Introduction
Intervertebral disc degeneration (IDD), which is
considered to be a global health threat, is a gateway for other
disc-related diseases and is associated with significant healthcare
costs (1,2). IDD precedes, or is associated with,
numerous clinical conditions, including lower back or limb pain,
spinal stenosis, and disc herniation (3). Clinically, IDD is characterized by a
loss of intervertebral disc (IVD) height and structural failure,
due to proteolytic degradation of the extracellular matrix (ECM)
and infiltration of blood vessels and nerve fibers (2,4). IDD is
generally accepted as an inescapable consequence of the aging
process; it affects 90–100% of individuals aged >63 years, and
is the predominant cause of ~40% of cases of lower back pain
(5,6). As a multifactorial disease, IDD may be
caused by genetic or environmental factors, among which mechanical
stress to the spine is the primary factor, although age, heavy
lifting, smoking, and biochemical influences may also contribute to
the risk of IDD (7,8). Currently, there is no cure for IDD, and
it is instead managed via surgical procedures, including vertebral
fusion and disc excision, which may relieve the pain in the short
term but alter the biomechanics of the spine in the long term,
resulting in degeneration of the surrounding tissues and adjacent
discs (9,10). Miyagi et al (11) detected elevated levels of
interleukin-6 (IL-6) in the sera of patients with IDD; therefore,
the present meta-analysis aimed to investigate the association of
IL-6 with IDD, in order to hasten the development of early
therapeutic intervention strategies.
IL-6 is a 26-kDa protein, which was initially
described as a B-cell activating factor secreted by T-cells, and
can be mapped to chromosome 7p15-p21 (12). IL-6 is a well-established
pro-inflammatory cytokine that stimulate the growth and
proliferation of numerous immune cell types during host immune
defense responses (13).
Furthermore, it is considered to be a key regulator of human
chronic inflammatory diseases (14).
IL-6 is able to initiate various effects on the nervous system,
vascular tissue, immune response and stress response, by modulating
gene expression and cell survival, proliferation, and
differentiation (15). Numerous
studies have associated elevated serum levels of IL-6 with
cardiovascular diseases, type 2 diabetes, rheumatoid arthritis,
multiple sclerosis, Crohn's disease, and lymphatic, renal, bladder
and colorectal cancers (13,16). In addition, IL-6 has been associated
with IDD pathogenesis (17–19); however, Liu et al (20) was unable to detect an association
between IL-6 and disc-associated disorders. Within a meta-analysis
framework, the present study investigated the correlation between
serum IL-6 protein expression levels and IDD pathology, with the
intention of developing early intervention strategies.
Materials and methods
Search strategy
The present study was conducted on the basis of the
guidelines of the Preferred Reporting Items for Systematic Reviews
and Meta-analysis (PRISMA guidelines; http://www.prisma-statement.org/). A comprehensive and
systematic literature search of numerous electronic databases,
including PubMed (http://www.ncbi.nlm.nih.gov/pubmed/), EBSCO
(http://search.ebscohost.com/), Ovid
(http://gateway.ovid.com/), Medline (http://www.medline.com/), Springerlink (http://link.springer.com/), Wiley Online Library
(http://onlinelibrary.wiley.com/), Web of
Science (http://wok.mimas.ac.uk/), the Chinese
Biomedical Database (http://www.sinomed.ac.cn/), the Chinese Journal
Full-Text Database (part of the China National Knowledge
Infrastructure; http://www.cnki.net/), the Wanfang
Database (http://www.wanfangdata.com.cn/) and the VIP Database
(http://www.cqvip.com/), was performed. In
addition, the reference lists from numerous original and review
articles were manually searched. A combination of Medical Subject
Headings, Medline medical index terms and free words, were used to
retrieve studies broadly associated with the topic of interest. The
search criteria were as follows: ‘Intervertebral disc
degeneration’; ‘intervertebral disk degeneration’; ‘disk
degeneration’; ‘disk degradation’; ‘disc degeneration’; ‘disc
degradation’; ‘degeneration, disc’; ‘degenerative intervertebral
disc’; ‘degenerative intervertebral disks’; ‘lumbar disc
herniation’; ‘disc herniation’; ‘disk herniation’; ‘cervical disc
herniation’; ‘lumbar intervertebral disc herniation’ or ‘lumbar
intervertebral disk herniation’; and ‘interleukin-6’; ‘plasmacytoma
growth factor’; ‘B-cell differentiation factor-2’; ‘B-cell
stimulatory factor 2’; ‘BSF-2’; ‘hepatocyte-stimulating factor’;
‘hybridoma growth factor’; ‘IFN-beta 2’; ‘IL-6’; ‘IL 6’; ‘MGI-2’;
‘myeloid differentiation-inducing protein’; ‘B cell stimulatory
factor-2’; or ‘B-cell differentiation factor’.
