Introduction
Ménétrier's disease (MD), also known as hypertrophic
protein-losing gastropathy, was first described in 1888 by Pierre
Ménétrier (1). It is a rare clinical
entity of unknown etiology characterized by large gastric folds
associated with epithelial hyperplasia. As MD is a rare disease,
incidence and mortality rate data remain undetermined. Primarily
observed in male adults (mean age at diagnosis is 55 years), MD
presents with variable symptoms. Classic presentation includes
abdominal pain, nausea, vomiting, anemia, hypochlorhydria and
peripheral oedema, which is due to protein loss across the gastric
mucosa (2). The natural history of
the disease may vary considerably between adults and children. In
childhood, the disorder is typically arises suddenly and resolves
spontaneously, and has been associated with cytomegalovirus (CMV)
infection (2). By contrast, in
adults, the course of the disease is progressive with an insidious
onset (3). The aetiology of MD
remains unknown, although previous studies have demonstrated that
MD is associated with infections, including Helicobacter
pylori, CMV and herpes simplex virus (2,4,5). Furthermore, it has been shown that the
pathogenesis of MD is associated with the upregulation of
transforming growth factor (TGF)-α binding to gastric epidermal
growth factor receptor (EGF-R) (6).
However, targeted therapies such as H. pylori eradication
and treatment with antibiotics, prednisone, octreotide and
monoclonal antibodies have yielded inconsistent results in clinical
trials (7–9). Notably, a number of patients with MD
have presented gastric cancer, which may demonstrate that MD
carries an increased risk of cancer (3,10).
However, whether MD is a precancerous condition is yet to be
elucidated. Due to the low incidence of MD, a limited amount of
research has been performed to address the disease progression, its
tendency to be refractory and its association with gastric cancer.
In the current study, a case of adult-onset MD with a 9-year
follow-up is presented.
Case report
In August 2005, a 56-year-old man was admitted to
The First Affiliated Hospital of Sun Yat-sen University (Guangzhou,
China) with recurrent upper abdominal pain, bloating, acid
regurgitation, heartburn, hematochezia and weight loss of 7 kg
experienced for a duration of 1 year. The patient's initial symptom
of upper abdominal pain had occurred from April 2004 and the
patient had undergone an endoscopy on April 28, 2004, which only
revealed chronic non-atrophic gastritis. Subsequently, the patient
used Chinese herbal medicine to control the symptoms; however, the
categories of and ingredients within the traditional Chinese
medicines are unknown. The patient's medical history included
hyperlipemia and benign prostatic hyperplasia. The patient had been
a smoker for 20 years prior to quitting smoking in 2004, and denied
drinking alcohol. The family history was considered to be
noncontributory.
Upon physical examination the patient's face was
pale and no pretibial edema was detected. Laboratory studies
indicated iron-deficiency anemia (hemoglobin, 59 g/l; normal range,
120–160 g/l) and hypoalbuminemia (albumin, 31 g/l; normal range,
35–50 g/l), as displayed in Table I.
