Introduction
Peptic ulcer development is one of the most frequent
complications of type 2 diabetes mellitus (T2DM) due to the
increased likelihood of Helicobacter pylori (H.
pylori) infection in T2DM patients, resulting in symptoms such
as bleeding and perforation (1). The
symptoms of peptic ulcers also include a burning sensation,
belching, weight loss and poor appetite. Peptic ulcers affect 4% of
the population and were the cause of 301,000 deaths in 2013
(2,3). T2DM is a metabolic disorder
characterized by hyperglycemia in association with insulin
resistance and lack of insulin (4).
Furthermore, ulcers are the most common complication of metabolic
disorders, and are associated with severe pathological lesions,
such as extensive vascular lesions, and mucosa ischemic necrosis
(5).
Endothelial progenitor cells (EPCs) are biological
markers for vascular function, since they have an important role in
vascular repair and angiogenesis (6). The development of T2DM has been
demonstrated to be closely associated with low levels of
circulating EPCs (7). Furthermore, a
previous study has reported that peripheral vascular disease in
T2DM patients was associated with a low number of EPCs (8). Although the role of EPCs in ulcer
healing in humans has yet to be investigated, a reduction of EPCs
in patients with diabetic foot ulcers has been demonstrated
(9). Therefore, the hypothesis that
EPC injury is associated with T2DM and contributes to a poor
clinical outcome in peptic ulcer patients with T2DM requires
further investigation.
In the present study, circulating EPCs were obtained
from the blood samples of three groups, including peptic ulcer
patients with T2DM, peptic ulcer patients without T2DM and healthy
controls. The study aimed to examine the association of the
quantity and function of circulating EPCs with the curative effect
of various treatments, in order to provide novel strategies for the
treatment of peptic ulcers in patients with T2DM.
Patients and methods
Patients and groups
All subjects were recruited from the Department of
Gastroenterology, Gongli Hospital (Shanghai, China) between January
2011 and December 2013. In total, three groups of patients were
examined: Peptic ulcer patients with T2DM (group A; n=32;
age, 64.4±6.3 years; 18 male and 14 female); peptic ulcer patients
without T2DM (group B; n=32; age, 65.1±5.8 years; 17 male
and 15 female); and healthy control subjects (group C; n=32;
age, 64.8±6.9 years; 18 male and 14 female).
Prior to inclusion into the present study, subjects
underwent T2DM evaluation using the diagnostic criteria for DM as
determined by the World Health Organization (10), and peptic ulcer disease was evaluated
using the assessment of diagnosis of peptic ulcer reviewed by the
Editorial Board of Chinese Journal of Digestion (11). Furthermore, an endoscopy and H.
pylori infection diagnosis were conducted to further determine
the health conditions of the patients. The healthy control patients
were also subjected to blood glucose examination, the 14C-urea
breath test and gastric biopsies.
Subjects with the following characteristics were
excluded from the study: i) Malignant lesions in the gastric
ulcers, which were identified using pathology techniques; ii)
concurrent severe H. pylori infection and acidosis in the
patients with T2DM; iii) severe complications associated with the
ulcers; iv) drug administration, such as non-steroidal
anti-inflammatory drugs, corticosteroids or statins; v) acute
myocardial infarction, angina and peripheral vascular disease; or
vi) having undergone surgery of any kind within the last 24
months.
The present study was approved by the Ethics
Committee of Gongli Hospital and written informed consent was
obtained from all participants.
Treatment protocols
Peptic ulcer patients with T2DM were treated with 10
mg glipizide daily (Pfizer, Inc., New York, NY, USA) to lower the
blood glucose levels, and patients with characteristics of
hematemesis or hematochezia were treated with daily injections of 8
units novolin (Novo Nordisk, Bagsvaerd, Denmark). Omeprazole (20
mg; Hainan Haili Pharmaceutical Co. Ltd., Haikou, China) was
administered as an antiulcer proton pump inhibitor for 8 weeks in
peptic ulcer patients with or without T2DM. In addition, H. pylori
infection in peptic ulcer patients was treated with a combination
of amoxicillin (0.5 g every 8 h; CSPC Pharmaceutical Group,
Shijiazhuang, China), clarithromycin (250 mg every 12 h; Abbott
Laboratories, Lake Bluff, IL, USA) and metronidazole (1.2 g daily;
Novartis, Basel, Switzerland) for 2 weeks. This treatment regimen
was maintained for 8 weeks.