Inclusion and exclusion criteria
The published studies were included in the
meta-analysis if they met the following inclusion criteria: i) It
was a case-control study, which had investigated the association
between the expression levels of IL-6 and IDD; ii) the study had
included patients with IDD and healthy controls; iii) complete
data, including sample size, age, ethnicity, gender, pathological
types and the IL-6 protein expression levels, were available; and
iv) only the study with the largest sample size or the most recent
study was selected when the retrieved studies were published by the
same authors using identical case materials. The exclusion criteria
were as follows: i) The study was not relevant to the study topic;
ii) it was not a case-control study; iii) the data was incomplete;
iv) the study was from a non-English or non-Chinese publication; or
v) the study was a duplicate.
Data extraction and quality
assessment
Two independent investigators used a standard
reporting form to extract data from each study. The following
information was collated: Initials of the first author, year of
submission, the country of submission, language, ethnicity, study
design, disease, protein detection method, total number of included
cases, age, expression levels of IL-6, and the type of IDD.
Disagreements were resolved by reaching a consensus following a
discussion with numerous investigators during data extraction. The
quality of the selected studies were estimated independently by two
investigators based on the critical appraisal skills program (CASP)
score criteria (http://www.casp-uk.net/), which are as follows: i)
Unambiguous study issue (CASP01); ii) an appropriate method was
used to answer study issue (CASP02); iii) acceptable case selection
(CASP03); iv) acceptable selection of controls (CASP04); v)
accurate measure of exposure factors for minimizing bias (CASP05);
vi) other confounding factors were controlled (CASP06); vii)
complete study results were available (CASP07); viii) precise study
results (CASP08); ix) credible study results (CASP09); x) suitable
study results for the local population (CASP10); and xi) consensus
between the study results and other evidence (CASP11).
Statistical analysis
The correlations between the IL-6 protein expression
levels and IDD were estimated using the standardized mean
difference (SMD) and the corresponding 95% confidence interval (95%
CI). The pooled SMDs were determined using the Z-test. The
heterogeneity among the various studies was quantified using the
Cochran's Q-statistic and the I2 index (21,22). A
random-effects model was used in the case of significant
heterogeneity (P<0.05 or I2>50%), and a
fixed-effects model was used in the case of non-significant
heterogeneity (23). The potential
sources of heterogeneity were assessed via single-factor or
multi-factor meta-regression analyses, and the Monte Carlo
simulation was used for multiple calibration tests. Subsequently,
sensitivity analyses were performed, in order to evaluate whether
removing a single study influenced the results. Funnel plots
alongside the Egger's linear regression test were used to detect
the publication bias of the selected studies. All statistical
analyses were performed using the STATA version 12.0 software
(StataCorp LP, College Station, TX, USA).
Results
Baseline characteristics of included
studies
Fig. 1 demonstrates
the screening process of the included studies. A total of 112
publications were retrieved through electronic database and manual
searches, of which 104 were excluded for the following reasons: 14
studies were duplicates; two were letters or review articles; 11
involved non-human subjects; 44 were not associated with the topic
of interest; 11 had insufficient data; and 22 possessed data of a
low relevancy. Eight eligible case-control studies (24–31) were
pooled for the present meta-analysis, which analyzed the serum IL-6
protein expression levels in 263 IDD cases (with bulging,
protrusion and sequestered IDD subtypes), as compared with 129
controls. The eight studies were published between 2002 and 2014,
and included five studies involving Chinese subjects and three
studies involving Caucasians (one of each from Turkey, Italy and
Brazil). All of the included studies determined serum IL-6 protein
expression levels using ELISA. The baseline characteristics and
CASP quality assessments of the selected studies are presented in
Table I and Fig. 2, respectively.
 | Table I.Baseline characteristics of the
included studies. |
Table I.
