Introduction
Determination of a target gene is the crucial
initial step in DNA vaccine research. In recent years, researchers
around the world have screened protective antigens of varicella
zoster virus (VZV), exploring a series of antigen markers with
various degrees of immune protective effects (1). Previous studies have demonstrated that
the glycoprotein E (gE) antigen is one of the most promising
candidate antigen markers (2,3). gE is
one of the most important protective antigens in VZV, and since it
is capable of inducing cellular and humoral immunity, gE is
considered the most appropriate candidate antigen for a DNA vaccine
(4).
Various eukaryotic proteins exhibit very low
biological activity when synthesized in bacteria, due to incorrect
folding or low folding efficiency (5). When using a cloned gene,
post-translational processing, including disulfide bond formation,
glycosylation and phosphorylation, is often required to produce
eukaryotic proteins with true biological activity (6). However, post-translational processing
cannot be conducted in prokaryotic cells; therefore, mammalian cell
expression vectors are often required to express the secreted
protein with the desired biological function. By inducing the
corresponding gene into eukaryotic cells, proteins with high
bioactivities may be expressed (7).
Previous studies have demonstrated that
corresponding protein antigens may be successfully expressed
following DNA vaccine administration, and these protein antigens
are capable of simulating a natural infection and producing a more
comprehensive immune response (8,9). The
antigen-specific cellular and humoral immune responses must be
detected following administration of a DNA vaccine directed against
a pathogenic microorganism or malignant tumor, in order to evaluate
the immune effects of the DNA vaccine. Furthermore, it is helpful
to elucidate the detailed mechanism underlying how a DNA vaccine
induces cellular and humoral immune responses in an organism. The
results obtained may provide a crucial experimental basis for the
development of novel and highly efficient DNA vaccines against
pathogenic microorganisms or malignant tumors (10,11).
pcDNA-VZV gE is a eukaryotic expression plasmid of
VZV gE that was constructed in the present study, and was used as a
DNA vaccine to immunize BALB/c mice via intramuscular injection.
The specific antibody levels, spleen lymphocyte proliferation
activity and specific cytotoxic T lymphocyte (CTL) responses were
analyzed in the mice, and compared with those of mice immunized
with the pcDNA3.1 plasmid. The results obtained laid the foundation
for future research into a VZV DNA vaccine.
Materials and methods
Materials
Specific pathogen-free BALB/c female mice, aged 4–6
weeks old and weighing 18–20 g were purchased from Shanghai SLAC
Laboratory Animal Co., Ltd. (Shanghai, China). The pcDNA3.1
plasmid, recombinant COS7 cell lines (kidney fibroblasts
transformed by SV40 viral genes) were purchased from Amresco, LLC
(Solon, OH, USA); whereas Taq DNA polymerase, T4 DNA ligase,
TRIzol® (Invitrogen) and various restriction
endonucleases were purchased from Thermo Fisher Scientific, Inc.
(Waltham, MA, USA). Liposome, VZV gE monoclonal antibodies
(ab52549; Abcam, Cambridge, UK) and VZV gE were purchased from
Shanghai Baoman Biological Technology Co., Ltd. (Shanghai, China);
methylthiazolyltetrazolium (MTT) was purchased from Beijing Bole
Life Science Development Co., Ltd. (Beijing, China); a two-step
reverse transcription-polymerase chain reaction (RT-PCR) kit was
purchased from Shanghai Sunred Biological Technology Co., Ltd.
(Shanghai, China); horseradish peroxidase (HRP)-labeled goat
anti-mouse immunoglobulin (Ig)G (E030110-02; EarthOx, Millbrae, CA,
USA) was purchased from Sigma-Aldrich (St. Louis, MO, USA); an
ultraviolet spectrophotometer (Ci6x; X-Rite, Inc., Grand Rapids,
MI, USA). The gel imaging system was purchased from GE Healthcare
Bio-Sciences (Pittsburgh, PA, USA), the microcentrifuge was
purchased from Beijing Jingli Centrifuge Co. Ltd. (Beijing, China).
The study was approved by the ethics committee of Inner Mongolia
Medical University (Hohhot, China).
Primer design
Primer sequences were designed using Primer 5.0
design software (Premier Biosoft, Palo Alto, CA, USA), according to
the previously published VZV gE DNA sequence (7). The upstream primer was introduced with
a BamHI restriction site (underlined sequence), as follows:
5′-TCCAGAATGGCGCTTATACTC-3′; whereas the downstream primer
was introduced with a termination codon and XbaI restriction site
(12) (underlined sequence), as
follows: 5′-TTCCACCAGGTCTAATCTATTCCT-3′. Primers used to
amplify the VZV gE gene were synthesized by Shanghai Jiang Lai
Biotechnology Co., Ltd. (Shanghai, China).
Double digestion of recombinant
plasmid
Double digestion was initiated by adding 15 µl
recombinant plasmid DNA into a 1.5 ml centrifuge tube, recombinant
plasmid according to previously described methods (13). The double digestion reaction system
included: 9 µl ddH2O; 1.5 µl BamHI (1 U/µl); 1.5 µl XbaI (1
U/µl); 3 µl buffer; and 15 µl recombinant plasmid (20 µl was
prepared in total), incubated at 37°C for 3 h. Following double
digestion, 10 µl reaction mixture was used to detect the digestion
effect using 1% agarose gel electrophoresis.
