Introduction
Negative pressure wound therapy (NPWT) has been
extensively used for the treatment of acute and chronic wounds, and
the underlying molecular mechanisms has been elucidated (1). It has recently been suggested that
cutaneous wound healing is a processes involving elevated levels of
neovascularization (2), and three
different processes of neovascularization serve as a the minimum
requirements for poor wound healing, including the following
(3): Capillary regeneration
(angiogenesis) and pericyte recruitment (vessel maturation),
followed by conductance microvascular growth (arteriogenesis)
(4). Recently, it has been revealed
that microvascular maturation may be an important therapeutic
target for neovascularization, as the lack of microvessel mature
processes would weaken efficient perfusion of microvessels and
prosperous growth of conductance microvessels (5–7).
Neovascularization is a multistage process involving
the formation of arteries and veins, and is considered to have an
important role in tissue repair and reconstruction. In the initial
phases, angiogenesis is dependant on the degradation of the
extracellular matrix and pre-existing blood vessels (8), followed by the formation of new
endothelial tube composed of tip cells on the top of stalk cells
(3). Subsequently, the cascaded
growth factors and various cytokines that act on the vascular
endothelial tubes are guided into the avascular area. As a result,
the diameter of blood vessels increases and pericyte and vascular
smooth cells are recruited to endothelial tubules and cover nascent
endothelial cell tubules during arteriogenesis (3). Finally, the mature, functional
microvascular circulatory system formed through neovascularization
during the wound healing process, experiences enhanced vascular
stability and blood flow perfusion is regulated (9). Mature capillaries provide increased
blood flow perfusion, and provide an abundance of oxygen and
nutrients to the wound tissue. Conversely, immature microvessels
are typically inclined toward capillary destabilization and
regression due to the absence of pericytes and, subsequently, blood
flow perfusion is affected (10,11).
The positive therapeutic effects of NPWT have been
generally accepted, including its ability to increase the quantity
of angiogenesis and to increase the expression levels of growth
factors and cytokines in the wound, thereby increasing blood flow
perfusion and accelerating the wound healing process (12–15).
However, whether the new blood vessels were mature, integrated and
functional following NPWT, and how the relevant signal pathway
regulates angiogenesis and vessel maturation, has yet to be
reported conclusively in the literature. Furthermore, the process
by which neovascularization was altered at various stages of the
wound healing process, and the association between blood vessel
maturity and wound prognosis, are only partially understood.
A recent study suggested that angiogenesis and
vessel maturation were regulated primarily by the angiogenin (Ang)
family (3). The family is comprised
of 4 ligands, including Ang-1, Ang-2, Ang-3 (in mice) and Ang-4 (in
humans), that bind the endothelial receptor tyrosine kinase, Tie-2
(11). It has previously been
demonstrated that Tie-2 is primarily expressed in endothelial cells
in neovascularized sites and is associated with microvascular
sprouting, branching, remodeling, maturation and stabilization
(3). Ang-1 is primarily expressed in
blood vessel mural cells (pericytes) (16,17), and
has an important role in regulating vessel maturation and
endothelial cell migration, adhesion and survival (18). By contrast, Ang-2 acts as an
antagonist and inhibits Ang-1 induced phosphorylation of Tie-2 in
the endothelium (16,19,20). In
addition, Ang-2 is able to disrupt the connection between cells in
the endothelium and perivascular cells, promoting vascular
regression and destabilization (18). Ang-3 is a Tie-2 receptor agonist, and
is expressed in the endothelial cells of mice, whilst Ang-4 served
is also an agonist of the Tie-2 receptor, and is exclusively
expressed in human lung tissue (21). Based upon the aforementioned
findings, the present study primarily assessed the expression
levels of Ang-1, Ang-2 and Tie-2.
In the current study, 48 clinical patients with soft
tissue defects were recruited and treated with NPWT or a petrolatum
gauze at various time-points, and the relevant detection methods
were applied. The effect of NPWT on the process of angiogenesis and
vessel maturation at different stages of wound healing was detected
and, simultaneously, the associated signal pathway was explored.
Finally, the association between wound prognosis and vessel
maturation was assessed.
Patients and methods
Patients and grouping
Between January 2013 and January 2015, 48 patients
with soft tissue defects were recruited. Patients treated with NPWT
(n=26) served as the experimental group, and the remaining patients
(n=22) were treated with a petrolatum gauze and served as the
control group. All patients were treated in the Department of
Orthopedics, Zhongnan Hospital of Wuhan University (Wuhan, China).
The present study was approved by the local ethical committee
(approval no. 2012039; Zhongnan Hospital of Wuhan University) and
written informed consent was obtained from all patients. The
inclusion criteria was as follows: i) Patients experiencing acute
soft tissue defects in the arms and legs, excluding those with any
contraindication for NPWT; ii) the patients were between 18 and 50
years old; iii) patients were without active bleeding or
malignancies; iv) patients did not have phlegmon; v) the etiology
of all wounds was trauma; and vi) all wounds underwent debridement
prior to the administration of NPWT or a petrolatum gauze.
In the present study, 23 men and 25 women with an
age range of 30–50 years old were recruited, and demographics and
the laboratory results of patient cohorts are displayed in Table I. Patients were assigned into the
experimental or control group, according to patient's condition and
inclusion criteria. Patients in the experimental group were further
divided into day 1, 3, 7 or 15 subgroups and the wound beds were
covered with a polyurethane foam dressing (Wuhan VSD Medical
Science and Technology Corp., Hubei, China), with the pressure
value constant set at continuous-125 mmHg to ensure that the
vacuum-assisted closure device (Wego Biotechnology Co. Ltd.,
Weihai, China) did not affect activity or rest. The patients
assigned to the control group were further divided into day 1, 3, 7
or 15 subgroups and administered with a petrolatum gauze dressing.
An antibiotic (2g cefpiramide; b.i.d; i.v.; Changlong Biochemical
Pharmaceutical Co., Ltd., Huinan, China) was administered according
to the results of a drug susceptibility test until infection had
been completely controlled. The polyurethane foam dressing was
replaced 2 times/week, and the gauze dressing was replaced as
necessary.
 | Table I.Patient demographics and laboratory
results for the two patient cohorts. |
Table I.
Patient demographics and laboratory
results for the two patient cohorts.
| Parameter | Experimental
group | Control group | P-value |
|---|
| Patients, n | 26 | 22 | – |
| Age, years | 39.09±6.45 | 40.27±6.00 | 0.516 |
| Gender, F:M | 15:11 | 10:12 | 0.752 |
| Initial defect
size, cm2 | 73.28±36.28 | 77.42±36.16 | 0.695 |
| Exposure of tendon
and bone, case | 20 (76.92) | 17 (77.27) | 0.978 |
| Post-operative
infection, case | 7 (26.92) | 15 (57.69) | 0.004 |
| Skin grafting,
case |
21(80.77) | 6 (27.27) | 0.000 |
| Transposition flap,
case | 5 (19.23) | 16 (72.73) | 0.000 |
| Duration of
hospital stay, days | 32.73±5.64 | 43.32±12.16 | 0.008 |
Wound blood flow and perfusion
detection
Wound surface blood flow perfusion was detected on
days 1, 3, 7, and 15 by a Laser Doppler Blood Perfusion Imager and
a PeriScan PIM 3 system Perimed Ltd. (Stockholm, Sweden). The
surface blood flow perfusion of each wound was assessed
non-invasively without disturbing the wound at a distance of 15 cm
and lasted for 6 min. Concurrently, fresh granulation tissue from
the center of the wound and margin were aseptically harvested by
punching biopsy (Hengchang Steel Co., Ltd., Tangshan, China) on
days 1, 3, 7 and 15. Samples were subdivided into two, with one
section fixed in 4% neutral paraformaldehyde (Aspen Bio, Wuhan,
China) for histopathological and immunofluorescent investigations
and the second section was stored in liquid nitrogen at −80°C for
protein analysis.