Baseline characteristics of the
included studies.
|
|
|
|
| Case | Control |
|---|
|
|
|
|
|
|
|
|---|
| First author
(reference) | Year | Ethnicity | Total | N | Age (years) | Gender (M/F) | N | Age (years) | Gender (M/F) |
|---|
| Ouyang (24) | 2014 | Asian | 70 | 50 | 43.9 (22–58) | 31/19 | 20 | 41.4 (27–65) | 15/5 |
| Ding (25) | 2014 | Asian | 50 | 30 | 41.32±7.24 | 19/11 | 20 | 42.35±6.49 | 12/8 |
| Li (26) | 2010 | Asian | 80 | 60 | 42.7±11.4 | 38/22 | 20 | 41.5±9.3 | 13/7 |
| Kraychete (27) | 2010 | Mixed | 33 | 23 | 42.8±7.0 | 12/11 | 10 | 39.5±4.5 | 6/4 |
| Song (28) | 2009 | Asian | 50 | 30 | 46.1 (34–57) | 22/8 | 20 | 42.5 (29–60) | 15/5 |
| Demircan (29) | 2007 | Caucasian | 32 | 12 | 42–63 | 20/12 | 20 | – | – |
| Dong (30) | 2004 | Asian | 40 | 28 | 45.4 (35–53) | 21/7 | 12 | – | 9/3 |
| Specchia (31) | 2002 | Caucasian | 37 | 30 | 40.7±6.3 | 19/11 | 7 | 42.2±5.9 | – |
Patients with IDD vs. normal individuals. Tests
indicated the existence of heterogeneity in the IL-6 protein
expression levels of patients with IDD; therefore, a random-effects
model was used (P<0.001; I2=95.9%). The IL-6 protein
expression levels markedly correlated with IDD (SMD=3.55; 95%
CI=1.77–5.32; P<0.001; Fig.
3A).
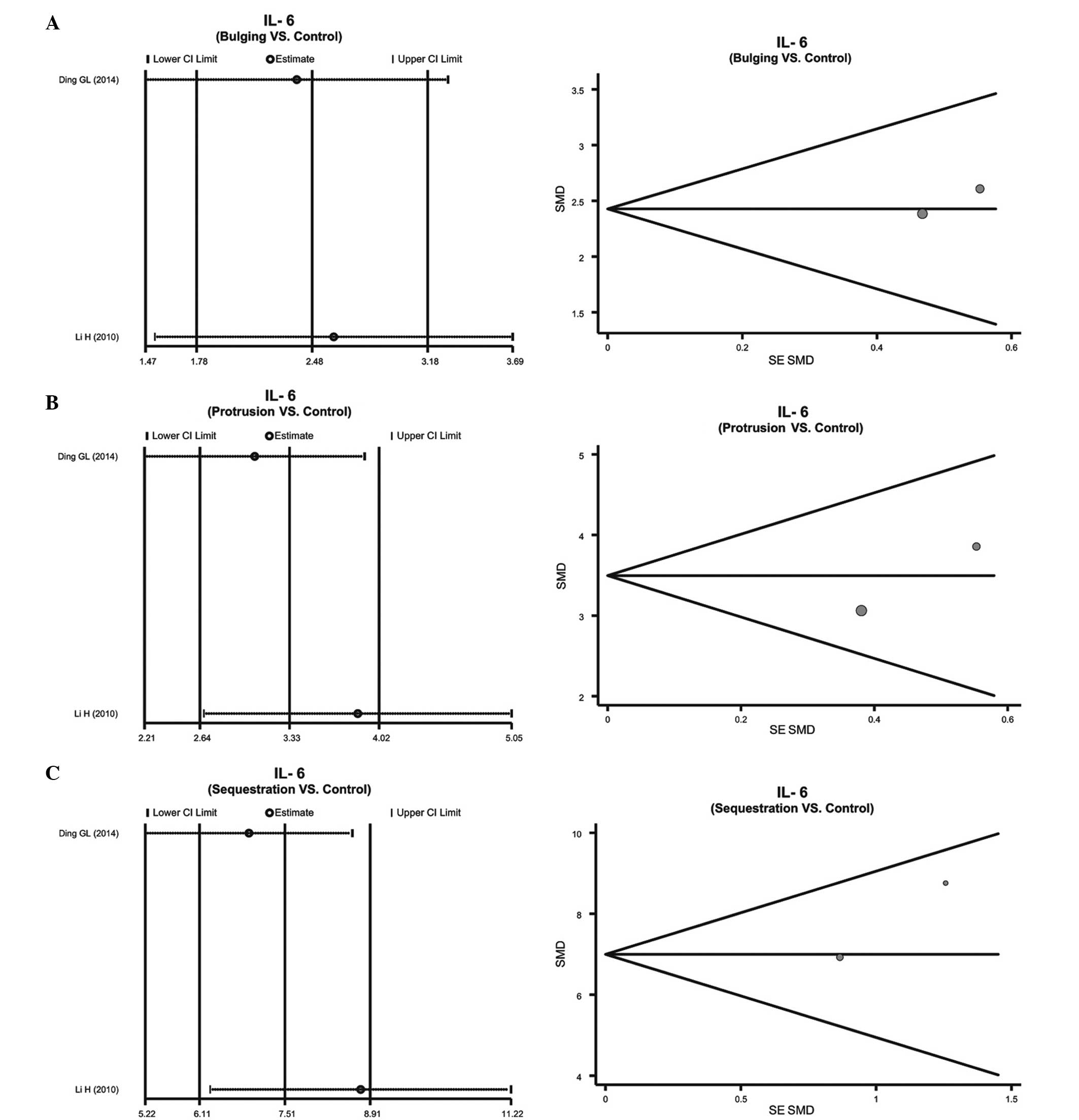
Patients with IDD subtypes vs. normal individuals. A
fixed-effects model was used due to the lack of heterogeneity in
the IL-6 protein expression levels in patients with the various IDD
subtypes, including bulging, protrusion and sequestered IDD
(bulging: P=0.757, I2=0.0%; protrusion: P=0.285,