Detection of expression product using
immunohistochemistry
Confluent COS7 cells, transfected with recombinant
plasmid pcDNA-VZV gE, were fixed with cold acetone and washed three
times with phosphate-buffered saline (PBS) for 5 min. Plasmid
transfection was conducted according to previously described
methods (14). Immunohistochemical
detection was conducted in a conventional manner. The process was
conducted as follows, the cover glass was sealed at room
temperature for 30 min using sealing liquid, following which,
anti-VZV gE monoclonal mouse antibody (1:50) was added and
incubated at 37°C overnight prior to washing three times with PBS
for 5 min. Biotin-labeled goat anti-mouse IgG (1:100) was added to
the cover glass, inoculated at 37°C for 30 min and washed with PBS
for 5 min, three times. Subsequently, HRP-labeled streptavidin
(1:100; Luwen, Wuhan, China) was added, incubated at 37°C for 30
min and washed three times with PBS for 5 min. Finally, the cover
glass was stained using diaminobenzidine (DAB) color buffer.
Following observation under a microscope (Olympus, Tokyo, Japan),
the cover glass was washed with tap water to terminate the reaction
and subsequently re-stained. Untransfected COS7 cells and COS7
cells transfected with the pcDNA3.1 null vector were used as
negative controls.
Immunization of mice with plasmid
DNA
A total of 30 BALB/c female mice aged 4–6 weeks old
were randomly divided into three groups: i) Negative control group
injected with null vector pcDNA3.1; ii) control group injected with
sterile saline; and iii) experimental group injected with
recombinant pcDNA-VZV gE (n=10 per group). Immunization was
performed as follows, the inner thigh hairs of the mice were
sheared off and the mice were grouped according to the sites marked
by picric acid. The quadriceps femoris injection sites were
pretreated with 50–100 µl bupivacaine hydrochloride (5 ml/l) 24 h
prior to injection. Each reagent was injected to the same site via
multi-point injection. The inoculation amount was 100 µg/100
µl/mouse and immunity was boosted every 2 weeks, three times in
total.
Sample collection and preparation
A total of 2 weeks after the final immunization, 60
mg quadriceps femoris tissue samples were harvested from the mice,
rinsed three times with PBS and treated with diethylpyrocarbonate
(DEPC). Subsequently, the quadriceps femoris sections were cut into
1-mm2 sections, placed in a DEPC-treated homogenate
tube, treated with 1 ml TRIzol® reagent and homogenized
into a uniform suspension in an ice bath. Subsequently, the
homogenate was transferred into a DEPC-treated 1.5-ml centrifuge
tube and incubated at room temperature for 10 min prior to
centrifugation at 5,000 × g for 15 min at 4°C. The supernatant was
subsequently discarded and the precipitate was washed with 75%
ethanol and vortexed. The precipitate and ethanol solution was
centrifuged at 5,000 × g for 5 min at 4°C. The supernatant was
discarded, the precipitate was re-washed using 75% ethanol and
centrifuged again as above. Subsequently, the supernatant was
discarded and the precipitate was naturally dried at room
temperature and dissolved using 50 µl DEPC-processed Tris EDTA
buffer. The concentration of the solution was measured using a
nucleic acid protein analyzer (Nano-200; HEBk, Xi'an, China) and
adjusted to 0.5 µg. The solution was subsequently stored at −20°C
prior to RT-PCR amplification using specific primers.
RT-PCR analysis
Total RNA from cultures was extracted using TRIzol,
followed by DNase treatment using the DNA-Free RNA Kit (Zymo
Research Corporation, Irvine, CA, USA) according to manufacturer's
instruction. The total RNA concentration and purity were determined
using UV spectrophotometry at 260 and 280 nm. cDNA was prepared
using the SuperScriptIII First-Strand Synthesis System (Invitrogen;
Thermo Fisher Scientific, Inc.) to reverse transcribe 1 µl total
RNA. RT-PCR was performed on the ViiA 7 Real-Time PCR System
(Applied Biosystems; Thermo Fisher Scientific, Inc.). Primer
template DNA sequences: VZV gE forward,
5′-ATGGATCCTATGGGGACAGTTAA-3′ and reverse:
5′-TCTCTAGATCACCGGGTCTTATCTAT-3′. Primer sequences for
glyceraldehyde 3-phosphate dehydrogenase (GAPDH) are described
elsewhere (15). A 20-µl reaction
system was constructed, composed of the following: 2 µl 10X Taq
Buffer (100 mM Tris HCl and 500 mM KCl); 2 µl MgCl2 (25
mM); 2µl dNTPs (100mM) 2µl; 0.5 µl each forward and reverse primers
(10 pM); 1 µl DNA template (~100 ng); 0.2 µl Taq DNA polymerase (5
U/µl); and nuclease-free water made the total volume up to 20 µl.
The reaction system was incubated in a thermalcycler, according to
the following protocol: Initial denaturation, 94°C for 4 min; 30
cycles of denaturation at 94°C for 30 sec, annealing at 55°C for 30
sec and extension at 72°C for 45 sec; and a final extension, 72°C
for 5 min. Two negative controls were used in the PCR: i) The
minus-RT control from the previous step; and ii) a minus-template
PCR. A BJS gel image analysis system (Shanghai Qiaofeng Industrial
Co., Ltd., Shanghai, China) was used to scan the band density, and
the results were normalized against GAPDH.