A local transposition flap or the use of a
full-thickness skin graft was utilized to provide full coverage of
the wound in the event that a red granulating wound bed was
confirmed following NPWT or petrolatum gauze treatment (22,23). The
selection of methods for the secondary wound coverage was
determined based on the growth of granulation tissue and exposure
of tendon and bone. Wounds with adequate growth of granulation
tissue were covered using the split or full thickness skin grafts;
whereas wounds with exposed bone or tendons were covered using the
transposition flap.
Immunohistochemical analysis
Samples were fixed in 4% neutral paraformaldehyde
and embedded in paraffin. Serial cuts were made into 5-µm slices,
sections underwent deparaffinized and were rehydrated, and the
slices were then placed on to glass slides and stained with
hematoxylin and eosin (Boster Biological Technology, Wuhan,
China).
For immunohistochemical staining, antibodies against
goat polyclonal Ang-1 (1:200; sc-6319), rabbit polyclonal Ang-2
(1:150; sc-20718) and Tie-2 (1:200; sc-9026), and mouse monoclonal
collagen type IV (1:200; sc-59814) and α-smooth muscle actin
(α-SMA; 1:200; sc-130616) were purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA) and served as primary
antibodies in the present study. Initially, endogenous peroxidase
was quenched with a 3% hydrogen dioxide; following this, citrate
buffer was used for antigen retrieval, sections underwent microwave
treatment (Galanz Group Ltd., Foshan, China) at 500 W for 5 min.
Following this, sections were incubated with the appropriate
primary antibody at 4°C overnight. Subsequently, sections were
washed three times with phosphate-buffered saline (PBS; Bioyear,
Wuhan, China), and incubated with horseradish peroxidase-conjugated
(HRP) goat anti-mouse (31430), goat anti-rabbit (31466) and rabbit
anti-goat (31402) IgG (H+L) secondary antibodies (all 1:500; all
Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) for
30 min. Sections were then incubated with an avidin-biotin complex
(VECTASTAIN Elite ABC kit; Vector Laboratories, Inc., Burlingame,
CA, USA) for 30 min. Subsequently, the reaction was visualized with
3′3-diaminobenzidine (Dako, Glostrup, Denmark), and nuclei were
stained using hematoxylin and eosin. Finally, all images were
captured on a fluorescence microscope (BX51WI; Olympus Corporation,
Tokyo, Japan).
Immunofluorescence analysis
To further observe the proliferation of
microvascular endothelial cells and pericyte coverage, a
double-labeling immunofluorescence technique was applied. Mouse
monoclonal anti-CD31 (1:200; ab9498; Abcam, Cambridge, UK) and
rabbit polyclonal anti-Ki67 (1:600; ab15580; Abcam) antibody
markers were used to observe the proliferation of microvascular
endothelial cells. Similarly, the pericyte coverage of microvessels
was assessed using anti-CD31 and α-SMA (1:400; ab124964; Abcam)
antibodies. Sections were blocked with bovine serum albumin (Roche
Diagnostics, Beijing, China) for 2 h, and incubated with the
required primary antibody for at 4°C overnight. Sections were
washed three times with PBS then incubated with fluorescein
isothiocyanate-conjugated goat anti-rabbit (65–6111) and Cyanine
3-conjugated goat anti-mouse (M30010) IgG (H+L) secondary
antibodies (both 1:400; both Invitrogen) for 1 h in a dark
environment. Following this, sections were incubated in
4′,6-diamidino-2-phenylindole (Aspen Bio) to stain and visualize
the nuclei. Images were captured using an Eclipse TE2000-E
fluorescence microscope (Nikon Corporation, Tokyo, Japan), analyzed
using Image-Pro Plus software (version 6.0; Media Cybernetics,
Inc., Rockville, MD, USA).
Quantitation of the proliferating
capillary index (PCI)
The PCI was used to evaluate the proliferation of
microvascular endothelial cells, and the ratio was expressed as
proliferating microvascular endothelial cells (as determined by
Ki67) divided by total number of microvessels (determined by CD31;
Ki67/CD31). PCI was quantified by three independent investigators
screening vessel hot spots with the highest microvessel density
under a magnification field of x200.
Quantification of microvessel density
(MVD)
MVD quantification methods were used to evaluate the
number of blood vessels number in the present study (11,24).
CD31 served as an endothelial cell marker, regardless of the
presence or absence of lumen, and 3 separate sections of each
sample were counted in 5 randomly selected areas with magnification
x200. MVD was quantified and the average number of microvessels in
each viewing field was recorded.
Quantification of the microvessel
pericyte coverage index (MPI)
MPI was applied to evaluated microvascular maturity,
and values are expressed as α-SMA divided by the number of
microvessels observed in CD31 staining (α-SMA/CD31). A previously
described method for quantification was applied to the present
study (11,25–28). A
single endothelial cell was deemed as 1 unit of quantify,
regardless of whether a tube was formed. A single pericyte was
defined as a single layer of α-SMA-positive cells colocalized with
CD31-positive cells. MPI was quantified in a minimum of five
non-overlapping microscopic areas per section, and sections were
analyzed by three independent investigators under double-blind
conditions. Pericyte coverage was expressed as the α-SMA/CD31
ratio.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RT-qPCR was performed to quantitatively analyze mRNA
expression levels. RNA (≤30 mg, depending on the tissue type) was
disrupted in Buffer RLT (Qiagen AB, Sollentuna, Sweden) and
homogenized. Total RNA was extracted using a RNeasy Mini kit
(Qiagen AB), and 5 µg RNA was reverse transcribed into cDNA using
the RevertAid First Strand cDNA Synthesis kit (Fermentas; Thermo
Fisher Scientific, Inc.) to a final reaction volume of 20 µl and an
S1000 Thermal Cycler (Bio-Rad Laboratories, Inc., Hercules, CA,
USA) at 65°C for 5 min, cooling on ice and 42°C for 60 min,
according to the manufacturer's protocol. The reaction was
terminated by heating at 70°C for 5 min. Primer sequences are
displayed in Table II.
 | Table II.Primer sequences for reverse
transcription-quantitative polymerase chain reaction. |
Table II.
