Introduction
Lymphangiomas are rare malformations of the
lymphatic system that present as benign submucosal tumors, and
largely dilated lymph channels and cisterns (1). These structures typically occur during
early childhood (90% occur in patients <2-years-old) and account
for ~4% of all vascular tumors in children. These tumors are
usually located in the head, neck and axilla (2). Nevertheless, lymphangiomas may occur at
any age and involve any part of the body; thus, a diagnosis of
lymphangioma should always be considered when investigating an
unexplained mass.
Although lymphangiomas of the gastrointestinal tract
are very rare, studies have previously reported such tumors
originating from the esophagus and stomach (3,4–7). The majority of cystic lymphangioma
cases are asymptomatic and, as such, misdiagnosis rates remain high
(8). Since lymphangioma are benign,
treatment is not usually required unless the legion is particularly
large. Common treatments include laparoscopy and surgical resection
(5).
The present study adds to the current literature by
reporting the case of 18-year-old female with cystic lymphangioma
arising from the cardia of the stomach. The patient presented with
abdominal distention and diagnosis of lymphangioma was confirmed by
histopathological analysis. The lesion was successfully treated
with endoscopic submucosal dissection (ESD). Following ESD, the
patient recovered quickly and fully, and no further symptoms or
complaints were reported. This distinctive case demonstrated that a
diagnosis of gastrointestinal lymphangioma should always be
considered at any age, and that ESD is an acceptable definitive
treatment strategy for such cases.
Case report
In September 2013, an 18-year-old female presented
at The First Hospital of Jilin University (Jilin, China) with
unexplained abdominal distention lasting 3 months. The patient had
no other gastrointestinal symptoms, such as stomachache, nausea or
emesis. Routine clinical tests, including a physical examination
and standard laboratory tests, were unremarkable and thus, an
endoscopic examination was required. The patient underwent
gastroscopy (Olympus GIF-Q260J; Olympus Corporation, Tokyo, Japan)
in September 2013 at the Department of Gastroenterology at The
First Hospital of Jilin University, which revealed a large
submucosal mass between the cardia and esophagogastric junction,
measuring 3.0×4.0 cm. The mass was soft and easily deformed by
pressure, and it had a smooth surface without ulceration or erosion
(Fig. 1A). The possibility of
submucosal lesions indicated further investigation using computed
tomography (CT), which revealed the presence of a low density
nodular mass with a diameter of ~4.0 cm protruding into the gastric
cavity and located anteromedial to the cardia, proximal to the
esophagogastric junction, and adjacent to the left lobe of the
liver (Fig. 1B). The aspect of the
mass was slightly enhanced when investigated using
contrast-enhanced CT (Ultravist 300; Bayer Corporation, Leverkusen,
Germany). Endoscopic ultrasonography (EUS) using a UM-DP 20–25R
probe (Olympus Corporation) was performed to further examine the
nature of the mass. This procedure resulted in the detection of a
homogenous and medium high-echo lesion, measuring 3.4×2.4 cm and
located in the submucosal layer (Fig.
1C). The lesion was not clearly separated from the muscle layer
and did not display evidence of invasion or a blood vessel
component. As it was suspected that the lesion was of a cystic
nature, further EUS-guided fine-needle aspiration biopsy collection
was not attempted.
Based on the aforementioned results, ESD with
intravenous 20 ml propofol (0.2 g; Fresenius Kabi AB Corporation,
Beijing, China) anesthesia was selected as the therapeutic
intervention for the preoperative diagnosis of a cystic lesion.
First, visualization of the lesion was improved by retroflexion of
the fundus of the stomach (Fig. 2A).