Detection of specific antibodies in
mice
On days 7, 21 and 35 following the final
immunization, blood samples were taken from the eyeballs of three
mice (Procaine injection anesthesia; Disha Pharm Co., 1%/2 ml) from
each group and were placed at 4°C overnight in order to collect the
serum, by subjecting whole blood to centrifugation at 5,000 × g for
15 min. The enzyme-linked immunosorbent assay (ELISA; Bio-Tek,
Winooski, VT, USA) plate was placed in a pure grade S ELISA plate
reader (BrandTech Scientific, Inc., Essex, CT, USA) and the OD
value of the serum samples was determined at 450 nm. The samples
were deemed positive for the antibody if: Mean OD450 of sample >
mean OD450 nm of control + 3 times of standard deviation (SD). The
highest dilution of the serum sample that demonstrated a positive
reaction was regarded as the antibody titer (16).
Detection of spleen T lymphocyte
subgroups
On day 21 following mice immunization, a 0.6-ml
suspension of spleen lymphocytes was harvested, centrifuged at
5,000 × g for 5 min, washed twice with 1 ml fluorescence lotion
(Nanjing SenBeijia Biotechnology Co., Ltd., Nanjing, China) and
equally divided into two aliquots. The two aliquots were
supplemented with 50 µl fluorescein isothiocyanate-marked
anti-mouse CD4+ (ab187745; Abcam, Cambridge, UK) and 50
µl phycoerythrin-labeled anti-mouse CD8+ (ab4055; Abcam)
monoclonal antibodies, respectively, and incubated at room
temperature for 15 min in the dark. Subsequently, the two aliquots
were centrifugally washed using 2 ml staining buffer and fixed
using 1% paraformaldehyde in a 500 µl/tube. Finally, the expression
levels of CD4+ and CD8+ on the cell surface were detected and
analyzed using a flow cytometer (Guava easyCyte 6HT-2L; Qzbiotech,
Beijing, China) and Cell Quest software (BD Biosciences, Franklin
Lakes, NJ, USA).
Lymphocyte proliferation activity as
detected by the MTT method
A spleen lymphocyte suspension (100 µl) was seeded
onto 96-well cell culture plates, 3 wells were prepared for the
lymphocytes of each mouse in each group. The blank control well was
not inoculated with any cells. The cell culture plate was placed in
an incubator containing 5% CO2 at 37°C for 48 h; and subsequently,
each well was supplemented with 20 µl MTT (5 mg/ml; MOER Co., Ltd.,
Hohhot, China) and incubated again as outlined. Following 4 h, the
cell culture plate was centrifuged at 5,000 × g for 5 min, the cell
culture liquid was removed and 100 µl hydrochloric acid isopropanol
(0.04 mol/l) was added to each well. The dark blue granules that
were generated were dissolved by mixing fully and a
spectrophotometer was used to measure the OD value of each well at
570 nm with 630 nm as the reference. The OD value of the pre-tested
sample was obtained by subtracting the obtained OD value from the
blank culture medium control OD value. The degree of lymphocyte
proliferation was expressed as the stimulation index (SI),
calculated as follows: SI = mean OD value of test group/mean OD
value of control group. The measured SI values of each group were
underwent a statistical analysis using t-test (17).
Statistical analysis
Data are presented as the mean ± SD of three
independent experiments. Statistical analysis was performed using
SPSS 15.0 software (SPSS, Inc., Chicago, IL, USA). Mean value
comparisons were performed between the groups using a Student's
t-test. P<0.05 was considered to indicate a statistically
significant difference.
Results
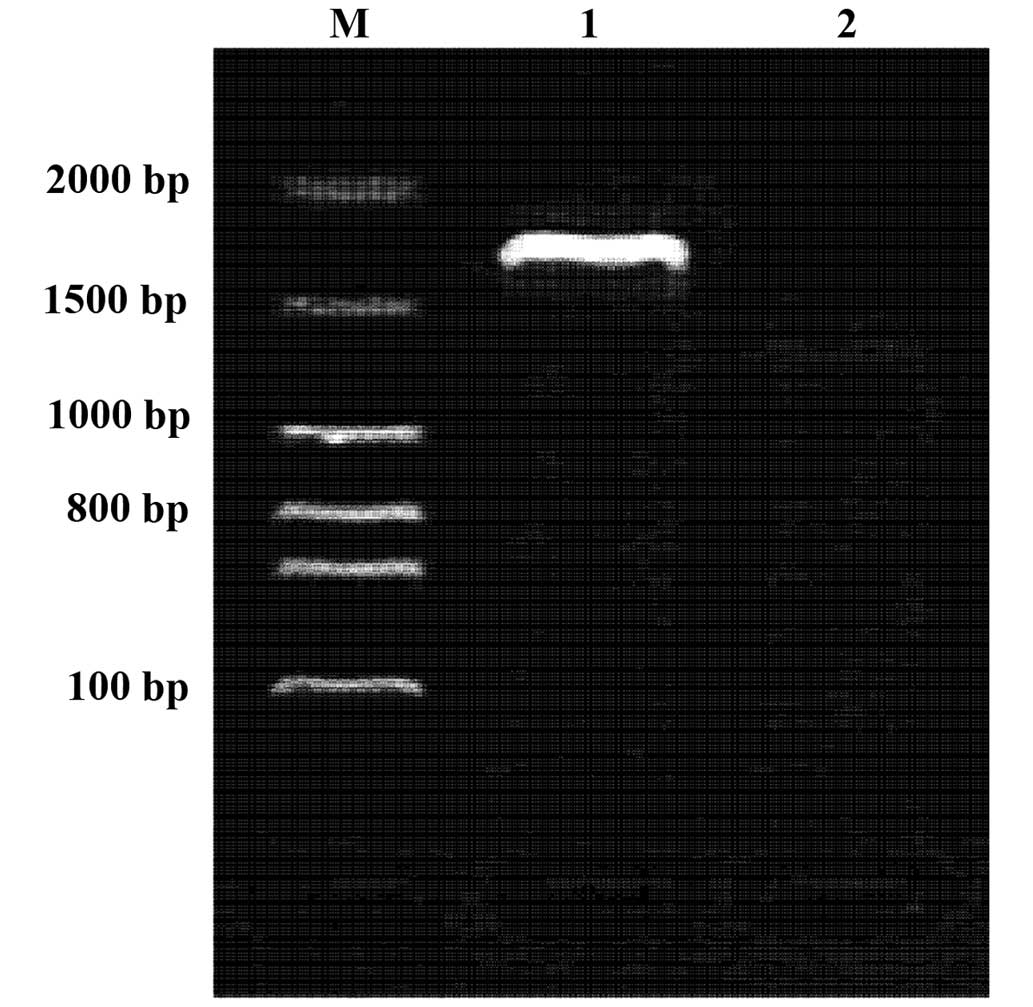
Double digestion of recombinant
plasmid pcDNA-VZV gE
The results of BamHI and XbaI double
digestion and subsequent electrophoresis demonstrated that ~2.7 kb
gE gene fragments were obtained following double digestion of the
recombinant plasmid. This result was consistent with the
predictions, suggesting that the plasmids were successfully
constructed (Fig. 1).