Serum tumor markers including carcinoembryonic antigen,
α-fetoprotein, carbohydrate antigen (CA)125 and CA199 were within
normal ranges. To exclude Zollinger-Ellison syndrome, serum gastrin
levels were assessed and revealed to be normal. A chest X-ray and
abdominal ultrasound were normal, as were the results of an
electrocardiogram. Upper gastrointestinal endoscopy was
subsequently performed with an Olympus GIF-Q180 gastroscope
(Olympus Corporation, Tokyo, Japan), which revealed large edematous
gastric folds throughout the fundus and body, with areas of
superficial punctate erosions and exudate, but sparing the antrum
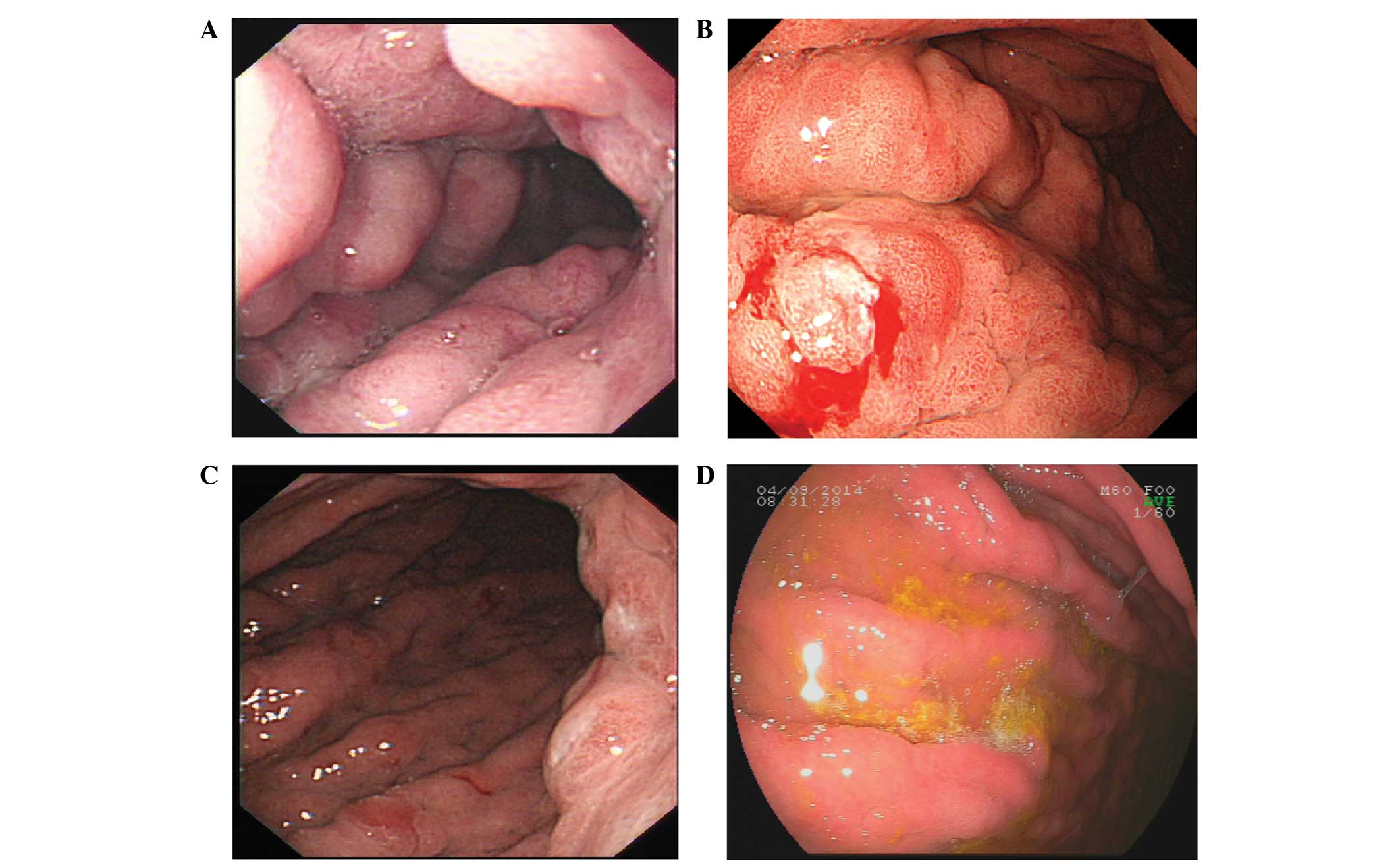
and pylorus (Fig. 1A). The stomach
was poorly distensible. Endoscopic ultrasound of the stomach was
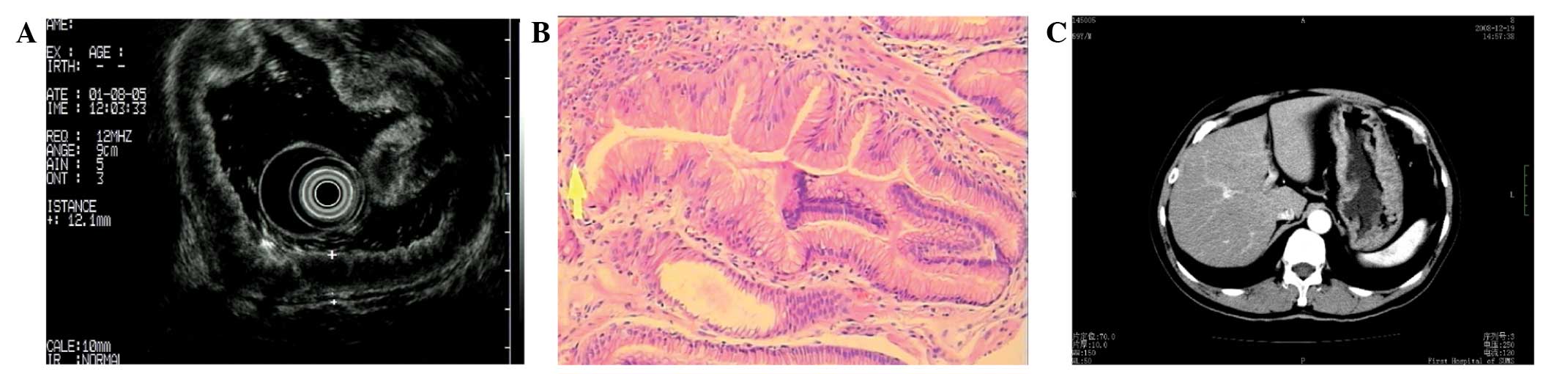
also performed (Olympus GF-UE260-AL5) and the results are displayed
in Fig. 2A.
 | Table I.Clinical and laboratory data of 9-year
follow-up for this case. |
Table I.
Clinical and laboratory data of 9-year
follow-up for this case.
| Variable | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 |
|---|
| Symptoms | Severe | Moderate | Mild | Mild | Absent | Absent | Absent | Absent | Absent | Absent |
| Laboratory tests |
|
|
|
|
|
|
|
|
|
|
|
Hemoglobin (g/l) | 59 | 85 | 64 | 120 | 160 | 140 | 150 | 153 | 160 | 160 |
| Total
protein (g/l) | 47 | 59 | – | – | 79 | – | – | 65 | 80.2 | – |
| Albumin
(g/l) | 31 | 37 | – | – | 45 | – | – | 36.6 | 48 | 47 |
|
Helicobacter
pylori | Negative | – | Negative | – | – | – | – | – | Negative | – |
|
Gastroscopy | See Fig. 1A | No change | No change | More severe | No change | Improved | Improved | Improved | Improved | Improved |