Evaluation of treatment effect
The curative effects of the treatments were
evaluated based on clinical symptoms and endoscopy results. Various
scales of treatment efficacy were defined: i) Complete recovery was
determined when clinical symptoms and signs of peptic ulcer,
including the mucosal defect, were no longer present, as determined
by gastroscopy; ii) partially effective treatment was determined in
cases where clinical symptoms and signs were markedly decreased,
but not absent, and >50% the mucosal defect area had been
repaired; iii) ineffective treatment was determined in cases where
the clinical symptoms and signs were increased or unchanged, and
the mucosal defect was not filled or was enlarged. In addition, a
negative result for H. pylori as determined by gastroscopic
biopsies, or a CO2 concentration <100 dpm/mM as
determined by a Hp [14C]-urea breath test indicated the
absence of H. pylori infection.
Isolation and culture of circulating
EPCs
Peripheral blood samples (20 ml) were obtained from
all three groups; these were drawn from the peptic ulcer patients
following anti-ulcer treatment for 8 weeks. Of these samples, 15 ml
was used for EPC isolation and culture and 5 ml for flow cytometry.
Peripheral blood mononuclear cells (PBMCs) were isolated by
magnetic bead selection. Briefly, a single-cell suspension
(1×108 cells/300 µl) was prepared by standard methods
(12). A total of 100 µl beads
(Miltenyi Biotec GmbH, Bergisch Gladbach, Germany), CD133 antigen
and 100 µl Fc-receptor (Miltenyi Biotec GmbH) were added to the
cell suspension and incubated for 40 min at 4°C. The cells were
subsequently placed in an LS Magnetic Cell Sorting column (Miltenyi
Biotec GmbH) in a magnetic field. The trapped cells were released
by placing the column in a Dynal magnet (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) for at least 1 min.
CD133+ cells isolated from the blood samples were
collected in Medium 199 (Sigma-Aldrich, St. Louis, MO, USA) with
the following supplements (all from PeproTech, Rocky Hill, NJ,
USA): Medium 199, 10% fetal bovine serum, 10 µg/l vascular
endothelial growth factor (VEGF) and 2 µg/l basic fibroblast growth
factor.