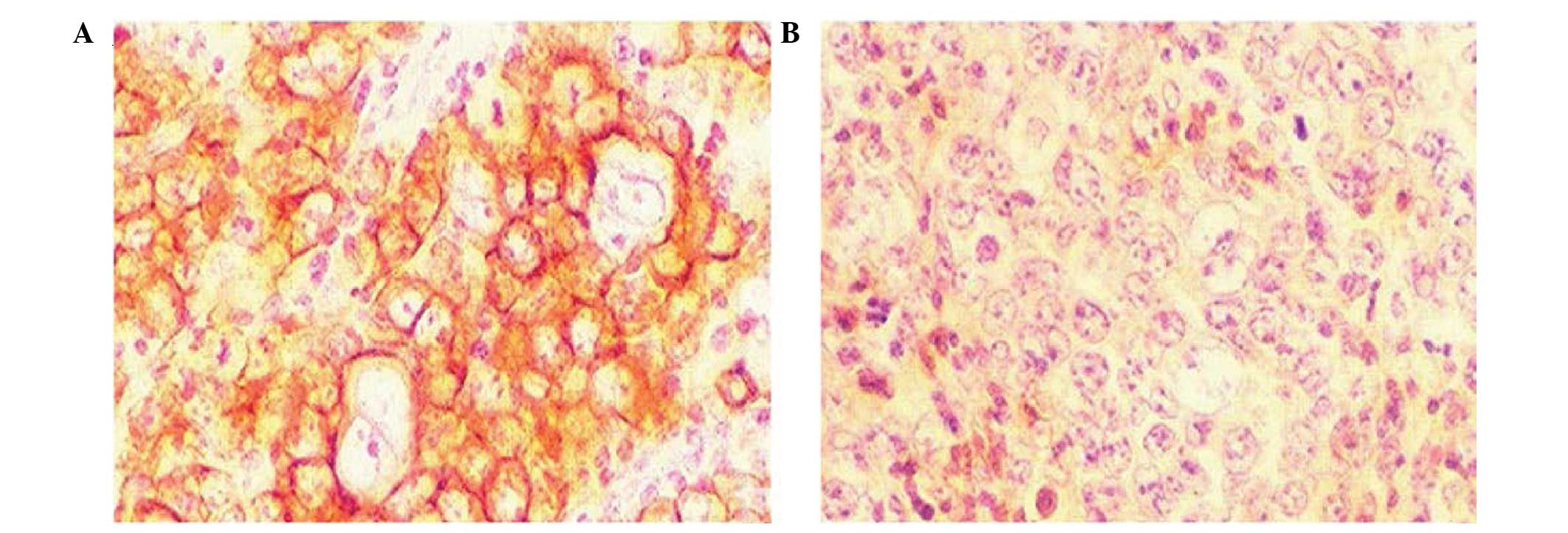
Immunohistochemistry
For the immunohistochemical detection of the
instantaneously expressed recombinant protein, the HRP-labeled
secondary antibody was added following generation of the pcDNA-VZV
gE cells and gE monoclonal antibody incubation. Following DAB
staining, orange particles were detected in the cytoplasm and cell
membrane using a microscope. The untransfected cells and those
transfected with the pcDNA3.1 null vector did not show staining
particles under the same conditions (Fig. 2).
In vivo expression of the pcDNA-VZV gE
recombinant plasmid
RT-PCR was conducted using the prepared primers and
total RNA from the muscles of mice immunized with pcDNA-VZV gE. The
corresponding amplified fragments were consistent with the size of
the predicted fragment (~2.7 kb). Under the same RT-PCR conditions,
the total RNA of the mice immunized with blank plasmid pcDNA did
not result in expression of the corresponding fragment. This result
suggests that the VZV gE recombinant plasmid induces mRNA
expression in vivo (Fig.
3).