The lesion was then elevated by submucosal injection of 0.4% sodium
hyaluronate (Jingfeng Corporation, Shanghai, China). Subsequently,
the mucosal surface was dissected, exposing a submucosal lesion
that was soft and exhibited a white fiber membrane surrounding its
surface (Fig. 2B and C). An electric
knife (KD640-L; Olympus Corporation) was used to open the lesion,
causing release of ample brown viscous fluid flowing from the core
of the lesion (Fig. 2D). Subsequent
to washing with 0.9% saline, a smooth wall with well-demarcated
hyperemic spots was observed (Fig. 2E
and F). The procedure ended with the cystic lumen remaining
open, without closure of the dissected site. The partial resection
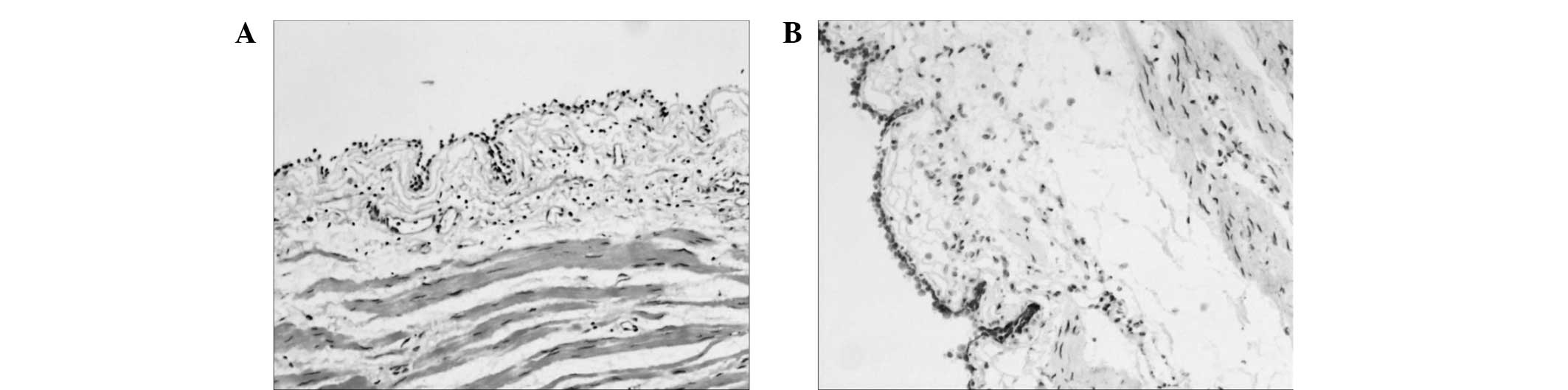
of the cystic wall and hyperemic mucosa were examined by
histopathology, including immunohistochemistry for the lymphatic
endothelium-specific 40-kDa O-linked sialoglycoprotein D240 using a
two-step Max-Vision kit, according to the manufacturer's protocol
(Zhongshan Jinqiao Biotechnology Co., Ltd., Beijing, China). The
results revealed the presence of highly-dilated lymphatic channels
and D2-40-positive lymph vessels (Fig.
3A and B), which were consistent with a diagnosis of submucosal
lymphangioma. The patient was discharged without any complications
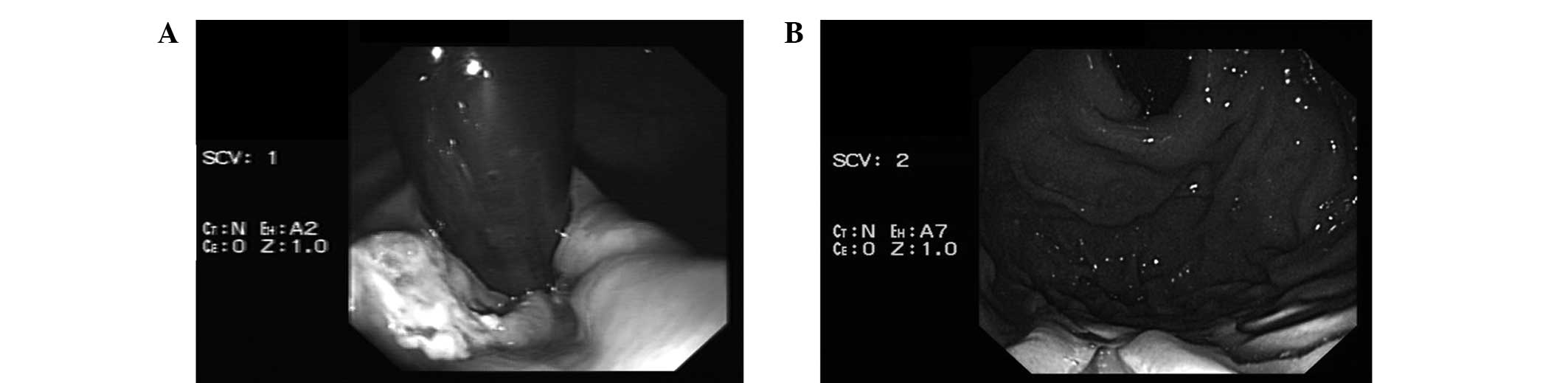
after 5 days and tolerated a regular diet. At 1 week after the
surgery, gastroscopy demonstrated ulceration at the site of ESD,
which was in line with the normal postoperative changes expected
following the procedure (Fig. 4A).
At 6-month follow-up, gastroscopy demonstrated that the ulcer had
healed and the mucosa of the cardia was smooth without any signs of
recurrence (Fig. 4B). The patient
reported no further symptoms or discomfort. The last follow-up was
in March 2015; gastroscopy demonstrated there was no recurrence and
the patient was not suffering from any discomfort. Written informed
consent was obtained from the patient prior to publication.
