determine BDNF absorbance
values at 450 nm. The standard linear regression equation was
calculated based on the concentration and corresponding OD values,
and then the sample BDNF concentration was calculated from the
regression equation.
Statistical analysis
All statistical analyses were performed using SPSS
(version 13.0 for Windows; SPSS Inc, Chicago, IL, USA). Data are
reported as the mean ± standard deviation. Statistical analysis was
performed by repeated measurement analysis of variance in order to
evaluate the stereotypy counts at different time points. A P-value
of <0.05 was considered to indicate a statistically significant
difference.
Results
Assessment of stereotypy
The results indicated that stereotypic TS behavior
was successfully established in the rats following intrastriatal
microinfusion of serum from TS patients. Stereotypic TS behavior
was recorded and quantified in the rats during a 30-min observation
period at 1, 7, 14 and 28 days after transplantation (Fig. 1). Statistical analysis indicated that
rats in the two TS groups exhibited significantly higher
stereotypic behavioral counts compared with those in the sham
surgery group (P<0.05). In addition, TS rats with MSC grafts
exhibited significantly decreased stereotypic behavior at 1 week
after transplantation (P<0.05; Fig.
1).
Effects of MSC transplantation on the
TS rat striatum neurotrophic factor
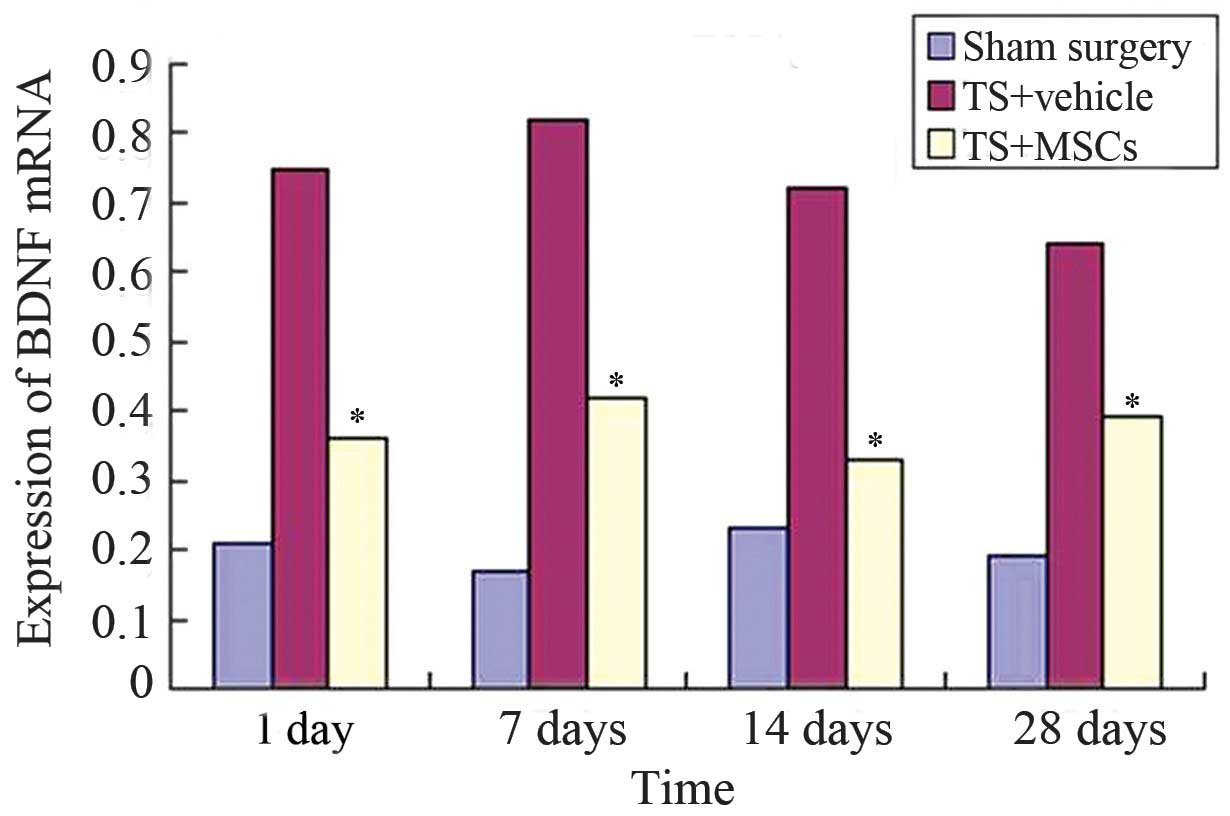
The mRNA expression levels in the rat striatum were
determined using RT-qPCR. Compared with the sham surgery group, the
mRNA expression levels of BDNF in the striatum of two TS rat groups
showed high levels (P<0.05). However, BDNF mRNA expression was
found to be reduced in the rat striatum following MSC
transplantation (TS+MSCs group), compared with that in the
TS+vehicle group (Fig. 2).