I2=12.4%; sequestered: P=0.230, I2=30.6%).
All of the IDD subtypes exhibited upregulation of the IL-6 protein,
as compared with the controls, which gradually increased with the
severity of IDD (bulging: SMD=2.48, 95% CI=1.78–3.18, P<0.01;
protrusion: SMD=3.33, 95% CI=2.64–4.02, P<0.01; sequestered:
SMD=7.51, 95% CI=6.11–8.91, P<0.01; Fig. 3B–D).
Sensitivity analysis and publication
bias
The sensitivity analyses demonstrated that no single
publication exerted influence on the SMD comparisons between
patients with IDD and healthy individuals (Fig. 4), and between patients with the
various subtypes of IDD and healthy individuals (Fig. 5). The funnel plots of IL-6 protein
expression levels were symmetrical, and the Egger's linear
regression test indicated no publication bias (P>0.05) (Fig. 4). However, a specific P-value could
not be determined, due to only two studies having reported on the
expression levels of the IL-6 protein in the various IDD subtypes
(Fig. 5).
Regression analysis
The results from the single factor meta-regression
analysis suggested that the observed heterogeneity was independent
of the year of submission, sample size, country and language (year
of submission, P=0.589; sample size, P=0.688; country, P=0.300;
language, P=0.159) (Fig. 6), and the
results from multi-factor meta-regression analysis further
demonstrated that ethnicity, year of submission, language and
sample size did not contribute to the observed heterogeneity
(Table II).
 | Table II.Meta-regression analyses of potential
sources of heterogeneity. |
Table II.