Primer sequences for reverse
transcription-quantitative polymerase chain reaction.
| Factor | Forward primer | Reverse primer |
|---|
| Ang-1 |
5′-CTACCACCAACAACAGTGTCCTTC-3′ |
5′-TTCTCTTCCTCTCTTTTTCCTCCC-3′ |
| Ang-2 |
5′-GCGTTGATTTTCAGAGGACTTG-3′ |
5′-GATGCTGCTTATTTTGCCGG-3′ |
| α-SMA |
5′-CTTGAGAAGAGTTACGAGTTGC-3′ |
5′-GATGCTGTTGTAGGTGGTTTC-3′ |
| Collagen IV |
5′-GGTGTTACAGGATTGGTGGGT-3′ |
5′-GAAGGACACTGTGGGTCATCTATT-3′ |
| GAPDH |
5′-GGTCGGAGTCAACGGATTTG-3′ |
5′-GGAAGATGGTGATGGGATTTC-3′ |
Following DNase treatment to remove genomic DNA,
RT-qPCR was performed to a final volume of 20 µl using 1 µl
template cDNA, 10 µl SYBR qPCR mix (2X; Toyobo Co., Ltd., Osaka,
Japan), 6.6 µl diethylpyrocarbonate-treated water, 1 µl forward
primer (5 µm), 1 µl reverse primer (5 µm), 0.4 µl ROX reference dye
(50X) on a iQ5 Real-Time PCR Detection System (Bio-Rad
Laboratories, Inc.). Respective negative (no cDNA) and RT controls
were used for each gene. The thermocycling profile for SYBR Green
RT-qPCR was as follows: Initially set at 95°C and sustained for 1
min for the initial denaturation step; followed by 40 cycles of
degeneration with the temperature set at 95°C, which was held for
15 sec, then set at 60°C and sustained for 15 sec for annealing;
and, finally, the elongation step, involving a 60 sec hold at 72°C.
Each sample was run in triplicate and quantified using the
2−∆∆Cq method (29) to
determine relative mRNA expression levels.
Western blot analysis
Samples were homogenized and total proteins were
extracted using radioimmunoprecipitation assay buffer (Beyotime
Institute of Biotechnology, Haimen, China). The concentrations of
proteins were determined using a bicinchoninic acid assay kit
(Beyotime Institute of Biotechnology). Proteins (40 µg) were loaded
onto a sodium dodecyl sulfate polyacrylamide gels (10%; Aspen Bio)
and run at 120 V for 90 min. Proteins were then transferred to
nitrocellulose membranes (Pall Life Sciences, Port Washington, NY,
USA), and incubated 2 h with non-fat dry milk (5%) in TBS-T (10 mM,
Tris base pH 7.5; 150 mM NaCl, 0.1% Tween-20; Aspen Bio) at room
temperature. The membranes were incubated at 4°C overnight with
primary antibodies against Ang-1 (1:1,000; sc-6319), Ang-2
(1:1,000; sc-20718), p-Tie-2 (1:500; sc-130607), α-SMA (1:2,000;
sc-130616), GAPDH (1:5,000; sc-25778) and collagen type IV
(1:1,500; sc-59814; all Santa Cruz Biotechnology, Inc.).
Subsequently, membranes were washed three times with TBS-T for 10
min. Finally, membranes were incubated with the required
HRP-conjugated goat anti-mouse (31430), goat anti-rabbit (31466)
and rabbit anti-goat (31402) IgG (H+L) secondary antibodies for 1 h
at room temperature, and an enhanced chemiluminescence substrate
(Beyotime Institute of Biotechnology) was used to detect the
membranes.
Statistical analysis
Data are presented as mean ± standard deviation.
Comparisons between the blood flow perfusion, proliferating
capillary index, microvascular density, number of pericytes,
microvessel pericyte coverage index, mRNA and protein expression
level changes between control and NPWT groups at the same time
point were conducted using Student's t-test. Differences between
the groups at different time points were compared using one-way
analysis of variance. All statistical analyses were performed using
SPSS software (version 19; IBM SPSS, Armonk, NY, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
Demographics and laboratory results of
patient cohorts
There were no statistical differences in the age,
gender, pre-operative initial defect size or exposure of tendon and
bone (P>0.05; Table I). However,
following the surgery the cultures of wound swabs revealed that the
infection rate in the experimental group were significantly lower
compared with the control group (P<0.05). All wounds were
covered with skin grafting or a transposition flap, and results
indicated that the cases of skin grafting in the experimental group
were significantly higher (P<0.01) compared with those of the
control group, while fewer cases were treated with transposition
flap compared with the control group (P<0.01), and the hospital
stay in experiment group was significantly shorter compared with
the control group (P<0.05).
Blood flow perfusion
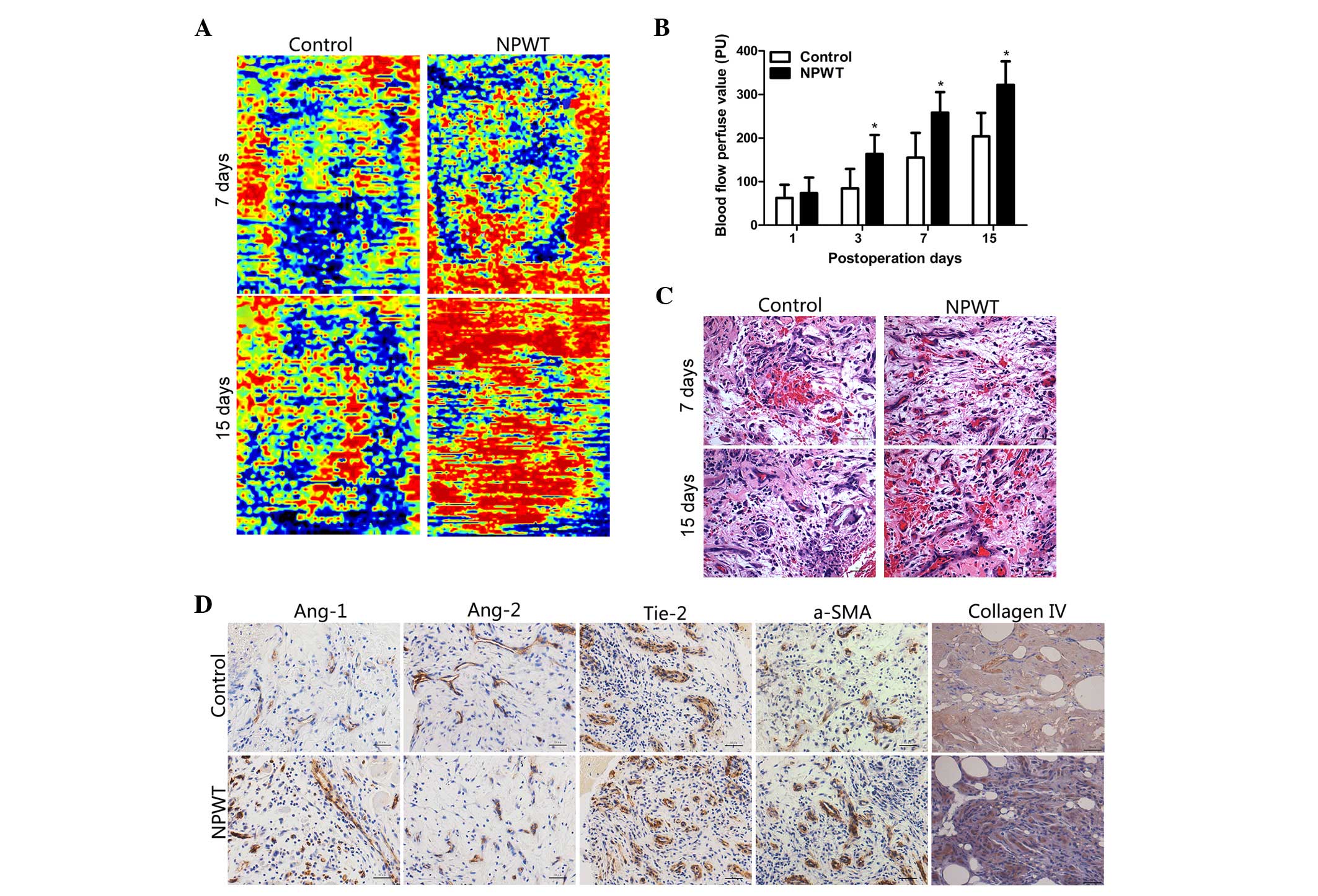
Blood flow perfusion results indicated that there
was an abundance of areas of hyperperfusion and reperfusion
following NPWT on days 7 and 15. However, in the control group, the
hyperperfusion and reperfusion areas were decreased during this
time point (Fig. 1A). The blood flow
perfusion values gradually increased between day 3 and day 15 in
the experimental group, in particular on days 7 and 15, where the
difference was statistically significant compared with the control
group (P<0.05; Fig. 1B).