The cells were seeded into fibronectin-treated
6-well culture plates (PeproTech) at a final density of
1×107 cells/well. At 48 h after seeding, nonadherent
cells (1.0×106 cells/well) were cultured at 37°C in an
atmosphere containing 5% CO2 for 21 days. The culture
medium was changed every 2–3 days. During culture, an inverted
phase contrast microscope (IX70-81FZ; Olympus Corporation, Tokyo,
Japan) was used to observe the EPC morphology and growth in
vitro at days 3, 7, 10, 14 and 21 of culture.
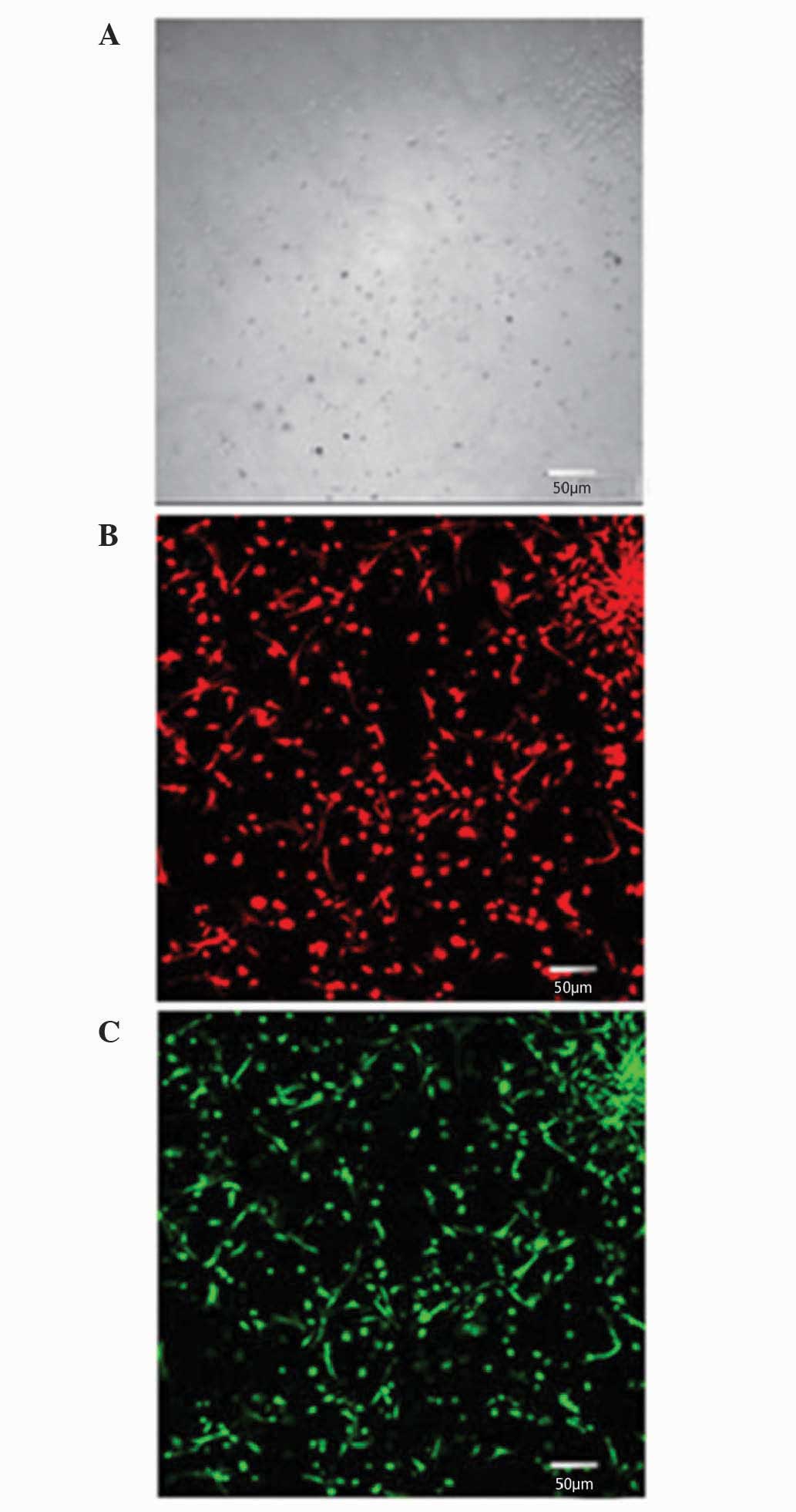
Identification of vascular endothelial
cells
To identify vascular endothelial cells, dual
staining for fluorescently labeled Dil-acetylated-low density
lipoprotein (Dil-ac-LDL; Molecular Probes; Thermo Fisher
Scientific, Inc.) and fluorescein isothiocyanate (FITC)-Ulex
europaeus agglutinin (UEA)-1 (Sigma-Aldrich) was performed on
day 4 of the culture. The cells were incubated at 37°C for 4 h with
10 µg/ml Dil-ac-LDL, and then fixed with 4% paraformaldehyde for 10
min. Subsequent to PBS washing, the cells were treated with 10.0
µg/ml FITC-UEA-1 for 30 min. A laser scanning confocal microscope
(TCS-SP5; Leica Microsystems GmbH, Wetzlar, Germany) was used for
observation, differentiation and identification.
Flow cytometry of circulating
EPCs
A 5-ml blood sample (from the aforementioned 20 ml
sample) was used for EPC counting. Approximately 0.2 ml mononuclear
cells obtained by magnetic bead selection was used for cell
counting. Briefly, 0.2 ml cell suspension was incubated with
monoclonal mouse phycoerythrin-conjugated anti-CD34 antibody
(dilution, 1:1,000; cat. no. FAB7227P; R&D Systems, Inc.,
Minneapolis, MN, USA), mouse FITC-conjugated anti-CD45 (dilution,
1:2,000; cat. no. 9625-02; SouthernBiotech, Birmingham, AL, USA)
and monoclonal mouse phycoerythrin-conjugated anti-type 2 VEGF
(VEGF-R2; dilution, 1:1,000; cat. no. FAB357P; R&D Systems,
Inc.) at room temperature for 20 min in the dark. The cells were
then blocked for non-specific binding by incubation in red blood
cell lysate for 15 min. Samples with a density of 1×106
cells were analyzed using a FC5000 cytometer (Beckman Coulter,
Inc., Brea, CA, USA).