Discussion
Endoscopy and EUS have become indispensable tools
for diagnosing and differentiating gastric submucosal lesions and
other gastric tumors (9). With the
increasing number of patients undergoing endoscopic evaluation, it
is to be expected that largely asymptomatic cases of
gastrointestinal system-associated masses will be detected more
frequently (10). In the present
study, a case of a giant cystic lymphangioma arising from the
cardia was reported in an 18-year-old patient complaining of
abdominal distention. The lesion was treated by ESD, and the
results of the pathological examination were consistent with a
diagnosis of cystic lymphangioma.
Lymphangioma originating from the gastrointestinal
tract is very rare. The condition typically occurs in the head,
neck and axilla, and the vast majority of cases occur in young
children (2). Its detection in
adolescents has been rarely reported; however, as the present study
demonstrates, with increased endoscopic surveillance the detection
of such cases may increase (3–5,10). Thus, gastroenterologists should be
aware of the possibility of this type of lesion. Although the
etiology of cystic lymphangiomas is not fully understood (11), anomalous development of the
lymphatics or inflammation and obstruction of developed lymphatic
channels have been proposed as possible mechanisms (3). To date, only a small number of studies
have described cystic lymphangiomas occurring intra-abdominally in
adolescent patients (12). In
particular, only four cystic lymphangiomas originating from the
lesser curvature of the stomach have been documented (3–5,10,13). The
present study described cystic lymphangioma arising from the cardia
and the anterior wall of the distal body. Although the majority of
cases occur in young children, clinical presentation can be highly
diverse and may include chronic abdominal pain, hunger pain, sour
regurgitation, nausea and weight loss (5). Clinically, the majority of cases are
asymptomatic and thus detected incidentally. In the current case,
the patient presented with a 3-month history of abdominal
distension, and the diagnosis of lymphangioma can be considered to
be coincidental based on its non-specific clinical presentation.
Therefore, it can be predicted that a large number of cases of
lymphangioma remain undetected.
With respect to the diagnosis of cystic
lymphangioma, CT constitutes an important imaging modality in which
the aberration presents as a non-enhancing extramucosal mass with
homogeneous attenuation, allowing evaluation of the extent of the
lesion before the treatment commences (14). In the case of gastric cystic
lymphangioma, the disease can manifest itself as a submucosal
polypoid mass with an endoscopically smooth surface. Upon physical
manipulation by forceps, the mass appears softer than its
surroundings. Furthermore, a cystic echo pattern during EUS
characterizes gastric cystic lymphangiomas (6,7).
Differential diagnoses for submucosal tumors of the stomach include
lipoma, hemangioma, mesenchymoma, leiomyoma and other tumors
(15). Clinically and
radiologically, it is difficult for these lesions to be
distinguished from each other. Endoscopy and EUS are important
diagnostic tools for this purpose, as they can establish whether
the tumor is located in the mucosa or submucosa, thus aiding the
selection of operative procedures, such as endoscopic resection or
surgery (16). Regarding
histopathology, D2-40 is a specific marker of lymphatic endothelial
cells, therefore if positive expression is detected in the lymph
vessels then reaching a diagnosis of cystic lymphangioma is
relatively straightforward. Cyst contents are variable and may
include serous, hemorrhagic, chylous or mixed fluids (17).
Lymphangiomas are benign and do not typically
require medical treatment, although patients may select surgical
removal for personal reasons or to prevent local invasion.
Treatment options include endoscopic removal of lesions <2 cm or
surgical resection of larger lesions (18). The main choice of treatment is
surgical resection, usually performed via laparoscopy, which has
good safety and efficacy. Generally, surgical resection is only
applied to larger lesions and endoscopy appears to be a viable
alternative. Endoscopy and EUS can be used to determine the size
and precise location of the lesion. Endoscopic mucosal resection
and ESD are the most widely used endoscopic resection methods
(6). They are considered to be
minimally invasive and safe, while complication rates are less than
those of surgery. Endoscopic minimally invasive treatment has
evident advantages over traditional treatments and was successfully
applied in the present case. Nevertheless, open surgery remains an
option for the management of lymphangioma for lesions that are
large or not suitable for endoscopy.
In conclusion, the present study reported an unusual
case of histologically confirmed cystic lymphangioma arising from
the cardia of the stomach, which was studied by gastroscopy, EUS
and CT, and treated by ESD. A review of the literature supports
that endoscopic treatment of lymphangioma, in relation to its size,
location and the absence of complications, is an appropriate
method. In addition, the present case demonstrated that
lymphangioma should be considered when adolescent patients present
atypical symptoms.