Histopathological assessments
Epidermal necrolysis and inflammatory cell
infiltration was detected in the control group in the early stage
of wound healing. In the later stage, there was an abundance of
neovascularization accompanied with compactly and regularly
arranged collagen fibers in the wounds of the experimental group.
By contrast, a smaller number of nascent blood vessels were present
in wounds in the control group, and collagen fibers had a
disorganized distribution (Fig.
1C).
Immunohistochemical detection
Immunohistochemical staining revealed that Ang-1 was
primarily expressed in pericytes, and in the experimental group,
the positive staining of Ang-1 was markedly higher compared with
the control group on the day 7 (Fig.
1D). By contrast, Ang-2 was predominately expressed in
endothelial cells, and the positive staining of Ang-2 in
experimental group was markedly lower compared with the control
group on day 7. Furthermore, Tie-2 was primarily present in
endothelial cell and results revealed that on day 7, the
Tie-2-positive stained area was greater in the experimental group
with the control group. In the experimental group, the area of
positive staining of collagen type IV and α-SMA were markedly
increased, as compared with the control group on day 7.
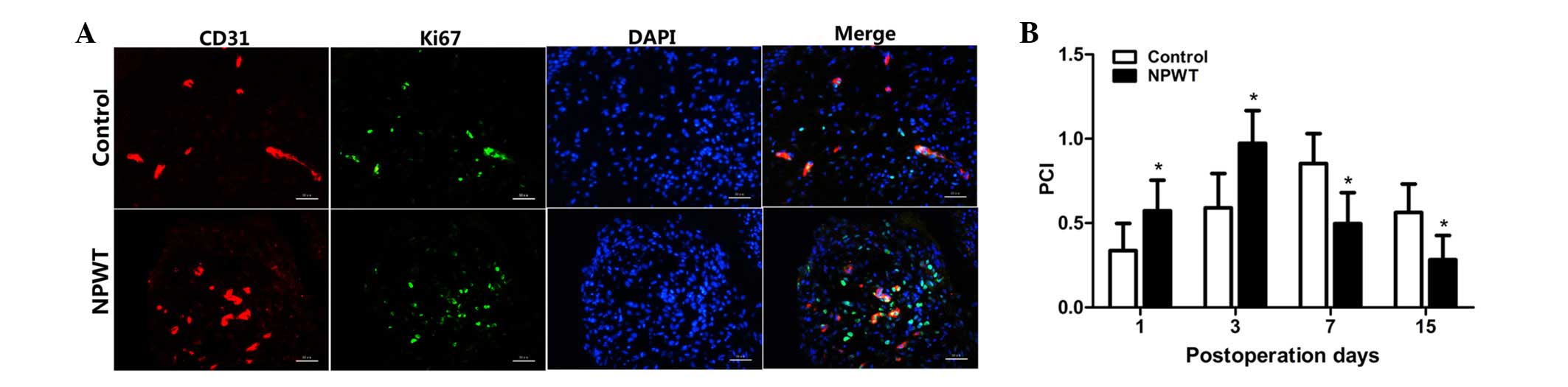
Endothelial cell proliferation in
human wounds
The PCI was used for evaluating proliferating
endothelial cells, and the results are displayed in Fig. 2A. PCI value was markedly increased
between days 1 and 3 in both groups; however, values were
significantly elevated in the experimental group compared with the
control group at the early stage of wound healing (P<0.05;
Fig. 2B). However, the PCI values
gradually decreased between days 7 and 15, and values were
significantly lower in the experimental group compared with the
control group during that time (P<0.05).
Microvessel density
CD31 and α-SMA served as markers for vascular
endothelial cells and pericytes, respectively. There was a small
quantity of CD31 positive endothelial cells in both groups on day 1
(Fig. 3A). Subsequently, a small
quantity of red-stained CD31-positive endothelial cells were
detected on day 3, and the number of endothelial cells was markedly
increased on day 7, and peaked on day 15. Statistical analysis
indicated that MVD was significantly higher in the experiment group
compared with the control group between days 3 and 15 (P<0.05;
Fig. 3B).
Pericytes count and microvessel
pericyte coverage index
Green-stained α-SMA positive pericytes were
observed, and a small number of pericytes were apparent in both
groups on day 3. There were a great number of pericytes
discontinuously covering endothelial cells on day 7. Subsequently,
on day 15, the endothelial cells were compactly wrapped with
pericytes in the experimental group. By contrast, there were fewer
pericytes wrapped with microvessels in the control group (Fig. 3A). Results revealed that the number
of pericytes were significantly higher in the experimental group
compared with the control group between days 3 and 15 (P<0.05;
Fig. 3C).
MPI was used to assess maturity of new blood vessels
and results showed there was no statistically significant
difference on day 1 between the groups. Subsequently, MPI was
significantly increased in both groups between days 7 and 15, in
contrast to the experimental group, where the MPI was significantly
higher compared control group at the aforementioned time-points
(P<0.05; Fig. 3D).