| Computed
tomography of abdomen | – | – | – | No tumor | – | – | – | – | – | – |
| Treatment |
|
|
|
|
|
|
|
|
|
|
| Red blood
cell transfusion | √ | – | √ | – | – | – | – | – | – | – |
| Oral
folic acid | √ | √ | – | – | – | – | – | – | – | – |
| Iron
supplement | √ (oral) | √ (oral) | √ (intravenous) | – | – | – | – | – | – | – |
| Proton
pump inhibitor | √ | √ | √ | √ | – | – | – | – | – | – |
|
Octreotide | – | – | – | – | – | √ | – | – | – | – |
To obtain more accurate histopathological evidence,
an endoscopic mucosal resection (EMR) was performed in the body of
the stomach. Briefly, the slices were fixed in 10% neutral buffered
formalin (Sigma-Aldrich, St. Louis, MO, USA) for ≥24 h and
subsequently embedded in paraffin wax (Wuhan Jiayuan Quantum Dots
Co., Ltd., Wuhan, China). Following dehydration with 95–75%
absolute ethyl alcohol (Wuhan Jiayuan Quantum Dots Co., Ltd.), the
slices were stained with hematoxylin (Sigma-Aldrich) for 10 min,
soaked in water for 5 min, rested in 1% hydrochloric acid alcohol
(Wuhan Jiayuan Quantum Dots Co., Ltd.) for 3 sec and rinsed with
water for 20 min prior to staining with eosin solution
(Sigma-Aldrich) for 10 min. Subsequently, the slices were
dehydrated with a graded alcohol series and sealed with half a drop
of neutral balsam (Wuhan Jiayuan Quantum Dots Co., Ltd.). Stained
slices were observed under an Olympus BX-43 optical microscope
(magnification, ×400; Olympus Corporation, Tokyo, Japan). Each
slice was randomly selected from five non-overlapping fields of
view, and double blind observation was conducted by two
pathologists, respectively. H. pylori was undetectable.