References
|
1
|
Okazaki T, Iwatani S, Yanai T, Kobayashi
H, Kato Y, Marusasa T, Lane GJ and Yamataka A: Treatment of
lymphangioma in children: Our experience of 128 cases. J Pediatr
Surg. 42:386–389. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Grasso DL, Pelizzo G, Zocconi E and
Schleef J: Lymphangiomas of the head and neck in children. Acta
Otorhinolaryngol Ital. 28:17–20. 2008.PubMed/NCBI
|
|
3
|
Leland HA, Lee JT, Tan JH, Romine LE and
Bansal V: Cystic lymphangioma of the lesser curvature of the
stomach-case report. J Radiol Case Rep. 5:31–37. 2011.PubMed/NCBI
|
|
4
|
Gockel I, Muller H, Kilic M, Eitelbach F,
Gaedertz Chr and Peters H: Giant cystic lymphangioma of the
stomach. Eur J Surg. 167:927–930. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
van Oudheusden TR, Nienhuijs SW, Demeyere
TB, Luyer MD and de Hingh IH: Giant cystic lymphangioma originating
from the lesser curvature of the stomach. World J Gastrointest
Surg. 5:264–267. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao ZF, Kuang L, Zhang N, Ma SR, Yang Z,
Han X, Zhao YF, Gao F, Gong ZJ and Yang L: Endoscopic diagnosis and
treatment of esophageal cavernous lymphangioma. Surg Laparosc
Endosc Percutan Tech. 23:299–302. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Arashiro M, Satoh K, Osawa H, Yoshizawa M,
Nakano H, Ajibe H, Miura Y, Yoshida T, Hirasawa T, Yamamoto H and
Sugano K: Endoscopic submucosal dissection of esophageal
lymphangioma: A case report with a review of the literature. Clin J
Gastroenterol. 3:140–143. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hoffmann J, Kirschniak A, Scharf G,
Feilitzsch VM, Konigsrainer A and Zdichavsky M: Laparoscopic
resection of a lymphangiomatous cyst of the colon: A case report. J
Med Case Rep. 5:431–434. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kang JH, Lim JS, Kim JH, Hyung WJ, Chung
YE, Choi JY, Park MS, Kim MJ and Kim KW: Role of EUS and MDCT in
the diagnosis of gastric submucosal tumors according to the revised
pathologic concept of gastrointestinal stromal tumors. Eur.
19:924–934. 2009.
|
|
10
|
Kim HS, Lee SY, Lee YD, Kim DH, Kwon JG,
Tak WY, Kweon YO, Kim SK, Choi YH and Chung JM: Gastric
lymphangioma. J Korean Med Sci. 16:229–232. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Weeda VB, Booij KA and Aronson DC:
Mesenteric cystic lymphangioma: A congenital and an acquired
anomaly? Two cases and a review of the literature. J Pediatr Surg.
43:1206–1208. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Roisman I, Manny J, Fields S and Shiloni
E: Intra-abdominal lymphangioma. Br J Surg. 76:485–489. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Alvite Canosa M, Alonso Fernandez L,
Seoane Vigo M, Pérez Grobas J, Berdeal Díaz M, Bouzón Alejandro A,
Carral Freire M, Budiño Ramos J and Gómez Freijoso C: Abdominal
cystic lymphangioma in a teenager. Rev Esp Enferm Dig. 100:517–518.
2008.PubMed/NCBI
|
|
14
|
Zhu H, Wu ZY, Lin XZ, Shi B, Upadhyaya M
and Chen K: Gastrointestinal tract lymphangiomas: findings at CT
and endoscopic imaging with histopathologic correlation. Abdom
Imaging. 33:662–668. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Papanikolaou IS, Triantafyllou K, Kourikou
A and Rösch T: Endoscopic ultrasonography for gastric submucosal
lesions. World J Gastrointest Endosc. 3:862011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hizawa K, Matsumoto T, Kouzuki T, Suekane
H, Esaki M and Fujishima M: Cystic submucosal tumors in the
gastrointestinal tract: endosonographic findings and endoscopic
removal. Endoscopy. 32:712–714. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Levy AD, Cantisani V and Miettinen M:
Abdominal lymphangiomas: Imaging features with pathologic
correlation. AJR Am J Roentgenol. 182:1485–1491. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Irisawa A and Bhutani MS: Cystic
lymphangioma of the colon: Endosonographic diagnosis with
through-the-scope catheter miniprobe and determination of further
management. Dis Colon Rectum. 44:1040–1042. 2001. View Article : Google Scholar : PubMed/NCBI
|