Meta-regression analyses of potential
sources of heterogeneity.
|
|
|
|
|
| 95% CI |
|---|
|
|
|
|
|
|
|
|---|
| Heterogeneity
factors | Coefficient | SE | t | P-value
(adjusted) | LL | UL |
|---|
| Year | −0.876 | 0.564 | −1.55 | 0.476 | −3.301 | 1.55 |
| Sample size | 0.059 | 0.209 | 0.28 | 0.939 | −0.841 | 0.958 |
| Country | −2.712 | 3.37 | −0.8 | 0.703 | −17.214 | 11.789 |
| Language | −1.451 | 8.204 | −0.18 | 0.99 | −36.75 | 33.848 |
Discussion
IDD is a significant clinical and financial burden,
due to the high rate of disability associated with the disease;
pronounced physical defects occur in 10% of patients with IDD,
stressing the importance of understanding the onset and progression
of the disease (32). In the present
study, a meta-analysis of published studies from numerous databases
was conducted, in order to identify a definitive correlation
between serum IL-6 expression levels, and IDD disease pathogenesis
and severity. The protein expression levels of IL-6 were
consistently higher in IVD protrusion tissue, as compared with
normal IVD tissue. Furthermore, IL-6 protein expression levels were
demonstrated to increase with increasing disease severity; thus
suggesting that IL-6 may effect IDD disease progression. IVD
predominantly consists of an inner nucleus pulposus (NP),
surrounded by the annulus fibrosus and hyaline cartilaginous
end-plate, which lie between adjacent vertebrae in the spine. The
gelatinous NP is regarded as an avascular tissue containing ECM,
which consists of hydrated proteoglycan and collagen (33). IDD has previously been demonstrated
to be associated with degradation of the ECM in the NP, and
decreased levels of disc ECM proteoglycans (2). Furthermore, IDD has been associated
with alterations in the collagen type content of the ECM, decreased
water disc content, and local inflammatory reactions (34). In a previous analysis of human
articular cartilage, IL-6 inhibited proteoglycan synthesis, which
maintains high NP tissue hydration, and prevents blood and
lymphatic vessel ingrowth, under normal physiological conditions
(19). Degrading IVD tissue has been
demonstrated to secrete IL-6 and other inflammatory cytokines
(35); therefore, this may explain
the upregulated expression levels of IL-6 in IVD protrusion tissue,
as compared with normal IVD tissue.
In order to assess factors confounding the validity
of the results, the present meta-analysis investigated whether IL-6
protein expression levels varied between the different IDD clinical
subtypes (bulging, protrusion and sequestered), as compared with
the controls. The serum IL-6 expression levels were similarly
upregulated in IVD protrusion tissue, as compared with normal IVD
tissue, irrespective of the IDD subtype, and the IL-6 levels
increased with increasing severity of IDD.
The limitations of the present meta-analysis include
its similarity to other published meta-analyses and the small
sample size; of the 112 studies initially identified, only eight
met the inclusion criteria and this may have negatively impacted on
the reliability of the results. In addition, a subgroup analysis
based on ethnicity could not be performed, which may have indicated
ethnicity bias in the results.
In conclusion, the present meta-analysis
demonstrated that the protein expression levels of IL-6 were
upregulated in IVD protrusion tissue, as compared with normal IVD
tissue, and this was irrespective of the IDD subtype. Therefore,
IL-6 expression levels may effect the progression of IDD. Future
studies should endeavor to elucidate the underlying mechanism by
which IL-6 effects the onset and progression of IDD.
References
|
1
|
Wang HQ, Yu XD, Liu ZH, Cheng X, Samartzis
D, Jia LT, Wu SX, Huang J, Chen J and Luo ZJ: Deregulated miR-155
promotes Fas-mediated apoptosis in human intervertebral disc
degeneration by targeting FADD and caspase-3. J Pathol.
225:232–242. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee JM, Song JY, Baek M, Jung HY, Kang H,
Han IB, Kwon YD and Shin DE: Interleukin-1β induces angiogenesis
and innervation in human intervertebral disc degeneration. J Orthop
Res. 29:265–269. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siemionow K, An H, Masuda K, Andersson G
and Cs-Szabo G: The effects of age, sex, ethnicity, and spinal
level on the rate of intervertebral disc degeneration: A review of
1712 intervertebral discs. Spine (Phila Pa). 36:1333–1339. 2011.