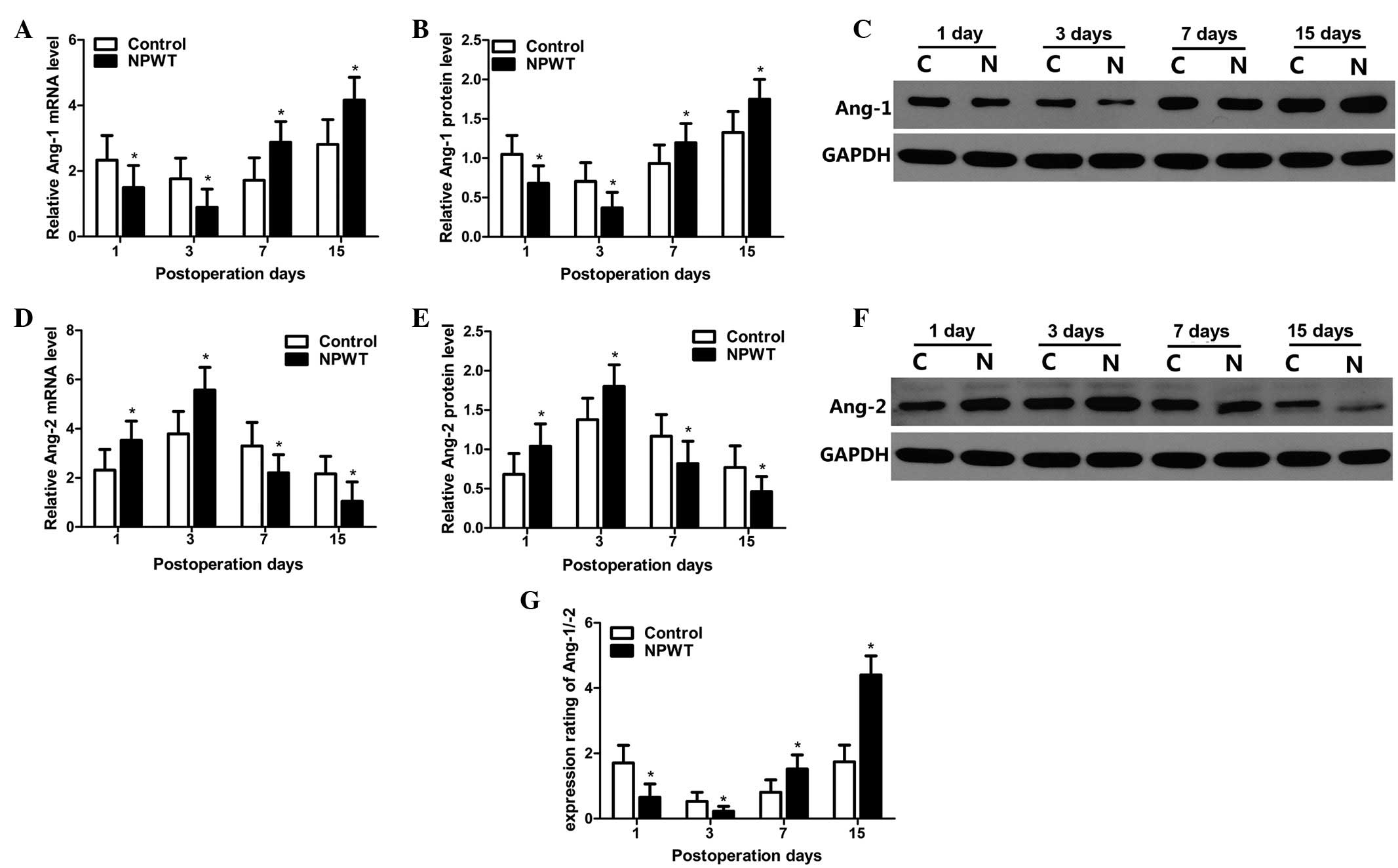
mRNA expression levels of Ang-1, Ang-2
and Ang-1/Ang-2
The RT-qPCR results indicated that mRNA expression
levels of Ang-1 gradually decreased between days 1 and 3 in the
experimental group, and expression levels were significantly lower
compared with the control group at the same time-point (P<0.05).
mRNA expression levels of Ang-1 gradually increased on day 7, and
peaked on day 15 in the two groups; however, its expression levels
in experimental group were significantly higher compared with the
control group between days 7 and 15 (P<0.05; Fig. 4A). Western blot analysis was used to
quantitatively analyze protein expression levels of Ang-1, as
indicted in Fig. 4B. Results
revealed that protein expression level trends of Ang-1 were in
agreement with the mRNA expression profile in both groups. Relevant
results regarding statistical analysis are displayed in Fig. 4C.
The mRNA expression levels of Ang-2 are shown in
Fig. 4D. Results indicated that mRNA
expression levels of Ang-2 gradually increased in the experimental
group from the 1st to the 3rd day, where levels peaked.
Furthermore, levels were significantly higher compared with the
control group during the aforementioned time-point (P<0.05). The
mRNA expression levels of Ang-2 sharply decreased between days 7
and 15, with levels in the experimental group significantly lower
compared with the control group (P<0.05). Protein expression
level trends for Ang-2 were consistent with its mRNA expression
level profile in the two groups. Results of statistical analysis
performed are displayed in Fig. 4E and
F.
The expression ratio of Ang-1/Ang-2 is displayed in
Fig. 4G. Results indicated that the
expression ratio was significantly lower in the experimental group
compared with the control group days 1 and 3 (P<0.05); however,
this ratio was gradually increased from between days 7 and 15, and
maintained a higher expression level, whilst the expression ratio
of Ang-1/Ang-2 in the experimental group was significantly higher
compared with the control group (P<0.05).