Circulating EPCs were negative for the leucocyte
marker CD45, positive for the prototypical stem cell marker CD34,
and positive for the endothelial cell marker VEGF-R2 (13).
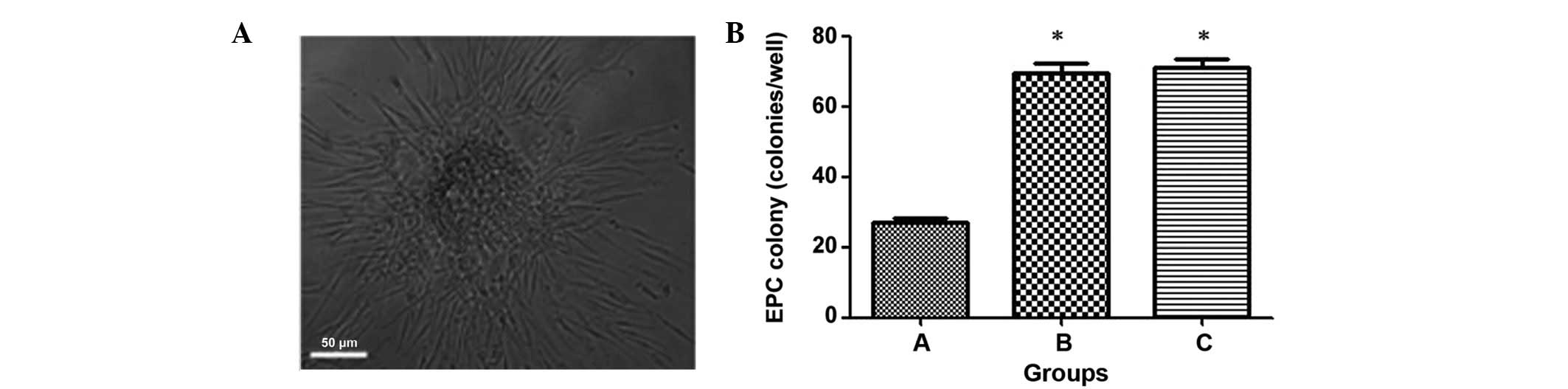
Circulating EPC colony counts
Colonies were evaluated after 7 days of culture, and
a colony was defined as a central core of ‘round’ cells with
elongated ‘sprouting’ cells at the periphery. Three researchers
independently counted the EPC colonies, and experiments were
conducted four times per patient.
Statistics analysis
Values are presented as the mean ± standard
deviation. The statistical significance of the data was first
assessed using a Kolmogorov-Smirnov test. Comparison of contingency
values and frequency was analyzed using a χ2 test.
Multiple comparisons were performed with a one-way analysis of
variance, and Student's t test was applied for single comparisons.
Statistical analyses were conducted using SPSS version 12 (SPSS,
Inc., Chicago, Il, USA). P<0.05 was considered to indicate a
statistically significant result.
Results
Treatment efficacy in the experimental
groups
The treatment efficacy in the experimental groups
was evaluated (Table I). Treatment
was partially effective in 12 of the 32 peptic ulcer patients with
T2DM, and ineffective in the remaining 20 patients. Notably, 25
patients were defined as completely recovered following treatment,
and the treatment was considered partially effective in 7 patients
among the 32 peptic ulcer patients without T2DM. The treatment was
therefore more effective in patients without T2DM than those with
T2DM.
 | Table I.Treatment effect for peptic ulcer
patients. |
Table I.
Treatment effect for peptic ulcer
patients.