Therefore, a diagnosis of MD was concluded based upon the sizeable
expansion of the mucosa and foveolar hyperplasia with a reduced
number of parietal cells and chief cells (Fig. 2B).
The patient received a transfusion of red blood cell
concentrate (600 ml) and was administered oral folic acid (10 mg
three times a day) and polysaccharide-iron complex capsules (0.15 g
daily) to treat his anemia. In order to control the patient's
symptoms of recurrent upper abdominal pain, bloating, acid
regurgitation, heartburn and hematochezia, esomeprazole (20 mg
daily) was administered. Three weeks later, the patient's
hypoalbuminemia was resolved after an albumin level of 37 g/l was
reached, and hemoglobin levels increased to 87 g/l. The patient was
discharged as an outpatient and received follow-up by surveillance
endoscopy once or twice per year. From August 2005 to June 2007,
the patient displayed hemoglobin levels ranging from 60 to 85 g/l
and received no blood transfusions. The symptom of upper abdominal
pain recurred occasionally. Using an ISOMAX 2000
Carbon13 breath infrared spectrometer (Isodiagnostika,
Inc., Edmonton, AB, Canada), a C13 urea breath test was
performed for a second time to detect H. pylori during this
period and returned negative. It was hypothesized that this result
may have been influenced by the use of esomeprazole. The patient
was treated empirically for H. pylori with a 14-day course
of amoxicillin (1 g twice a day), clarithromycin (0.5 g twice a
day) and esomeprazole (20 mg twice a day).
On November 7, 2007, the patient was admitted again
as a result of anemia with hemoglobin levels of 64 g/l. Serum
concentrations of folic acid and vitamin B12 were within
the normal range with the exception of ferritin (1.00 µg/l; normal
range, 16.4–323 µg/l). Subsequently, the patient received another
red blood cell concentrate transfusion (400 ml), the administration
of oral folic acid was discontinued and iron dextran was injected
intravenously for 1 week (100 mg/day). After 3 weeks, hemoglobin
levels recovered to 126 g/l. An endoscopy was performed again on
November 8, 2007, revealing no changes in the large gastric folds
and no evidence of gastric malignancy.
Subsequently, the patient displayed hemoglobin
levels >120 g/l and felt well. An endoscopy on December 17,
2008, revealed that the large edematous gastric folds of the
stomach body were more severe than they had previously been
(Fig. 1B); however, the gastric
biopsies revealed no carcinoma. There was a concern that the random
biopsies may not have decisively ruled out the possibility of a
carcinoma. Thus, to rule out gastric carcinoma with greater
certainty, contrast-enhanced computerized tomography of the abdomen
was performed on December 19, 2008, and showed significant
thickening of the gastric body and fundus with no mass and no
mesenteric lymph nodes swelling (Fig.
2C). It was found that the administration of 20 mg esomeprazole
as required was sufficient to control the symptoms. The patient had
gained 6 kg in weight and reported no symptoms on July 29, 2009;
however, the endoscopy revealed no signs of improvement (Fig. 1C). The oral administration of
esomeprazole was discontinued at this stage, based on the lack of
symptoms and weight gain.