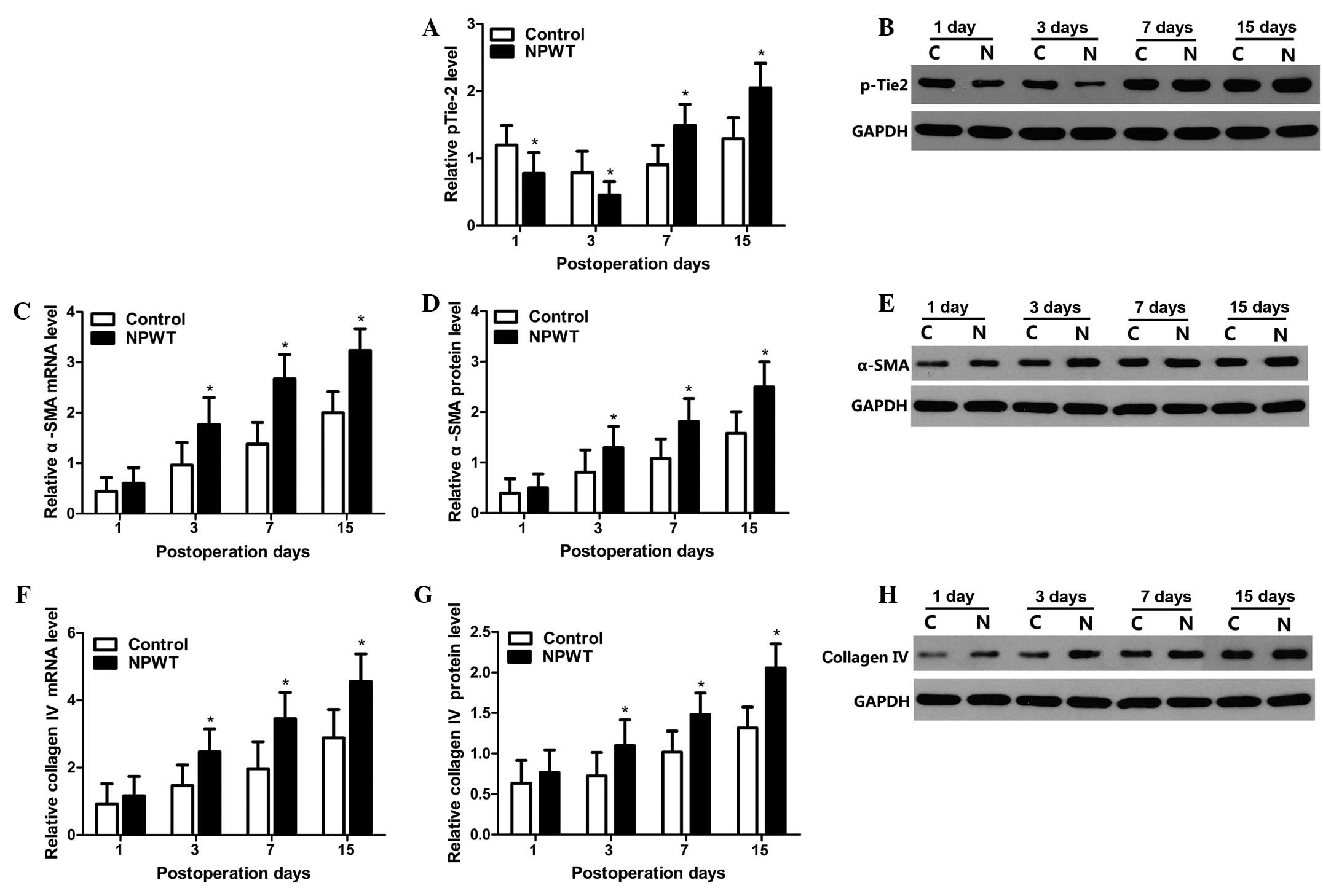
Expression levels of pTie-2, α-SMA and
collagen type IV
The tyrosine phosphorylated protein expression
levels of Tie-2 were detected by western blot analysis. The changes
in protein expression levels pTie-2 are displayed in Fig. 5A, and the results indicate that the
expression levels of pTie-2 gradually decreased between days 1 and
3 in both groups, whilst expression levels in the experimental
group were significantly lower compared with the control group
(P<0.05; Fig. 5B). Subsequently,
expression levels gradually increased between days 7 and 15, and
the expression levels of pTie-2 in the experimental group was
significantly higher compared with the control group (P<0.05).
The mRNA and protein expression level changes in α-SMA are
displayed in Fig. 5C–E. The results
indicated that α-SMA expression levels gradually increased between
days 3 and 15, and expression levels were significantly higher in
the experimental group compared with the control group (P<0.05).
The mRNA and protein expression level changes of collagen type IV
are displayed in Fig. 5F–H. The
expression levels of collagen type IV presented trends that were
concordant with the α-SMA expression profile in both groups.
Discussion
NPWT has been routinely used for the treatment of
various chronic and acute wounds, bone exposure, pressure ulcers
and diabetes mellitus wounds. Previous studies have demonstrated
that NPWT is able to promote the formation of granulation tissue
and increase the amount of angiogenesis (30–32),
accelerate wound neovascularization, increase blood flow perfusion
and thus accelerate wound re-epithelialization (33,34), in
addition to accelerating the speed of wound healing in wounds in
rats (34,35). However, the investigation of new
blood vessel maturation and the relevant signal pathway subsequent
to NPWT in human wound, is infrequent and limited. In the present
study, the changes in microvasculature were investigated at various
stages following NPWT in human wounds, and whether NPWT was capable
of promoting the maturation of new blood vessels was explored, in
addition to the corresponding signal pathway. Furthermore, the
relevant association between the maturation of nascent blood
vessels and the prognosis of wounds were investigated and
analyzed.
In normal and mature blood vessels, basement
membrane is shared by the vascular endothelial tube and pericytes
(11,36). Mature microvessels, predominantly
characterized by the vascular endothelial tube, were covered with
abundant pericytes and the basement membrane (11,20,37).
Pericytes serve as a specific structural component and marker of
vessel maturation, wrapping around microvascular endothelial cells
(11,38). Furthermore, pericytes are able to
contact endothelial cells through the hole of the basement membrane
and form special cell-cell contact, termed peg-and-socket contact
(39). Pericytes and endothelial
cells communicate with each other by the peg-and-socket contacts,
and control the proliferation and differentiation of endothelial
cells (37,39), and transmit mechanical contractile
forces to affect blood flow perfusion (20,40). The
characterization of pericytes was previously problematic due to the
absence of a specific molecular marker; however, several recent
studies have indicated that α-SMA is able to serve as marker of
pericytes (20,37,39,41).
Consequently, α-SMA served as a pericyte marker in the present
study.
To further elucidate the method by which NPWT is
able to regulate the process of angiogenesis and vessel maturation
in human wounds, the corresponding expression levels of
pro-angiogenesis factor Ang-2, and pro-maturation factor Ang-1,
which are able to regulate the sprouting of new blood vessels and
their maturation, were detected (42). Previous studies have demonstrated
that the process of angiogenesis and vessel maturation is regulated
by the Ang/Tie-2 system in the wounds of rats (11,39,41). It
has been demonstrated that Ang-1 functions as a pericyte-derived
blood vessels stabilizer and pro-maturation factor; binding to the
Tie-2 receptor and serving an important role in sustaining
quiescent microvasculature (43). In
the present study, RT-qPCR and western blot analysis indicated that
expression levels of Ang-1 and pTie-2 were gradually reduced in the
early stage of wound healing following NPWT. However, in the
control group the expression levels of Ang-1 and pTie-2 were
significantly higher compared with the experimental group in the
early stage. By contrast, as an antagonist, Ang-2 inhibited the
phosphorylation of Tie-2 in endothelial cells induced by Ang-1
(3,16,19), and
induced the destabilization and regression of microvasculature
(44). Brudno et al (42) proposed that a destabilized wound
microenvironment would be able to facilitate vessel sprouting and
angiogenesis. The results in the present study indicated that, in
the experimental group, the expression levels of Ang-2 were
significantly higher compared with the control group in the early
stage of wound healing following NPWT. Furthermore, the present
study observed that the lower expression ratio of Ang-1/Ang-2, and
the MVD and PCI were significantly higher in the experimental group
compared with the control group in the early stage. The
aforementioned results suggest that microvessels were regressive
and destabilized in the early stage of wound healing. The present
data demonstrated that NPWT was able to preferentially promote
microvessel regression and destabilization at the early stage, and
thus promote vascular endothelial cell sprouting and proliferation,
and increase the amount of angiogenesis.
Subsequent to microvascular endothelial lumen
formation, stabilization and maturation of the nascent blood
vessels takes place in the later stage of wound healing.
Pro-maturation factor Ang-1 has an important role in promoting
recruitment of mural cell and blood vessel maturation in the later
stage of wound healing (45).
Previous studies have demonstrated that pericytes are an important
component of microvessel maturation, and support microvascular
structural integrity and functional stabilization (46,47).