|
| Peptic ulcer patients
with T2DM (n=32) | Peptic ulcer patients
without T2DM (n=32) |
|---|
|
|
|
|
|---|
| Category | Partially
effective | Ineffective | Complete
recovery | Partially
effective |
|---|
| No. of patients,
n | 12 | 20 | 25 | 7 |
| Male/female patients,
n/n | 8/4 | 10/10 | 15/10 | 2/5 |
| Age, years | 63.9±5.2 | 64.3±5.4 | 65.3±3.8 | 64.6±5.5 |
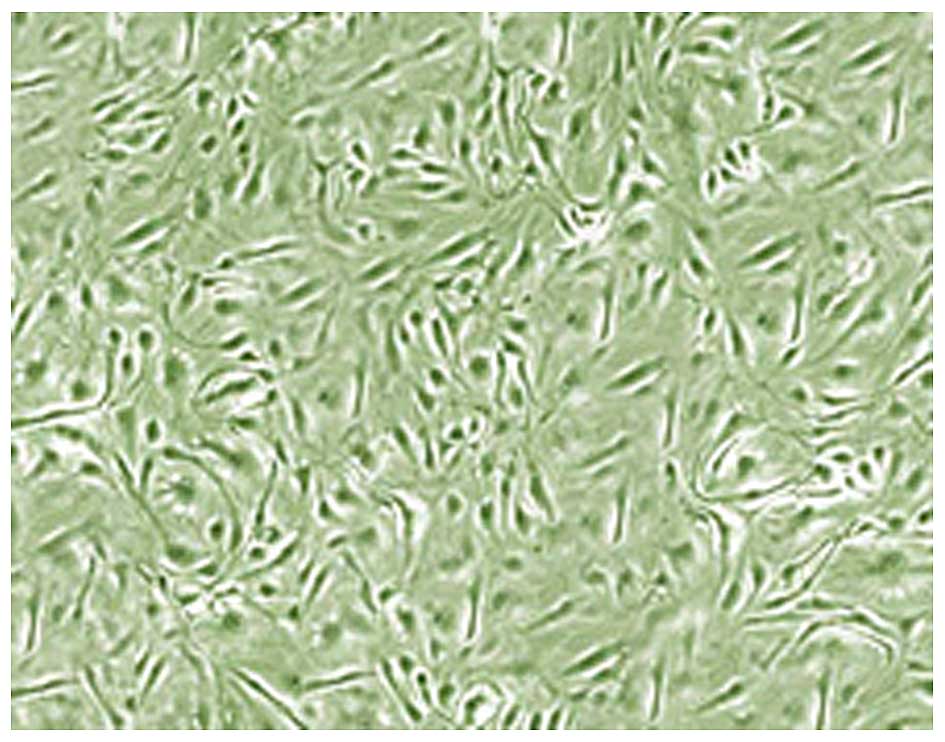
EPC characterization
EPC characterization was conducted using
fluorescence microscopy. PBMCs were found to exhibit circular
morphology and were suspended in the medium. The volume of adherent
cells increased following 3 days of culture, as well as the number
of diamond-shaped cells. Colonies of adherent cells began to appear
at day 7. The majority of the cells were of bipolar spindle shape
and exhibited a cable-like structure at day 14. As shown in
Fig. 1, the adherent cells displayed
cobble stone-like morphology at day 21. Notably, flow cytometry
immunophenotyping revealed that isolated cells expressed Dil-ac-LDL
and UEA-1 after 10 days of culturing (Fig. 2).
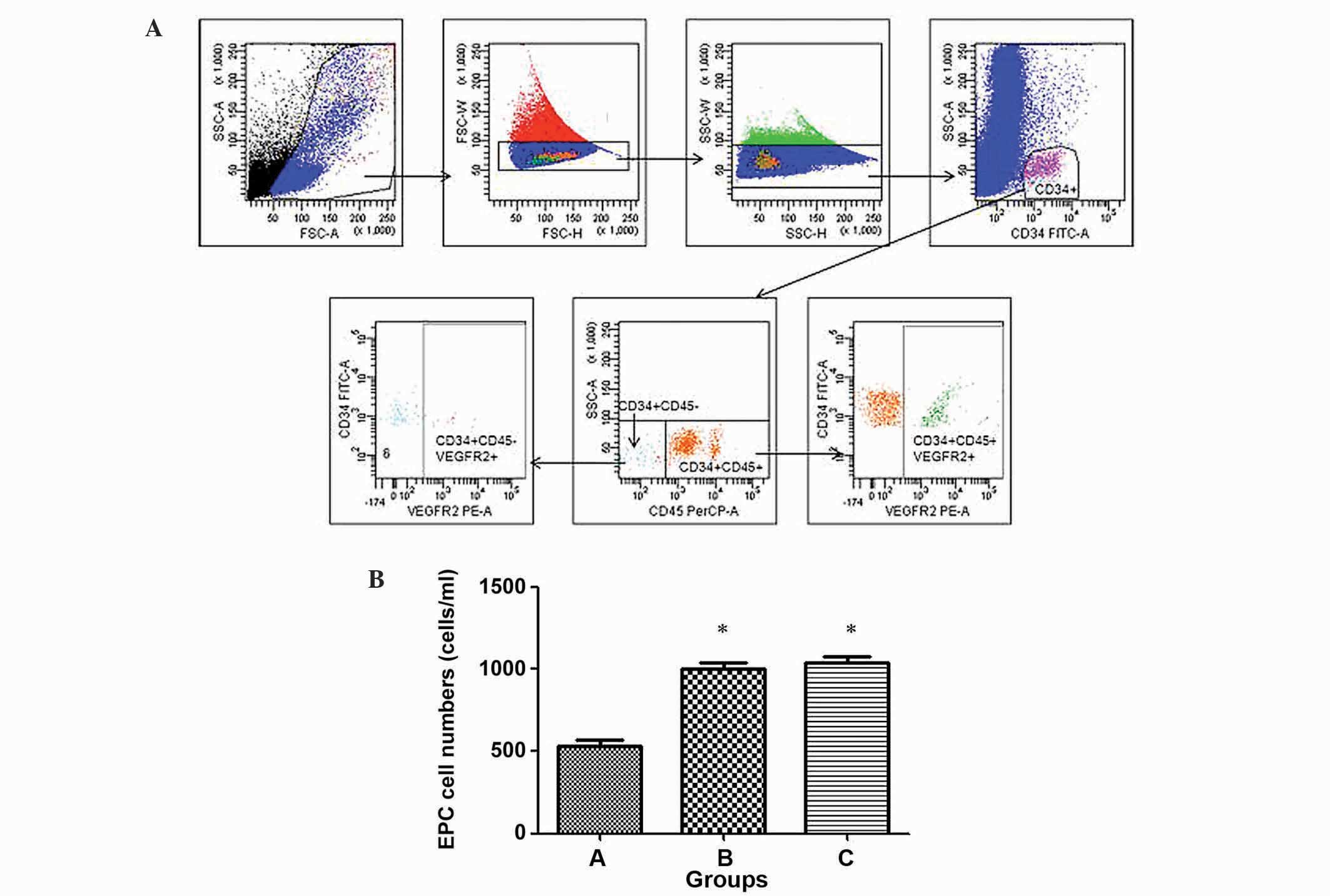
Comparison of changes in EPCs in the
three treatment groups
To evaluate the number of vascular endothelial cells
derived from circulating EPCs in the three groups, cells were
counted using flow cytometry. As shown in Fig. 3, group C (healthy control patients)
exhibited the highest circulating EPC-forming ability with
1,045±106 cells/ml, whereas group A (peptic ulcer patients with
T2DM) exhibited the lowest ability with 532±90 cells/ml. The number