ELISA was also used to determine the BDNF protein
expression in the striatum of TS rats. At 28 days after
transplantation, BDNF levels were found to be significantly
increased in the TS+MSC group (3.29±0.58 ng/g) compared with those
in the sham surgery group (1.67±0.31 ng/g; P<0.05). In addition,
the TS+MSC rats had significantly lower BDNF levels compared with
the TS+vehicle rats (2.59±0.52 ng/g vs. 3.29±0.58 ng/g,
respectively; P<0.05) (Fig.
3).
Therefore, the findings of the present study
demonstrated that transplantation of MSCs was able to reduce the
levels of BDNF in the TS rats and alleviate the symptoms of TS.
Discussion
Current therapeutic approaches for the treatment of
TS include psychological and behavioral therapy, drug
administration, immunomodulatory therapy and neurosurgery (14). Patients with malignant TS experience
self-injurious and life-threatening symptoms, which can not be
managed by neurosurgical procedures (such as deep brain
stimulation) or conservative treatments. Effective novel strategies
for the treatment of TS patients must be developed; thus, the
present study investigated an MSC transplantation procedure that
was performed into the striatum of a rat TS model. The results
demonstrated that transplantation of MSCs resulted in significant
improvement of stereotypic behavior in the TS rats (5).
The underlying mechanisms of the MSC transplantation
action in the treatment of central nervous system disorders may be
as follows (15): i) MSCs may
directionally migrate to the injury site and differentiated into
Nestin-positive cells, replacing any damaged or dead nerve cells;
ii) MSCs may regulate neurotrophic factors, including nerve growth
factor and BDNF, which can regulate neuronal survival, mediate
axonal growth and regulate neurotransmitter generation; and iii)
MSCs may function via immunomodulation.
In recent years, the role of neurotrophic factors in
the pathogenesis and treatment of neuropsychiatric disorders was
emphasized (16). A neurotrophic
factor is produced by the body and is able to promote nerve cell
survival, growth and differentiation. BDNF is a member of the
neurotrophic factor family and serves a key role in maintaining the
normal physiological function of neurons. BDNF promotes
neurotransmitter release and enhances neuromuscular transmission
excitation-contraction coupling. In addition, it regulates ion
channel activity through the high-affinity receptor protein, TrkB,
and the low-affinity receptor protein, p75 (17,18).
In the present study, according to the autoimmune
mechanism of TS, the rat striata were infused with serum from TS
patients, which contained a high concentration of ABGA, resulting
in striatal dysfunction and various stereotypic behaviors. A number
of endogenous neuroprotective responses are known to be generated
against noxious stimuli (19), with
nutrition regulation being an important neuroprotective mechanism.
In addition, noxious stimulation of the brain can cause
upregulation of BDNF (20). BDNF
stabilizes the metabolic function of damaged neurons, in which
synthesis and metabolism at low levels (21), and enhances neuron resilience to
hypoxia and survival in a damaged environment. Furthermore, BDNF is
involved in the development of striatal neurons,
nutrition-associated maintenance and neuroprotection (22,23).
Antigen-antibody immune response may lead to compensatory
protective response in the TS rat brain, which is associated with
the increased brain BDNF expression levels.
ABGA is able to destroy cells and cause
corresponding neurological deficits. However, it may also increase
the expression and secretion of endogenous neuroprotective factors,
and ultimately induce the expression of neurotrophic factors, such
as BDNF, initiating the self-protection and repair mechanisms of
the body (24).