Blood vessel maturation is predominately characterized by an
abundance of pericytes wrapping around vascular endothelial tubes
(3,11). Previous studies have demonstrated
that immature vessels induced vessel hemorrhage, tissue oedema and
vessels occlusion, and eventually led to obstruction of the
transportation of nutrients and oxygen (18,48). The
results of the present study indicated that at the later stage of
wound healing, the expression levels of Ang-1 and pTie-2 gradually
increased in the experimental group following NPWT, and the
difference was statistically significant compared with the control
group. However, expression levels of Ang-2 gradually decreased in
the experimental group, compared with the control group in the
later stage of the wound healing process. Furthermore, the results
for the expression ratio of Ang-1/Ang-2 were significantly
increased in the NPWT group, as compared with the control group at
a later stage of wound healing. Additionally, in the experimental
group, the blood flow perfusion was significantly increased and
α-SMA and collagen IV also increased gradually, thus MPI was
relatively higher in the experimental group between days 7 and 15.
Results suggested that microvessels were gradually stabilized in
the later stage, and the stabilized microvascular microenvironment
contributed to mediate the recruitment of pericytes to vessel
tubes, and promoted the maturation of new blood vessels. NPWT
predominately promoted microvessel stabilization and maturation in
the later stage of wound healing in human wounds, and thus
increased blood flow perfusion and accelerated the speed of wound
healing.
Finally, all wounds were covered with skin grafting
or underwent the transposition flap technique according to the
quality of granulation tissue following NPWT or petrolatum gauze
treatment. Previous research has demonstrated that a granulation
tissue wound may be treated via covering with skin grafting, which
is preferable to the transposition flap when red, fresh and
abundant tissue granulation is detected (22,49).
Combining the results of patient demographics, laboratory results
of patient cohorts and blood flow changes, the present study
indicated that in the experimental group, there was a greater
number of cases of skin grafting and blood flow perfusion of the
wound, and a lower number of cases of requiring the transposition
flap technique, in addition to a shorter hospital stay, compared
with the control group. The aforementioned results may be
associated with blood vessel maturation. Due to the gradual
maturation of microvessels at the later stage of wound healing in
the experimental group, in addition to the increased number of
pericytes, which serve as a blood flow regulators, in later stage
of wound healing (22,50), blood flow perfusion was increased at
the site of the wound. As a result, notable granulation tissue was
generated, and subsequently the wound healing process was markedly
accelerated by NPWT. It was further suggested that NPWT was able to
promote angiogenesis and microvessel maturation at different stages
during wound healing, and increased blood flow perfusion, which may
eventually be able to influence wound prognosis.
In summary, NPWT was able to promote microvessel
destabilization and regression, and consequently promote vessel
sprouting and increase the quantity of microvessels in the early
stage of wound healing. In addition, NPWT was able to promote the
structural integrity and functional stabilization of microvessels,
and then promote microvascular maturation during the later stage of
human wound healing. The present study also demonstrated that
mature microvessels were able to influence wound prognosis in human
wounds.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81572163) and by
Hubei National Natural Science Fund projects (grant no.
2014CFB751). The authors would also like to acknowledge the Wuhan
VSD Medical Science & Technology, Co., Ltd. (Wuhan, China) for
supplying the vacuum material. Finally, thanks is given to the
Medical Science Experimentation Center of Wuhan University for
providing the experimental equipment.
References
|
1
|
Zhang DM, Yang ZH, Zhuang PL, Wang YY,
Chen WL and Zhang B: Role of negative-pressure wound therapy in the
management of submandibular fistula after reconstruction for
osteoradionecrosis. J Oral Maxillofac Surg. 74:401–405. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ram M, Singh V, Kumawat S and Kumar D,
Lingaraju MC, Singh Uttam T, Rahal A, Tandan Kumar S and Kumar D:
Deferoxamine modulates cytokines and growth factors to accelerate
cutaneous wound healing in diabetic rats. Eur J Pharmacol.
764:9–21. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Qin D, Trenkwalder T, Lee S, Chillo O,
Deindl E, Kupatt C and Hinkel R: Early vessel destabilization
mediated by Angiopoietin-2 and subsequent vessel maturation via
Angiopoietin-1 induce functional neovasculature after ischemia.
PLoS One. 8:e618312013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hinkel R, Trenkwalder T and Kupatt C: Gene
therapy for ischemic heart disease. Expert Opin Biol Ther.
11:723–737. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jain RK: Molecular regulation of vessel
maturation. Nat Med. 9:685–693. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Greenberg JI, Shields DJ, Barillas SG,
Acevedo LM, Murphy E, Huang J, Scheppke L, Stockmann C, Johnson RS,
Angle N and Cheresh DA: A role for VEGF as a negative regulator of
pericyte function and vessel maturation. Nature. 456:809–813. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kupatt C, Hinkel R, Pfosser A, El-Aouni C,
Wuchrer A, Fritz A, Globisch F, Thormann M, Horstkotte J, Lebherz
C, et al: Cotransfection of vascular endothelial growth factor-A
and platelet-derived growth factor-B via recombinant
adeno-associated virus resolves chronic ischemic malperfusion role
of vessel maturation. J Am Coll Cardiol. 56:414–422. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kilarski WW, Samolov B, Petersson L,
Kvanta A and Gerwins P: Biomechanical regulation of blood vessel
growth during tissue vascularization. Nat Med. 15:657–664. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Patel-Hett S and D'Amore PA: Signal
transduction in vasculogenesis and developmental angiogenesis. Int
J Dev Biol. 55:353–363. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dor Y, Djonov V, Abramovitch R, Itin A,
Fishman GI, Carmeliet P, Goelman G and Keshet E: Conditional
switching of VEGF provides new insights into adult
neovascularization and pro-angiogenic therapy. Embo J.
21:1939–1947. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao J, Chen L, Shu B, Tang J, Zhang L,
Xie J, Qi S and Xu Y: Granulocyte/macrophage colony-stimulating
factor influences angiogenesis by regulating the coordinated
expression of VEGF and the Ang/Tie system. PLoS One. 9:e926912014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Greene AK, Puder M, Roy R, Arsenault D,
Kwei S, Moses MA and Orgill DP: Microdeformational wound therapy:
Effects on angiogenesis and matrix metalloproteinases in chronic
wounds of 3 debilitated patients. Ann Plast Surg. 56:418–422. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen SZ, Li J, Li XY and Xu LS: Effects of
vacuum-assisted closure on wound microcirculation: An experimental
study. Asian J Surg. 28:211–217. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Grimm A, Dimmler A, Stange S, Labanaris A,
Sauer R, Grabenbauer G and Horch RE: Expression of HIF-1 alpha in
irradiated tissue is altered by topical negative-pressure therapy.
Strahlenther Onkol. 183:144–149. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Labler L, Rancan M, Mica L, Harter L,
Mihic-Probst D and Keel M: Vacuum-assisted closure therapy
increases local interleukin-8 and vascular endothelial growth
factor levels in traumatic wounds. J Trauma. 66:749–757. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Reiss Y, Droste J, Heil M, Tribulova S,
Schmidt MH, Schaper W, Dumont DJ and Plate KH: Angiopoietin-2
impairs revascularization after limb ischemia. Circ Res. 101:88–96.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gaengel K, Genove G, Armulik A and
Betsholtz C: Endothelial-mural cell signaling in vascular
development and angiogenesis. Arterioscler Thromb Vasc Biol.