of circulating EPCs in group A was significantly decreased compared
with groups B (peptic ulcer patients without T2DM; 1,002±93
cells/ml) and C (P<0.05), while no significant difference was
observed between groups B and C (P>0.05).
To evaluate the colony forming ability of the
circulating EPCs in the three groups, colonies were counted by
three independent researchers (Fig.
4). Similarly, group C exhibited the highest circulating EPC
forming ability (70±9 colonies/ml), whereas group A exhibited the
lowest ability (28±5 colonies/ml). The number of circulating EPC
colonies in group A were significantly increased compared with
groups B (68±8 colonies/ml) and C (P<0.05), whereas group B did
not reveal significantly different circulating colony numbers to
group C (Fig. 4) (P>0.05). This
indicates that the EPC forming ability may be significantly
disrupted in group A.
Discussion
A previous study demonstrated that circulating EPCs
were associated with microvascular disease (7), whereas studies have yet to report the
existence of an association between circulating EPCs and ulcer
treatment. The results of the present study indicated that,
compared with peptic ulcer patients without T2DM, peptic ulcer
patients with T2DM exhibited poor treatment outcomes. In addition,
the number of EPCs was most significantly decreased in peptic ulcer
patients with T2DM, compared with the other two groups.
Furthermore, the lowest colony forming ability of circulating EPCs
was present in peptic ulcer patients with T2DM.