Detection of the gE antibody in the
serum of immunized mice
On days 7, 21 and 35 following immunization, blood
samples were collected from the inner canthus of three mice in each
group. The serum samples were separated and used to detect specific
antibodies. The serum titers of the antigen-specific antibodies
were determined using an indirect ELISA. The results demonstrated
that the pcDNA-VZV gE group was positive for antigen-specific
antibodies following immunization, whereas the pcDNA3.1 and saline
groups were negative for gE antibodies. Therefore, by immunizing
mice with the pcDNA-VZV gE plasmid, a humoral immune response was
induced. On day 21 following immunization, the pcDNA-VZV gE group
demonstrated the highest antibody titer; however the titer of the
antibody had decreased by day 35 (Table
I and Fig. 4).
 | Table I.Titers of antigen specific antibody
in the serum of mice following immunization strengthening (IS). |
Table I.
Titers of antigen specific antibody
in the serum of mice following immunization strengthening (IS).
| Days post-IS | Saline | pcDNA3.1 | pcDNA-VZV gE |
|---|
| 7 | 0 | 0 | 1:32 |
| 21 | 0 | 0 | 1:256 |
| 35 | 0 | 0 | 1:64 |
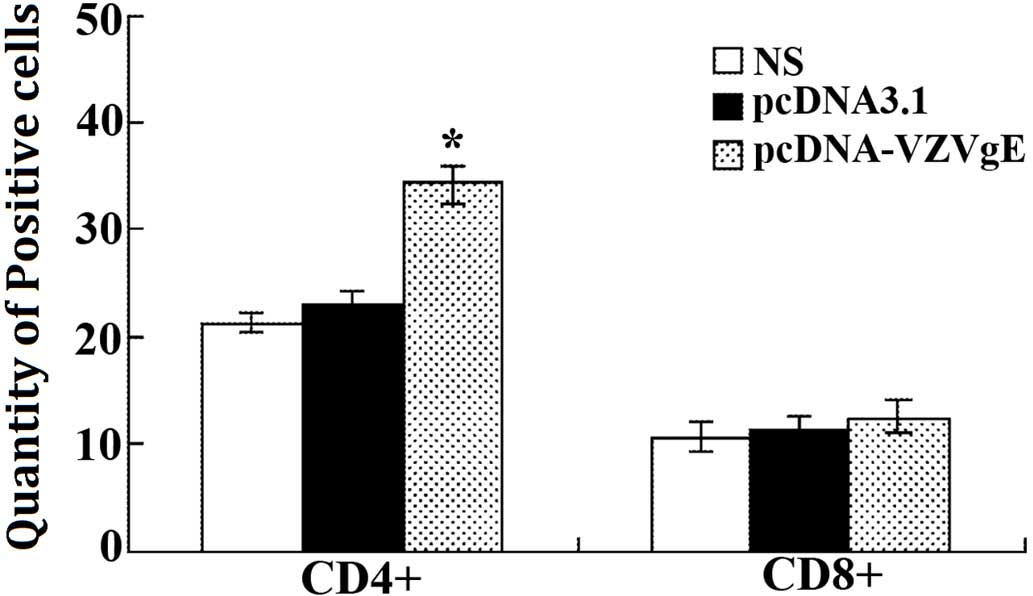
Changes in the number of T cell
subgroups in immunized mice
A flow cytometer was used to detect the number of
CD4+ and CD8+ positive T cells. The results
demonstrated that the total number of T cells in the pcDNA-VZV gE
plasmid-immunized group increased, as compared with the control
groups (Table II and Fig. 5). In particular, the number of
CD4+ positive cells significantly increased (P<0.01).
These results suggest that immunization with a recombinant plasmid
may effectively increase the number of T cells and effectively
strengthen the immune system of immunized mice.
 | Table II.Changes in the number of lymphocyte
subgroups in the spleen following immunization with the DNA
vaccine. |
Table II.
Changes in the number of lymphocyte
subgroups in the spleen following immunization with the DNA
vaccine.
| Marker | Saline | pcDNA3.1 | pcDNA-VZVgE |
|---|
|
CD4+ | 21.36 | 22.93 | 34.17a |
|
CD8+ | 10.62 | 11.28 | 12.45 |
Detection of spleen lymphocyte
proliferation activity in immunized mice
On days 7, 21 and 35 following immunization
strengthening, spleen lymphocytes were isolated from three mice in
each group under sterile conditions and lymphocyte proliferation
activity was determined using the MTT method. The lymphocyte
proliferation was significantly greater (P<0.01) in the
pcDNA-VZV gE group, as compared with that of the saline and
pcDNA3.1 groups on days 7, 21 and 35 following immunization
strengthening (Table III and
Fig. 6).
 | Table III.Spleen lymphocyte proliferation
activity following immunization strengthening (IS) in mice. |
Table III.
Spleen lymphocyte proliferation
activity following immunization strengthening (IS) in mice.
| Days post-IS | Saline | pcDNA3.1 | pcDNA-VZV gE | SI |
|---|
| 7 | 0.3244±0.0136 | 0.3315±0.0152 |
0.5216±0.0184a | 1.5735 |
| 21 | 0.3461±0.0128 | 0.3517±0.0157 |
0.9554±0.0162a | 2.7165 |
| 35 | 0.3647±0.0137 | 0.3582±0.0147 |
0.8965±0.0169a | 2.5028 |
Discussion
Nucleotide vaccines, such as DNA vaccines, contain
DNA molecules that are capable of immunizing a host against a
pathogen or disease (18). The DNA
vaccination process is performed as follows, the exogenous genes
are cloned into a eukaryotic expression vector and the recombinant
plasmid DNA is injected directly into the animal. These exogenous
genes are subsequently expressed in vivo, and the generated
antigens activate the immune system, triggering an immune reaction
(19,20). DNA vaccines are different from
traditional attenuated pathogen, protein or polypeptide vaccines
and, as such, DNA vaccines have particular advantages. DNA vaccines
can be simply and effectively produced using molecular biology.