On the basis of preliminary reports detailing the
use of octreotide for the successful treatment of Ménétrier's
disease (11), three doses of
octreotide acetate injection (20 mg/month) were administered the
patient in the present study from January to March 2010, which were
tolerated well. However, a repeat endoscopy on October 13, 2010,
revealed no remission of the gastric mucosa. The final follow-up
was performed on September 4, 2014. The findings on endoscopy
indicated a slight improvement in the gastric mucosa compared with
previous results (Fig. 1D). All
other clinical and laboratory data during 9-year follow-up are
summarized in Table I.
Discussion
MD is a disease which predominantly presents in
adulthood with markedly hypertrophied gastric mucosal folds, and is
typically associated with hypoalbuminemia and anemia (12). In the current study, a case of MD is
presented with a 9-year follow-up period. As the majority of cases
of MD in pediatric patients are typically benign and self-limited
with symptoms resolving within 5 weeks (13), the treatment is predominately
supportive. However, the disease in adults is associated with
considerable morbidity and even mortality, associated with surgical
resection and a potential risk of malignant transformation
(3,10). Targeted therapies such as H.
pylori eradication, antibiotics, prednisone, octreotide and
monoclonal antibodies have yielded differing benefits in previous
studies (7–9). There remains a lack of research to
address the disease progression, refractory tendency and
association with gastric cancer following treatment. Thus, a
follow-up subsequent to treatment for MD is required.
Patients with MD may be debilitated as a result of
epigastric pain, vomiting, weight loss, hypoalbuminemia and edema
(12). In addition, previous cases
of MD have also presented with severe iron-deficiency anemia
(14,15). In the present case, anemia and
hypoproteinemia were successfully eliminated by red cell
transfusion and intravenous iron therapies. On the basis of the
rapid response, it is hypothesized that there may be possible iron
and protein leakage from large folds and disruption of the iron
absorption function due to inflammatory changes in the mucosa of
stomach. The iron deficiency may alter mucosal function, which
leads to protein leakage. Therefore, intravenous iron therapy
rather than oral iron therapy is recommended in cases of MD with
iron-deficiency anemia.
The etiology of MD remains as of yet largely
unknown. One prominent report indicated that >90% of cases
displayed H. pylori following biopsies in 138 patients with
hypertrophic gastropathy (16).
Following the initial successful treatment of MD with cephalexin,
several studies have reported that the eradication of H.
pylori may lead to complete clinical and morphologic recovery
(15,17,18).
Thus, mounting evidence suggests that H. pylori serves an
etiological role in MD. However, H. pylori was not detected
in the present case and further empirical treatment for H.
pylori did not affect endoscopic morphology. It is indicated
that H. pylori may not be a precursor factor in the disease
process, but rather a contributing factor involved in the
progression of the disease. Furthermore, in cases of H.
pylori-negative MD, the refractoriness of the disease is to be
expected following antimicrobial combination therapy. Notably, the
patient in the present study had taken Chinese herbal medicine for
1 year prior to diagnosis. The results of the endoscopy the patient
underwent prior to taking Chinese herbal medicine revealed no
morphologic indications of MD. Whether Chinese herbal medicine had
a casual role in the pathogenesis of the present case was unclear.
However, it is possible that an infection or other as yet unknown
cause may trigger an immunologic reaction through the action of
cytokines such as TGF-α. The continuous antigenic stimuli may lead
to the development of MD.
Overproduction of TGF-α, one of several ligands of
the EGF-R, in the stomach has been suggested to explain several
clinical features of MD, including decreased acid production,
increased hyperplasia of surface mucous cells, oxyntic atrophy and
increased mucin production (6).
Molecular evidence suggests that octreotide may modulate the EGF-R
signaling pathway (19,20). On the basis of this molecular
mechanism, and the successful use of octreotide in adults with MD
(7,11), the use of octreotide in the present
study was prompted. However, the repeat endoscopy indicated that
the large gastric folds were only marginally improved compared with
the earlier endoscopy, but no remission of the abnormalities of the
gastric mucosa. In 2000, Burdick et al (8) reported a severe case of MD that was
unresponsive to numerous therapies, including octreotide, but was
successfully treated with a monoclonal antibody against EGF-R,
cetuximab. Since then, a number of clinical trials have reported
the successful use of cetuximab in MD. Thus, in the present case,
cetuximab therapy may be considered as a subsequent treatment.