29:630–638. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fagiani E and Christofori G: Angiopoietins
in angiogenesis. Cancer Lett. 328:18–26. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Maisonpierre PC, Suri C, Jones PF,
Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J,
Aldrich TH, Papadopoulos N, et al: Angiopoietin-2, a natural
antagonist for Tie2 that disrupts in vivo angiogenesis. Science.
277:55–60. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Armulik A, Genové G and Betsholtz C:
Pericytes: Developmental, physiological and pathological
perspectives, problems and promises. Dev Cell. 21:193–215. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee HJ, Cho CH, Hwang SJ, Choi HH, Kim KT,
Ahn SY, Kim JH, Oh JL, Lee GM and Koh GY: Biological
characterization of angiopoietin-3 and angiopoietin-4. Faseb J.
18:1200–1208. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou M, Qi B, Yu A, Pan Z, Zhu S, Deng K
and Tao S: Vacuum assisted closure therapy for treatment of complex
wounds in replanted extremities. Microsurgery. 33:620–624.
2013.PubMed/NCBI
|
|
23
|
Matsunaga T, Warltier DC, Tessmer J,
Weihrauch D, Simons M and Chilian WM: Expression of VEGF and
angiopoietins-1 and −2 during ischemia-induced coronary
angiogenesis. Am J Physiol Heart Circ Physiol. 285:H352–H358. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Weidner N: Tumoural vascularity as a
prognostic factor in cancer patients: The evidence continues to
grow. J Pathol. 184:119–122. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yonenaga Y, Mori A, Onodera H, Yasuda S,
Oe H, Fujimoto A, Tachibana T and Imamura M: Absence of smooth
muscle actin-positive pericyte coverage of tumor vessels correlates
with hematogenous metastasis and prognosis of colorectal cancer
patients. Oncology. 69:159–166. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao W, Jiang AH, Li CY, Yang WZ, Xu CC
and Liu ZG: Pericytes are correlated with the permeability of rat
corneal neovascular vessels induced by alkali burn. Chin Med J
(Engl). 120:274–279. 2007.PubMed/NCBI
|
|
27
|
O'Keeffe MB, Devlin AH, Burns AJ, Gardiner
TA, Logan ID, Hirst DG and McKeown SR: Investigation of pericytes,
hypoxia and vascularity in bladder tumors: Association with
clinical outcomes. Oncol Res. 17:93–101. 2008.PubMed/NCBI
|
|
28
|
Kalinski T, Sel S, Kouznetsova I, Röpke M
and Roessner A: Heterogeneity of angiogenesis and blood vessel
maturation in cartilage tumors. Pathol Res Pract. 205:339–345.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Armstrong DG and Lavery LA: Diabetic Foot
Study Consortium: Negative pressure wound therapy after partial
diabetic foot amputation: A multicentre, randomised controlled
trial. Lancet. 366:1704–1710. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Apelqvist J, Armstrong DG, Lavery LA and
Boulton AJ: Resource utilization and economic costs of care based
on a randomized trial of vacuum-assisted closure therapy in the
treatment of diabetic foot wounds. Am J Surg. 195:782–788. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Blume PA, Walters J, Payne W, Ayala J and
Lantis J: Comparison of negative pressure wound therapy using
vacuum-assisted closure with advanced moist wound therapy in the
treatment of diabetic foot ulcers: A multicenter randomized
controlled trial. Diabetes Care. 31:631–636. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tuncel U, Turan A, Markoc F, Erkorkmaz U,
Elmas C and Kostakoglu N: Loofah sponge as an interface dressing
material in negative pressure wound therapy: Results of an in vivo
study. Ostomy Wound Manage. 60:37–45. 2014.PubMed/NCBI
|
|
34
|
Xia CY, Yu AX, Qi B, Zhou M, Li ZH and
Wang WY: Analysis of blood flow and local expression of
angiogenesis-associated growth factors in infected wounds treated
with negative pressure wound therapy. Mol Med Rep. 9:1749–1754.
2014.PubMed/NCBI
|
|
35
|
Li X, Liu J, Liu Y, Hu X, Dong M, Wang H
and Hu D: Negative pressure wound therapy accelerates rats diabetic
wound by promoting agenesis. Int J Clin Exp Med. 8:3506–3513.
2015.PubMed/NCBI
|
|
36
|
Kim N and Cho SG: Clinical applications of
mesenchymal stem cells. Korean J Intern Med. 28:387–402. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gokcinar-Yagci B, Uçkan-Çetinkaya D and
Çelebi-Saltik B: Pericytes: Properties, functions and applications
in tissue engineering. Stem Cell Rev. 11:549–559. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Stratman AN, Malotte KM, Mahan RD, Davis
MJ and Davis GE: Pericyte recruitment during vasculogenic tube
assembly stimulates endothelial basement membrane matrix formation.
Blood. 114:5091–5101. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ribatti D, Nico B and Crivellato E: The
role of pericytes in angiogenesis. Int J Dev Biol. 55:261–268.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bergers G and Song S: The role of
pericytes in blood-vessel formation and maintenance. Neuro Oncol.
7:452–464. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Aguilera KY and Brekken RA: Recruitment
and retention: Factors that affect pericyte migration. Cell Mol
Life Sci. 71:299–309. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Brudno Y, Ennett-Shepard AB, Chen RR,
Aizenberg M and Mooney DJ: Enhancing microvascular formation and
vessel maturation through temporal control over multiple
pro-angiogenic and pro-maturation factors. Biomaterials.
34:9201–9209. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Thomas M and Augustin HG: The role of the
Angiopoietins in vascular morphogenesis. Angiogenesis. 12:125–137.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Scharpfenecker M, Fiedler U, Reiss Y and
Augustin HG: The Tie-2 ligand angiopoietin-2 destabilizes quiescent
endothelium through an internal autocrine loop mechanism. J Cell
Sci. 118:771–780. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Brindle NP, Saharinen P and Alitalo K:
Signaling and functions of angiopoietin-1 in vascular protection.
Circ Res. 98:1014–1023. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Fagiani E, Lorentz P, Kopfstein L and
Christofori G: Angiopoietin-1 and −2 exert antagonistic functions
in tumor angiogenesis, yet both induce lymphangiogenesis. Cancer
Res. 71:5717–5727. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hirschi KK, Rohovsky SA, Beck LH, Smith SR
and D'Amore PA: Endothelial cells modulate the proliferation of
mural cell precursors via platelet-derived growth factor-BB and
heterotypic cell contact. Circ Res. 84:298–305. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Bhushan M, Young HS, Brenchley PE and
Griffiths CE: Recent advances in cutaneous angiogenesis. Br J
Dermatol. 147:418–425. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Stannard JP, Singanamala N and Volgas DA:
Fix and flap in the era of vacuum suction devices: What do we know
in terms of evidence based medicine? Injury. 41:780–786. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hamilton NB, Attwell D and Hall CN:
Pericyte-mediated regulation of capillary diameter: A component of
neurovascular coupling in health and disease. Front
Neuroenergetics. 2:52010. View Article : Google Scholar : PubMed/NCBI
|