The present study measured the most currently used
phenotypic markers for assessing vascular endothelial cells,
including CD34, CD133 and VEGR-R2 (14). The results indicated that peptic
ulcer patients with T2DM had significantly reduced numbers of
circulating EPCs, compared with peptic ulcer patients without T2DM
and healthy controls. Furthermore, following treatment with peptic
ulcer drugs, patients with and without T2DM exhibited significantly
reduced colony forming abilities, as compared with healthy
controls. It is widely known that angiogenesis and tissue repair
are required for ulcer healing (15). In addition, previous studies
demonstrated that EPC injury reduces regeneration, contributing to
low microvascular density, slow blood vessel formation and delayed
cellular renewal, suggesting an important role for EPCs in
endogenous vascular repair (16,17). The
results of the present study suggested that ulcer treatment may be
associated with EPC impairment.
Patients with diseases associated with T2DM were
demonstrated to have lower levels of circulating EPCs (18,19),
results which were not concordant with a hypothesis of association
between ulcer treatment and EPC injury. However, the lower
colony-forming ability of circulating EPCs from peptic ulcer
patients compared with that in healthy controls suggested a
correlation between EPC injury and peptic ulcers. Further studies
that examine the association between EPCs and ulcer treatment, and
adjust to EPC changes induced by T2DM are therefore required.
Recent studies have sought stem cell-based
approaches to harness vascular regeneration in view of its capacity
for self-renewal and directed differentiation (20–22).
Given the hypothetical ability of EPCs to differentiate and form
new blood vessels, the use of EPCs for vascular regeneration was
presented by Asahara et al (23) in 1997. Recently, stem cell-based
therapy has been studied in clinical trials (24,25). The
present study demonstrated the role of EPCs in ulcer treatment, and
we therefore propose that EPC therapy may also be used in peptic
ulcer-associated diseases.
In conclusion, the results of the present
investigation indicated that ulcer treatment is associated with
reduction in circulating EPC number. In addition, the ability of
circulating EPCs to differentiate into vascular endothelial cells
was lowest in peptic ulcer patients with T2DM, suggesting the
important role of circulating EPCs in ulcer treatment. However,
further studies are required in order to examine the association
between EPCs and ulcer treatment following adjustment to the effect
of T2DM on circulating EPCs.
Acknowledgements
The present study was supported by grants from the
Key Discipline Construction Project of the Pudong Health Bureau of
Shanghai (no. PWZx2014-03), the General Program of Shanghai
Municipal Health Bureau (no. 2007094) and the Subject Leader Plan
of Pudong Health Bureau of Shanghai (no. pWRd2014-02). The authors
would like to thank colleagues in the gastroscopy, laboratory and
endocrinology departments for their assistance with this study.
References
|
1
|
Tacheci I and Bures J: Peptic ulcer
disease in patients with diabetes mellitus. Vnitr Lek. 57:347–350.
2011.(In Czech). PubMed/NCBI
|
|
2
|
Najm WI: Peptic ulcer disease. Prim Care.
38:383–394. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
GBD 2013 Mortality and Causes of Death
Collaborators: Global, regional, and national age-sex specific
all-cause and cause-specific mortality for 240 causes of death,
1990–2013: A systematic analysis for the Global Burden of Disease
Study 2013. Lancet. 385:117–171. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dey L, Attele AS and Yuan CS: Alternative
therapies for type 2 diabetes. Altern Med Rev. 7:45–58.
2002.PubMed/NCBI
|
|
5
|
Lorenzi M: Glucose toxicity in the
vascular complications of diabetes: The cellular perspective.
Diabetes Metab Rev. 8:85–103. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Urbich C and Dimmeler S: Endothelial
progenitor cells: Characterization and role in vascular biology.
Circ Res. 95:343–353. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Werner N, Kosiol S, Schiegl T, Ahlers P,
Walenta K, Link A, Böhm M and Nickenig G: Circulating endothelial
progenitor cells and cardiovascular outcomes. N Engl J Med.