Follow-up for patients with MD is essential as there
is an increased risk of gastric-associated malignancies, primarily
gastric carcinoma and gastric lymphoma (3,10). This
provides evidence for the hypothesis that MD is a premalignant
lesion. However, the association with cancer has yet to be
confirmed due to the low incidence rate of MD. Despite this
limitation, it is recommended that patients are treated with
particular attention and that lesions are monitored with regular
endoscopic biopsy examination. In the present case, the patient
received a surveillance endoscopy 1–2 times per year. There was no
indication of gastric carcinoma in the following 9 years.
Typically, in the cases that have been presented in the literature,
MD and carcinoma were diagnosed simultaneously (21,22).
However, Wood et al (23)
reported a patient with early gastric cancer 3.5 years subsequent
to a diagnosis of MD and another report described a case that
presented after 13 years (24). The
longest follow-up period for a case of MD was 16 years, until the
patient succumbed to mortality as a result of hepatocellular
carcinoma (3). Therefore, although
malignant transformation of this lesion remains controversial,
patients should be followed up for a substantial period of time. If
the patient is concerned about the potential development of cancer,
a partial or total gastrectomy is recommended.
MD is a rare type of hypertrophic gastropathy and
currently does not have a gold-standard therapy. In the present
case, it is suggested that intravenous iron therapy rather than
oral iron therapy is preferable in MD with iron-deficiency anemia.
Treatment with octreotide had little effect on the gastric mucosa,
while antimicrobial combination therapy provided no benefit in
H. pylori-negative MD. Furthermore, although there was no
evidence of carcinoma in the present patient at 9-year follow-up,
malignant transformations in MD should not be overlooked, and it is
necessary to monitor the gastric mucosa via routine endoscopy.
References
|
1
|
Coffey RJ Jr and Tanksley J: Pierre
Ménétrier's and his disease. Trans Am Clin Climatol Assoc.
123:126–134. 2012.PubMed/NCBI
|
|
2
|
Eisenstat DD, Griffiths AM, Cutz E, Petric
M and Drumm B: Acute cytomegalovirus infection in a child with
Ménétrier's disease. Gastroenterology. 109:592–595. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Scharschmidt BF: The natural history of
hypertrophic gastrophy (Menetrier's disease). Report of a case with
16 year follow-up and review of 120 cases from the literature. Am J
Med. 63:644–652. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Badov D, Lambert JR, Finlay M and Balazs
ND: Helicobacter pylori as a pathogenic factor in
Menetrier's disease. Am J Gastroenterol. 93:1976–1979. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jun DW, Kim DH, Kim SH, Song MH, Lee HH,
Kim SH, Jo YJ and Park YS: Menetrier's disease associated with
herpes infection: Response to treatment with acyclovir.
Gastrointest Endosc. 65:1092–1095. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dempsey PJ, Goldenring JR, Soroka CJ,
Modlin IM, McClure RW, Lind CD, Ahlquist DA, Pittelkow MR, Lee DC,
Sandgren EP, et al: Possible role of transforming growth factor
alpha in the pathogenesis of Ménétrier's disease: Supportive
evidence form humans and transgenic mice. Gastroenterology.
103:1950–1963. 1992.PubMed/NCBI
|
|
7
|
Yeaton P and Frierson HF Jr: Octreotide
reduces enteral protein losses in Ménétrier's disease. Am J
Gastroenterol. 88:95–98. 1993.PubMed/NCBI
|
|
8
|
Burdick JS, Chung E, Tanner G, Sun M,
Paciga JE, Cheng JQ, Washington K, Goldenring JR and Coffey RJ:
Treatment of Ménétrier's disease with a monoclonal antibody against
the epidermal growth factor receptor. N Engl J Med. 343:1697–1701.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Di Vita G, Patti R, Aragona F, Leo P and
Montalto G: Resolution of Ménétrier's disease after Helicobacter
pylori eradicating therapy. Dig Dis. 19:179–183. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mosnier JF, Flejou JF, Amouyal G, Gayet B,
Molas G, Henin D and Potet F: Hypertrophic gastropathy with gastric
adenocarcinoma: Menetrier's disease and lymphocytic gastritis? Gut.