BDNF has been reported to have a significant
nutritional effect on dopamine neurons and to increase the number
of dopamine receptors in the brain (25). In certain cases, excess BDNF is
harmful. Excessive expression of BDNF may damage the neural
circuitry, which could affect memory and cognitive function,
increasing the risk of seizures (26). Dopamine receptor levels have been
shown to increase in the brains of infants with TS (27). The experimental results of the
current study showed that BDNF levels were elevated in TS rats.
Thus, we hypothesize that BDNF participates in the pathogenesis of
TS by increasing the number of dopamine receptors and by
upregulating the excitatory ion channels and downregulating the
inhibitory ion channels in order to release neurotransmitters.
MSC has immunosuppressive properties in vivo.
In an inflammation environment, MSC may induce the immune
regulation process, limit inflammation to facilitate self-survival
and form an immunosuppressive microenvironment. Furthermore, MSC
has anti-inflammatory and immunomodulatory effects, and inhibits
the proliferation of T lymphocytes (28,29).
The present results indicate that antigen-antibody
reaction may cause immune damage and lead to a series of
stereotypic behaviors in autoimmune TS rats. Upon transplantation
into the striatum of TS rats, MSCs inhibit the immune response and
repair local damage in the microenvironment to restore homeostasis,
resulting in the elevated BDNF levels in TS rats returning to the
normal levels. In the present study, following MSC transplantation,
the BDNF level was reduced in the striatum of TS rats. The
excitatory neurotransmitter channels were closed and the inhibiting
ion channels were increased, thereby improving neuromotor function
and reducing stereotypy in the TS rats. In conclusion, MSC
transplantation in TS rats may reduce BDNF levels and reduce the
stereotyped behavior. However, the mechanism underlying MSC
transplantation for the treatment of TS requires further study.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (no. 81101017), the Department
of Science and Technology of Shandong Province (no. BS2010F030),
and the Yantai Science and Technology Development project (no.
2010148-24).
References
|
1
|
Tamara P: Tourette syndrome and other tic
disorders of childhood. Handb Clin Neurol. 112:853–856. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cheung MY, Shahed J and Jankovic J:
Malignant tourette syndrome. Mov Disord. 22:1743–1750. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lv W, Li WY, Xu XY, Jiang H and Bang OY:
Bone marrow mesenchymal stem cells transplantation promotes the
release of endogenous erythropoietin after ischemic stroke. Neural
Regen Res. 10:1265–1270. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Choi HS, Kim HJ, Oh JH, Park HG, Ra JC,
Chang KA and Suh YH: Therapeutic potentials of human
adipose-derived stem cells on the mouse model of Parkinson's
disease. Neurobiol Aging. 36:2885–2892. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu X, Wang Y, Li D and Ju X:
Transplantation of rat neural stem cells reduces stereotypic
behaviors in rats after intrastriatal microinfusion of Tourette
syndrome sera. Behav Brain Res. 186:84–90. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu XM, Wang YW and Yi MJ: Effects of
human mesenchymal stem cell transplantation in the bilateral corpus
striatum in a rat model of Tourette's syndrome. Neural Regen Res.
5:1285–1290. 2010.
|
|
7
|
van Velthoven CT, Braccioli L, Willemen
HL, Kavelaars A and Heijnen CJ: Therapeutic potential of
genetically modified mesenchymal stem cells after neonatal
hypoxic-ischemic brain damage. Mol Ther. 22:645–654. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu X, Wang X, Li L, Wang H and Jiao X:
Influence of mesenchymal stem cell transplantation on stereotypic
behavior and dopamine levels in rats with Tourette syndrome. PLoS
One. 8:e621982013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen A, Xiong LJ, Tong Y and Mao M: The
neuroprotective roles of BDNF in hypoxic ischemic brain injury.
Biomed Rep. 1:167–176. 2013.PubMed/NCBI
|
|
10
|
Hornig M and Lipkin WI: Immune-mediated
animal models of Tourette syndrome. Neurosci Biobehav Rev.
37:1120–1138. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Singer HS, Loiselle CR, Lee O, Minzer K,
Swedo S and Grus FH: Anti-Basal Ganglia Antibodies in PANDAS. Mov
Disord. 19:406–415. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Macri S, Onori MP, Roessner V and Laviola
G: Animal models recapitulating the multifactorial origin of
Tourette syndrome. Int Rev Neurobiol. 112:211–237. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Taylor JL, Rajbhandari AK, Berridge KC and
Aldridge JW: Dopamine receptor modulation of repetitive grooming
actions in the rat: Potential relevance for Tourette syndrome.