353:999–1007. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fadini GP, Miorin M, Facco M, Bonamico S,
Baesso I, Grego F, Menegolo M, de Kreutzenberg SV, Tiengo A,
Agostini C and Avogaro A: Circulating endothelial progenitor cells
are reduced in peripheral vascular complications of type 2 diabetes
mellitus. J Am Coll Cardiol. 45:1449–1457. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tecilazich F, Dinh T, Pradhan-Nabzdyk L,
Leal E, Tellechea A, Kafanas A, Gnardellis C, Magargee ML, Dejam A,
Toxavidis V, et al: Role of endothelial progenitor cells and
inflammatory cytokines in healing of diabetic foot ulcers. PLoS
One. 8:e833142013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Alberti KG and Zimmet PZ: Definition,
diagnosis and classification of diabetes mellitus and its
complications Part 1: diagnosis and classification of diabetes
mellitus provisional report of a WHO consultation. Diabet Med.
15:539–553. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Editorial Board of Chinese Journal of
Digestion: Suggestion on standardized diagnosis and treatment of
peptic ulcer disease (2008, Huang Shah). Zhong Hua Xiao Hua Za Zhi.
28:447–450. 2008.(In Chinese).
|
|
12
|
Asahara T, Murohara T, Sullivan A, Silver
M, van der Zee R, Li T, Witzenbichler B, Schatteman G and Isner JM:
Isolation of putative progenitor endothelial cells for
angiogenesis. Science. 275:964–967. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hirschi KK, Ingram DA and Yoder MC:
Assessing identity, phenotype and fate of endothelial progenitor
cells. Arterioscler Thromb Vasc Biol. 28:1584–1595. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bertolini F, Mancuso P, Braidotti P,
Shaked Y and Kerbel RS: The multiple personality disorder
phenotype(s) of circulating endothelial cells in cancer. Biochim
Biophys Acta. 1796:27–32. 2009.PubMed/NCBI
|
|
15
|
Tarnawski AS: Cellular and molecular
mechanisms of gastrointestinal ulcer healing. Dig Dis Sci. 50(Suppl
1): S24–S33. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schmidt-Lucke C, Rössig L, Fichtlscherer
S, Vasa M, Britten M, Kämper U, Dimmeler S and Zeiher AM: Reduced
number of circulating endothelial progenitor cells predicts future
cardiovascular events: Proof of concept for the clinical importance
of endogenous vascular repair. Circulation. 111:2981–2987. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hristov M, Erl W and Weber PC: Endothelial
progenitor cells: Mobilization, differentiation and homing.
Arterioscler Thromb Vasc Biol. 23:1185–1189. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Brunner S, Hoellerl F, Schmid-Kubista KE,
Zeiler F, Schernthaner G, Binder S and Schernthaner GH: Circulating
angiopoietic cells and diabetic retinopathy in type 2 diabetes
mellitus, with or without macrovascular disease. Invest Ophthalmol
Vis Sci. 52:4655–4662. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li M, Ho JC, Lai KW, Au KK, Xu A, Cheung
BM, Lam KS and Tse HF: The decrement in circulating endothelial
progenitor cells (EPCs) in type 2 diabetes is independent of the
severity of the hypoadiponectemia. Diabetes Metab Res Rev.
27:185–194. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Leeper NJ, Hunter AL and Cooke JP: Stem
cell therapy for vascular regeneration: Adult, embryonic, and
induced pluripotent stem cells. Circulation. 122:517–526. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tang YL, Zhao Q, Qin X, Shen L, Cheng L,
Ge J and Phillips MI: Paracrine action enhances the effects of
autologous mesenchymal stem cell transplantation on vascular
regeneration in rat model of myocardial infarction. Ann Thorac
Surg. 80:229–237. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rafii S and Lyden D: Therapeutic stem and
progenitor cell transplantation for organ vascularization and
regeneration. Nat Med. 9:702–712. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Asahara T, Murohara T, Sullivan A, Silver
M, van der Zee R, Li T, Witzenbichler B, Schatteman G and Isner JM:
Isolation of putative progenitor endothelial cells for
angiogenesis. Science. 275:964–967. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sen S, McDonald SP, Coates PT and Bonder
CS: Endothelial progenitor cells: Novel biomarker and promising
cell therapy for cardiovascular disease. Clin Sci (Lond).
120:263–283. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao YH, Yuan B, Chen J, Feng DH, Zhao B,
Qin C and Chen YF: Endothelial progenitor cells: Therapeutic
perspective for ischemic stroke. CNS Neurosci Ther. 19:67–75. 2013.
View Article : Google Scholar : PubMed/NCBI
|