Furthermore, the encoded antigen is capable of long-term stable
in vivo expression due to transcriptional control by an
appropriate promoter, thus inducing antibody and cell immunity.
These properties suggest a solid foundation for the widespread
application of DNA vaccines (21,22). The
biggest limitation of a traditional subunit vaccine is that the
antigen cannot be expressed in host cells, therefore cell immunity
cannot be induced (23). DNA
vaccines are capable of stimulating the synthesis of antigens in
the host cells, in a manner similar to the formation of antigens
following a pathogenic microorganism infection. The naturally
formed antigen is then processed and modified in a normal manner
prior to presentation to the immune system, which subsequently
stimulates an immune response (24,25).
Therefore, DNA vaccines possess the safety of recombinant subunit
vaccines and the high efficiency of live attenuated vaccines in
inducing a comprehensive immune response (26), and these immunogenic and protective
effects have been demonstrated in numerous animal models and
preliminary human clinical trials (27,28).
In the present study, a eukaryotic plasmid of the
VZV gE antigen, pcDNA-VZV gE, was successfully constructed,
transfected into COS7 cells in vitro and stably expressed.
This plasmid was subsequently used as a DNA vaccine, and
antigen-specific humoral and cellular immune responses were
detected on days 7, 14 and 21 following immunization via
antigen-specific antibody levels. The results of the present study
demonstrated that the VZV gE DNA group presented superior
immunogenicity, as compared with the pcDNA3.1 immunization group.
Superior immunogenicity was demonstrated in the increased
antigen-specific antibody levels generated by the pcDNA-VZV gE DNA
vaccine in the immunized mice, the lymphocyte proliferation
activity of the immunized mice following in vitro induction
culturing. However, by day 35 following immunization strengthening,
the specific antibody levels and the cytotoxic activity of
lymphocytes in the spleen had decreased in the DNA
vaccine-immunized mice. This decrease may be due to an independent
replication failure of the plasmid DNA in the mice; therefore,
antigen expression levels may gradually decrease with time due to
the decomposition of plasmid DNA by host nucleic acid enzymes.
In addition to the detection of immunogenicity
through animal experimentation, it is also important to further
elucidate the mechanisms by which DNA vaccines immunize hosts in
order to enhance the immunogenicity of future DNA vaccines
(29,30). For example, intramuscular injection
is the predominant method of administration for DNA vaccines.
Following inoculation, the corresponding antigen proteins of the
DNA vaccine may express in muscle cells; therefore it was initially
hypothesized that muscle cells were the predominant cells exerting
antigen-presenting function following immunization with a DNA
vaccine. However, further research has demonstrated that muscle
cells do not express costimulatory molecules, including CD80, also
known as B71, and CD86, also known as B7H; therefore, muscle cells
do not have a key role in antigen presentation (31,32).
Previously, Zhang et al (33)
coated gold particles with plasmid DNA and immunized turbot
(Scophthalmus maximus) using a gene gun. By analyzing local
draining lymph nodes, Zhang et al demonstrated that the
reporter gene and the expression products of the golden particles
were predominantly located in dendritic cells; thus demonstrating
that professional antigen-presenting cells remained the dominant
mechanism of antigen presentation following DNA vaccine
immunization. This theory is supported by the majority of research
at present (34). Subsequent studies
demonstrated that antigen presentation following DNA vaccine
immunization was not merely achieved by the passive direct
transfection of professional antigen-presenting cells, as it was
demonstrated that antigen-presenting cells were capable of uptaking
other cells, including muscle cells (35). In this way, these non-professional
antigen-presenting cells could be taken up following administration
of the DNA vaccine, and the intracellular protein may be released
following the physiological apoptosis or pathological necrosis of
the cells. That is to say, the antigens expressed following DNA
vaccine administration may be presented to CD8+ T cells in
combination with major histocompatibility complex (MHC) class I
molecules as endogenous antigens. Furthermore, the antigens may be
presented to CD4+ T cells by combining with MHC class II molecules
as exogenous antigens, resulting in a broader T cell response
(36,37). Therefore, the factors which are
capable of promoting the uptake, antigen processing and
upregulation of MHC class I and MHC class II molecules' expression
levels on antigen presenting cells, including monocytes,
macrophages and dendritic cells, are likely to enhance the
immunogenicity of DNA vaccines (38).
In the present study, a eukaryotic expression
plasmid of the VZV gE protein antigen gene, pcDNA-VZV gE, was
constructed and used as a DNA vaccine. This DNA vaccine was used to
immunize BALB/c mice and the related indices of cellular and
humoral immunity were detected at various time points. The mice
that had received the DNA vaccine exhibited increased in
vivo antigen-specific antibody levels, spleen lymphocyte
proliferation activity following in vitro induction culture
and T cell numbers, as compared with that of the pcDNA3.1 group.
Therefore, these results suggested that specific humoral and
cellular immune responses were induced in the mice following
immunization with the VZV gE gene DNA vaccine constructed in the
present study. These results may lay the foundation for further
research into a VZV DNA vaccines.
Acknowledgements
This study was funded by the following projects: The
Nature Science Foundation of Inner Mongolia Autonomous Region
(2013MS1224); Science and Technology Innovation Fund of Provincial
Department of Finance, Inner Mongolia Autonomous Region;
Collaborative Innovation Project of Mogolian Medicine, Inner
Mongolia Autonomous Region; Technology Reserve Project of
Provincial Department of Science and Technology, Inner Mongolia
Autonomous Region.