View Article : Google Scholar
|
|
4
|
Akhatib B, Onnerfjord P, Gawri R, Ouellet
J, Jarzem P, Heinegård D, Mort J, Roughley P and Haglund L:
Chondroadherin fragmentation mediated by the protease HTRA1
distinguishes human intervertebral disc degeneration from normal
aging. J Biol Chem. 288:19280–19287. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Phillips KL, Jordan-Mahy N, Nicklin MJ and
Le Maitre CL: Interleukin-1 receptor antagonist deficient mice
provide insights into pathogenesis of human intervertebral disc
degeneration. Ann Rheum Dis. 72:1860–1867. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hughes SP, Freemont AJ, Hukins DW,
McGregor AH and Roberts S: The pathogenesis of degeneration of the
intervertebral disc and emerging therapies in the management of
back pain. J Bone Joint Surg Br. 94:1298–1304. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Adams MA and Dolan P: Intervertebral disc
degeneration: Evidence for two distinct phenotypes. J Anat.
221:497–506. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang M, Wang HQ, Zhang Q, Yan XD, Hao M
and Luo ZJ: Alterations of ADAMTSs and TIMP-3 in human nucleus
pulposus cells subjected to compressive load: Implications in the
pathogenesis of human intervertebral disc degeneration. J Orthop
Res. 30:267–273. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Erwin WM, Islam D, Inman RD, Fehlings MG
and Tsui FW: Notochordal cells protect nucleus pulposus cells from
degradation and apoptosis: Implications for the mechanisms of
intervertebral disc degeneration. Arthritis Res Ther. 13:R2152011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mwale F, Masuda K, Pichika R, Epure LM,
Yoshikawa T, Hemmad A, Roughley PJ and Antoniou J: The efficacy of
Link N as a mediator of repair in a rabbit model of intervertebral
disc degeneration. Arthritis Res Ther. 13:R1202011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Miyagi M, Ishikawa T, Orita S, Eguchi Y,
Kamoda H, Arai G, Suzuki M, Inoue G, Aoki Y, Toyone T, et al: Disk
injury in rats produces persistent increases in pain-related
neuropeptides in dorsal root ganglia and spinal cord glia but only
transient increases in inflammatory mediators: Pathomechanism of
chronic diskogenic low back pain. Spine (Phila Pa). 36:2260–2266.
2011. View Article : Google Scholar
|
|
12
|
Hirano T, Yasukawa K, Harada H, Taga T,
Watanabe Y, Matsuda T, Kashiwamura S, Nakajima K, Koyama K,
Iwamatsu A, et al: Complementary DNA for a novel human interleukin
(BSF-2) that induces B lymphocytes to produce immunoglobulin.
Nature. 324:73–76. 1986. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Neurath MF and Finotto S: IL-6 signaling
in autoimmunity, chronic inflammation and inflammation-associated
cancer. Cytokine Growth Factor Rev. 22:83–89. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ataie-Kachoie P, Pourgholami MH and Morris
DL: Inhibition of the IL-6 signaling pathway: A strategy to combat
chronic inflammatory diseases and cancer. Cytokine Growth Factor
Rev. 24:163–173. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yao J, Feng XW, Yu XB, Xie HY, Zhu LX,
Yang Z, Wei BJ, Zheng SS and Zhou L: Recipient IL-6-572C/G genotype
is associated with reduced incidence of acute rejection following
liver transplantation. J Int Med Res. 41:356–364. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Heikkilä K, Ebrahim S and Lawlor DA:
Systematic review of the association between circulating
interleukin-6 (IL-6) and cancer. Eur J Cancer. 44:937–945. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Studer RK, Vo N, Sowa G, Ondeck C and Kang
J: Human nucleus pulposus cells react to IL-6: Independent actions
and amplification of response to IL-1 and TNF-α. Spine (Phila Pa
1976). 36:593–599. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang KY, Lin RM, Chen WY, Lee CL, Yan JJ
and Chang MS: IL-20 may contribute to the pathogenesis of human
intervertebral disc herniation. Spine (Phila Pa 1976).