Brain Res. 1322:92–101. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Malaty IA and Akbar U: Updates in medical
and surgical therapies for Tourette syndrome. Curr Neurol Neurosci
Rep. 14:4582014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Phinney DG and Isakova IA: Mesenchymal
stem cells as cellular vectors for pediatric neurological
disorders. Brain Res. 1573:92–107. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang F, Wang YY, Liu H, Lu YF, Wu Q, Liu
J and Shi JS: Resveratrol produces neurotrophic effects on cultured
dopaminergic neurons through prompting astroglial BDNF and GDNF
release. Evid Based Complement Alternat Med. 2012:9376052012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kordi-Tamandani DM, Sahranavard R and
Torkamanzehi A: DNA methylation and expression profiles of the
brain-derived neurotrophic factor (BDNF) and dopamine transporter
(DAT1) genes in patients with schizophrenia. Mol Biol Rep.
39:10889–10893. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ferrini F and De Koninck Y: Microglia
control neuronal network excitability via BDNF signalling. Neural
Plast. 2013:4298152013.PubMed/NCBI
|
|
19
|
Paulsen JS: Cognitive impairment in
Huntington disease: Diagnosis and treatment. Curr Neurol Neurosci
Rep. 11:474–483. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shimada H, Makizako H, Doi T, Yoshida D,
Tsutsumimoto K, Anan Y, Uemura K, Lee S, Park H and Suzuki T: A
large, cross-sectional observational study of serum BDNF, cognitive
function and mild cognitive impairment in the elderly. Front Aging
Neurosci. 6:692014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li Y, Yui D, Luikart BW, McKay RM, Li Y,
Rubenstein JL and Parada LF: Conditional ablation of brain-derived
neurotrophic factor-TrkB signaling impairs striatal neuron
development. Proc Natl Acad Sci USA. 109:15491–15496. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Niitsu T, Shirayama Y, Matsuzawa D,
Hasegawa T, Kanahara N, Hashimoto T, Shiraishi T, Shiina A, Fukami
G, Fujisaki M, et al: Associations of serum brain-derived
neurotrophic factor with cognitive impairments and negative
symptoms in schizophrenia. Prog Neuropsychopharmacol Biol
Psychiatry. 35:1836–1840. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mang CS, Campbell KL, Ross CJ and Boyd LA:
Promoting neuroplasticity for motor rehabilitation after stroke:
Considering the effects of aerobic exercise and genetic variation
on brain-derived neurotrophic factor. Phys Ther. 93:1707–1716.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Martino D, Chiarotti F, Buttiglione M,
Cardona F, Creti R, Nardocci N, Orefici G, Veneselli E and Rizzo R:
Italian Tourette Syndrome Study Group: The relationship between
group A streptococcal infections and Tourette syndrome: A study on
a large service-based cohort. Dev Med Child Neurol. 53:951–957.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yeh TK, Hu CY, Yeh TC, Lin PJ, Wu CH, Lee
PL and Chang CY: Association of polymorphisms in BDNF, MTHFR, and
genes involved in the dopaminergic pathway with memory in a healthy
Chinese population. Brain Cogn. 80:282–289. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Binder DK, Croll SD, Gall CM and Scharfman
HE: BDNF and epilepsy: Too much of a good thing? Trends Neurosci.
24:47–53. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Denys D, de Vries F, Cath D, Figee M,
Vulink N, Veltman DJ, van der Doef TF, Boellaard R, Westenberg H,
van Balkom A, et al: Dopaminergic activity in Tourette syndrome and
obsessive-compulsive disorder. Eur Neuropsychopharmacol.
23:1423–1431. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Stagg J and Galipeau J: Mechanisms of
immune modulation by mesenchymal stromal cells and clinical
translation. Curr Mol Med. 13:856–867. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Brennen WN, Denmeade SR and Isaacs JT:
Mesenchymal stem cells as a vector for the inflammatory prostate
microenvironment. Endocr Relat Cancer. 20:R269–R290. 2013.
View Article : Google Scholar : PubMed/NCBI
|