32:1565–1567. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rothenberg M, Pai R and Stuart K:
Successful use of octreotide to treat Ménétrier's disease: A rare
cause of abdominal pain, weight loss, edema, and hypoalbuminemia.
Dig Dis Sci. 54:1403–1407. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rich A, Toro TZ, Tanksley J, Fiske WH,
Lind CD, Ayers GD, Piessevaux H, Washington MK and Coffey RJ:
Distinguishing Ménétrier's disease from its mimics. Gut.
59:1617–1624. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kaneko T, Akamatsu T, Gotoh A, Shimodaira
K, Shimizu T, Kiyosawa K, Katsuyama T and Momose A: Remission of
Menetrier's disease after a prolonged period with therapeutic
eradication of Helicobacter pylori. Am J Gastroenterol.
94:272–273. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Singh AK, Cumaraswamy RC and Corrin B:
Diffuse hypertrophy of gastric mucosa (Menetrier's disease) and
iron-deficiency anaemia. Gut. 10:735–737. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yoshimura M, Hirai M, Tanaka N, Kasahara Y
and Hosokawa O: Remission of severe anemia persisting for over 20
years after eradication of Helicobacter pylori in cases of
Menetrier's disease and atrophic gastritis: Helicobacter
pylori as a pathogenic factor in iron-deficiency anemia. Intern
Med. 42:971–977. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bayerdorffer E, Ritter MM, Hatz R, Brooks
W and Stolte M: Menetrier's disease and Helicobacter pylori.
N Engl J Med. 329:601993. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kawasaki M, Hizawa K, Aoyagi K, Nakamura S
and Fujishima M: Ménétrier's disease associated with
Helicobacter pylori infection: Resolution of enlarged
gastric folds and hypoproteinemia after antibacterial treatment. Am
J Gastroenterol. 92:1909–1912. 1997.PubMed/NCBI
|
|
18
|
Raderer M, Oberhuber G, Templ E, Wagner L,
Pötzi R, Wrba F, Hejna M and Base W: Successful symptomatic
management of a patient with Ménétrier's disease with long-term
antibiotic treatment. Digestion. 60:358–362. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pinski J, Halmos G and Schally AV:
Somatostatin analog RC-160 and bombesin/gastrin-releasing peptide
antagonist RC-3095 inhibit the growth of androgen-independent
DU-145 human prostate cancer line in nude mice. Cancer Lett.
71:189–196. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Watt HL, Kharmate GD and Kumar U:
Somatostatin receptors 1 and 5 heterodimerize with epidermal growth
factor receptor: Agonist-dependent modulation of the downstream
MAPK signalling pathway in breast cancer cells. Cell Signal.
21:428–439. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vandenborre KM, Ghillebert GL, Rutgeerts
LJ, Geboes KR, Rutgeerts PJ, Verbanck JJ and Tanghe WR:
Hypertrophic lymphocytic gastritis with a gastric carcinoma. Eur J
Gastroenterol Hepatol. 10:797–801. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Choi SB, Park SS, Oh SY, Kim JH, Kim WB,
Lee JH, Choi JW, Kim SJ, Kim CS and Mok YJ: Primary squamous cell
carcinoma of the stomach that developed with Menetrier's disease.
Dig Dis Sci. 52:1722–1724. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wood MG, Bates C, Brown RC and Losowsky
MS: Intramucosal carcinoma of the gastric antrum complicating
Menetrier's disease. J Clin Pathol. 36:1071–1075. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ramia JM, Sancho E, Lozano O, Santos JM
and Domínguez F: Ménétrier's disease and gastric cancer. Cir Esp.
81:153–154. 2007.(In Spanish). PubMed/NCBI
|