33:2034–2040. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hamamoto H, Miyamoto H, Doita M, Takada T,
Nishida K and Kurosaka M: Capability of nondegenerated and
degenerated discs in producing inflammatory agents with or without
macrophage interaction. Spine (Phila Pa 1976). 37:161–167. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu Z, Tang NL, Cao XB, Liu WJ, Qiu XS,
Cheng JC and Qiu Y: Lack of association between the promoter
polymorphisms of MMP-3 and IL-6 genes and adolescent idiopathic
scoliosis: A case-control study in a Chinese Han population. Spine
(Phila Pa 1976). 35:1701–1705. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jackson D, White IR and Riley RD:
Quantifying the impact of between-study heterogeneity in
multivariate meta-analyses. Stat Med. 31:3805–3820. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Peters JL, Sutton AJ, Jones DR, Abrams KR
and Rushton L: Comparison of two methods to detect publication bias
in meta-analysis. JAMA. 295:676–680. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zintzaras E and Ioannidis JP:
Heterogeneity testing in meta-analysis of genome searches. Genet
Epidemiol. 28:123–137. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ouyang B, Su JC, Zeng YD and Bao J:
Expression and significance of IL-6, IL-10 and MCP-1 in degenerate
human lumbar intervertebral disc tissues. Hainan Yixueyuan Xuebao.
3:381–383. 2014.(In Chinese).
|
|
25
|
Ding GL, Li XY and Lv MX: Expression and
clinical study of IL-1 and IL-6 in lumbar disc herniation.
Chongqing Yixue. 43:1919–1922. 2014.(In Chinese).
|
|
26
|
Li H: Expression and significance of
protrusion of intervertebral disc degeneration in patients with
lumbar disc tissue in IL-6, IL-8, TNF-alpha. Shangdong Yixue.
50:65–66. 2010.(In Chinese).
|
|
27
|
Kraychete DC, Sakata RK, Issy AM, Bacellar
O, Santos-Jesus R and Carvalho EM: Serum cytokine levels in
patients with chronic low back pain due to herniated disc:
Analytical cross-sectional study. Sao Paulo Med J. 128:259–262.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Song YZ and Deng XZ: Detection and
significance of lumbar intervertebral disc nucleus pulposus tissue
TNF-alpha, IL-1, IL-6. Shangdong Yixue. 49:87–88. 2009.(In
Chinese).
|
|
29
|
Demircan MN, Asir A, Cetinkal A, Gedik N,
Kutlay AM, Colak A, Kurtar S and Simsek H: Is there any
relationship between proinflammatory mediator levels in disc
material and myelopathy with cervical disc herniation and
spondylosis? A non-randomized, prospective clinical study. Eur
Spine J. 16:983–986. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dong GH, Huang JQ, Cai XJ, Han JH and Xia
BJ: Expression of IL-1 and IL-6 in the herniated lumbar disc
tissues. Zhongguo Yishi Zazhi. 6:1134–1135. 2004.(In Chinese).
|
|
31
|
Specchia N, Pagnotta A, Toesca A and Greco
F: Cytokines and growth factors in the protruded intervertebral
disc of the lumbar spine. Eur Spine J. 11:145–151. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wilke HJ, Urban J and Kümin M: The
benefits of multi-disciplinary research on intervertebral disc
degeneration. Eur Spine J. 23(Suppl 3): S303–S304. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sakai D, Nakamura Y, Nakai T, Mishima T,
Kato S, Grad S, Alini M, Risbud MV, Chan D, Cheah KS, et al:
Exhaustion of nucleus pulposus progenitor cells with ageing and
degeneration of the intervertebral disc. Nat Commun. 3:12642012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kalichman L and Hunter DJ: The genetics of
intervertebral disc degeneration. Associated genes. Joint Bone
Spine. 75:388–396. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Holm S, Mackiewicz Z, Holm AK, Konttinen
YT, Kouri VP, Indahl A and Salo J: Pro-inflammatory, pleiotropic,
and anti-inflammatory TNF-alpha, IL-6, and IL-10 in experimental
porcine intervertebral disk degeneration. Vet Pathol. 46:1292–1300.
2009. View Article : Google Scholar : PubMed/NCBI
|