References
|
1
|
Schub D, Janssen E, Leyking S, Sester U,
Assmann G, Hennes P, Smola S, Vogt T, Rohrer T, Sester M and
Schmidt T: Altered phenotype and functionality of varicella zoster
virus-specific cellular immunity in individuals with active
infection. J Infect Dis. 211:600–612. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim YH, Hwang JY, Lee KM, Choi JH, Lee TY,
Choi JS and Park HS: Seroepidemiologic survey of varicella-zoster
virus in korean adults using glycoprotein enzyme immuno assay and
fluorescent antibody to membrane antigen test. Ann Dermatol.
23:39–43. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Suenaga T, Matsumoto M, Arisawa F, Kohyama
M, Hirayasu K, Mori Y and Arase H: Sialic acids on varicella-zoster
virus glycoprotein B are required for cell-cell fusion. J Biol
Chem. 290:19833–19843. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dendouga N, Fochesato M, Lockman L,
Mossman S and Giannini SL: Cell-mediated immune responses to a
varicella-zoster virus glycoprotein E vaccine using both a TLR
agonist and QS21 in mice. Vaccine. 30:3126–3135. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Grahn A, Studahl M, Nilsson S, Thomsson E,
Bäckström M and Bergström T: Varicella-zoster virus (VZV)
glycoprotein E is a serological antigen for detection of
intrathecal antibodies to VZV in central nervous system infections,
without cross-reaction to herpes simplex virus 1. Clin Vaccine
Immunol. 18:1336–1342. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thomsson E, Persson L, Grahn A, Snäll J,
Ekblad M, Brunhage E, Svensson F, Jern C, Hansson GC, Bäckström M
and Bergström T: Recombinant glycoprotein E produced in mammalian
cells in large-scale as an antigen for varicella-zoster-virus
serology. J Virol Methods. 175:53–59. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Oliver SL, Sommer MH, Reichelt M, Rajamani
J, Vlaycheva-Beisheim L, Stamatis S, Cheng J, Jones C, Zehnder J
and Arvin AM: Mutagenesis of varicella-zoster virus glycoprotein I
(gI) identifies a cysteine residue critical for gE/gI heterodimer
formation, gI structure and virulence in skin cells. J Virol.
85:4095–4110. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zerboni L, Berarducci B, Rajamani J, Jones
CD, Zehnder JL and Arvin A: Varicella-zoster virus glycoprotein E
is a critical determinant of virulence in the SCID mouse-human
model of neuropathogenesis. J Virol. 85:98–111. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Permyakova NV, Zagorskaya AA, Belavin PA,
Uvarova EA, Nosareva OV, Nesterov AE, Novikovskaya AA, Zav'yalov
EL, Moshkin MP and Deineko EV: Transgenic carrot expressing fusion
protein comprising M. tuberculosis antigens induces immune
response in mice. Biomed Res Int. 2015:4175652015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Berarducci B, Rajamani J, Zerboni L, Che
X, Sommer M and Arvin AM: Functions of the unique N-terminal region
of glycoprotein E in the pathogenesis of varicella-zoster virus
infection. Proc Natl Acad Sci USA. 107:282–287. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ali MA, Li Q, Fischer ER and Cohen JI: The
insulin degrading enzyme binding domain of varicella-zoster virus
(VZV) glycoprotein E is important for cell-to-cell spread and VZV
infectivity, while a glycoprotein I binding domain is essential for
infection. Virology. 386:270–279. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sauerbrei A, Wiesener N, Zell R and
Wutzler P: Sequence analysis of the glycoprotein E gene of
varicella-zoster virus strains of clades 1, 3 and 5. Arch Virol.
156:505–509. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang M, Tang JW, Song B, Wang B, Zhang J,
Wei YY, Zhang YH and Liu JW: Construction and identification of
recombinant eukaryotic plasmid pcDNA3.1(+)-ECH1. Zhong Hua Bing Li
Xue Za Zhi. 40:334–335. 2011.(In Chinese).
|
|
14
|
Zhang Y, Chang S, Sun J, Zhu S, Pu C, Li
Y, Zhu Y, Wang Z and Xu RX: Targeted microbubbles for ultrasound
mediated short hairpin RNA plasmid transfection to inhibit survivin
gene expression and induce apoptosis of ovarian cancer A2780/DDP
Cells. Mol Pharm. 12:3137–3145. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu JC, Lengner CJ, Gaur T, Lou Y, Hussain
S, Jones MD, Borodic B, Colby JL, Steinman HA, van Wijnen AJ, et
al: Runx2 protein expression utilizes the Runx2 P1 promoter to
establish osteoprogenitor cell number for normal bone formation. J
Biol Chem. 286:30057–30070. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Laderman EI, Whitworth E, Dumaual E, Jones
M, Hudak A, Hogrefe W, Carney J and Groen J: Rapid, sensitive and
specific lateral-flow immunochromatographic point-of-care device
for detection of herpes simplex virus type 2-specific
immunoglobulin G antibodies in serum and whole blood. Clin Vaccine
Immunol. 15:159–163. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shipkova M and Wieland E: Surface markers
of lymphocyte activation and markers of cell proliferation. Clin
Chim Acta. 413:1338–1349. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Im EJ, Borducchi EN, Provine NM, McNally
AG, Li S, Frankel FR and Barouch DH: An attenuated Listeria
monocytogenes vector primes more potent simian immunodeficiency
virus-specific mucosal immunity than DNA vaccines in mice. J Virol.
87:4751–4755. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chesnut G, McClain D and Galeckas K:
Varicella-zoster virus in children immunized with the varicella
vaccine. Cutis. 90:114–116. 2012.PubMed/NCBI
|
|
20
|
Ghanem A, Healey R and Adly FG: Current
trends in separation of plasmid DNA vaccines: A review. Anal Chim
Acta. 760:1–15. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Storlie J, Maresova L, Jackson W and Grose
C: Comparative analyses of the 9 glycoprotein genes found in
wild-type and vaccine strains of varicella-zoster virus. J Infect
Dis. 197(Suppl 2): S49–S53. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Berarducci B, Rajamani J, Reichelt M,
Sommer M, Zerboni L and Arvin AM: Deletion of the first
cysteine-rich region of the varicella-zoster virus glycoprotein E
ectodomain abolishes the gE and gI interaction and differentially
affects cell-cell spread and viral entry. J Virol. 83:228–240.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Polack FP, Lydy SL, Lee SH, Rota PA,
Bellini WJ, Adams RJ, Robinson HL and Griffin DE: Poor immune
responses of newborn rhesus macaques to measles virus DNA vaccines
expressing the hemagglutinin and fusion glycoproteins. Clin Vaccine
Immunol. 20:205–210. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Meng M, He S, Zhao G, Bai Y, Zhou H, Cong
H, Lu G, Zhao Q and Zhu XQ: Evaluation of protective immune
responses induced by DNA vaccines encoding Toxoplasma gondii
surface antigen 1 (SAG1) and 14–3-3 protein in BALB/c mice. Parasit
Vectors. 5:2732012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Galli V, Simionatto S, Marchioro SB, Fisch
A, Gomes CK, Conceição FR and Dellagostin OA: Immunisation of mice
with Mycoplasma hyopneumoniae antigens P37, P42, P46 and P95
delivered as recombinant subunit or DNA vaccines. Vaccine.
31:135–140. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Herrada AA, Rojas-Colonelli N,
González-Figueroa P, Roco J, Oyarce C, Ligtenberg MA and Lladser A:
Harnessing DNA-induced immune responses for improving cancer
vaccines. Hum Vaccin Immunother. 8:1682–1693. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fredriksen AB, Sandlie I and Bogen B:
Targeted DNA vaccines for enhanced induction of idiotype-specific B
and T cells. Front Oncol. 2:1542012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yu YZ, Li N, Ma Y, Wang S, Yu WY and Sun
ZW: Three types of human CpG motifs differentially modulate and
augment immunogenicity of nonviral and viral replicon DNA vaccines
as built-in adjuvants. Eur J Immunol. 43:228–239. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Martinez-Lopez A, Encinas P,
García-Valtanen P, Gomez-Casado E, Coll JM and Estepa A: Improving
the safety of viral DNA vaccines: Development of vectors containing
both 5′ and 3′ homologous regulatory sequences from non-viral
origin. Appl Microbiol Biotechnol. 97:3007–3016. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cai MS, Deng SX and Li ML: Comparison of
the immune responses in BALB/c mice following immunization with
DNA-based and live attenuated vaccines delivered via different
routes. Vaccine. 31:1353–1356. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li L, Saade F and Petrovsky N: The future
of human DNA vaccines. J Biotechnol. 162:171–182. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hartikka J, Bozoukova V, Morrow J, Rusalov
D, Shlapobersky M, Wei Q, Boutsaboualoy S, Ye M, Wloch MK, Doukas
J, et al: Preclinical evaluation of the immunogenicity and safety
of plasmid DNA-based prophylactic vaccines for human
cytomegalovirus. Hum Vaccin Immunother. 8:1595–1606. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang M, Hu YH, Xiao ZZ, Sun Y and Sun L:
Construction and analysis of experimental DNA vaccines against
megalocytivirus. Fish Shellfish Immunol. 33:1192–1198. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jiang DB, Sun YJ, Cheng LF, Zhang GF, Dong
C, Jin BQ, Song CJ, Ma Y, Zhang FL and Yang K: Construction and
evaluation of DNA vaccine encoding Hantavirus glycoprotein
N-terminal fused with lysosome-associated membrane protein.
Vaccine. 33:3367–3376. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhai Y, Zhou Y, Li X and Feng G:
Immune-enhancing effect of nano-DNA vaccine encoding a gene of the
prME protein of Japanese encephalitis virus and BALB/c mouse
granulocyte-macrophage colony-stimulating factor. Mol Med Rep.
12:199–209. 2015.PubMed/NCBI
|
|
36
|
Malavige GN, Jones L, Black AP and Ogg GS:
Varicella zoster virus glycoprotein E-specific CD4+ T cells show
evidence of recent activation and effector differentiation,
consistent with frequent exposure to replicative cycle antigens in
healthy immune donors. Clin Exp Immunol. 152:522–531. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Schmidt-Chanasit J, Bleymehl K, Schäd SG,
Gross G, Ulrich RG and Doerr HW: Novel varicella-zoster virus
glycoprotein E gene mutations associated with genotypes A and D. J
Clin Microbiol. 46:325–327. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Connolly RJ, Chapman T, Hoff AM, Kutzler
MA, Jaroszeski MJ and Ugen KE: Non-contact helium-based plasma for
delivery of DNA vaccines: Enhancement of humoral and cellular
immune responses. Hum Vaccin Immunother. 8:1729–1733. 2012.
View Article : Google Scholar : PubMed/NCBI
|