Introduction
Doxorubicin (DOX) is an effective anthracycline
antitumor antibiotic and is extensively used for treatment of
numerous types of malignant tumors, including osteosarcoma, breast
carcinoma and lung cancer (1–3).
However, because of cumulative and dose-dependent cardiotoxicity,
the clinical application of DOX is limited. Although the exact
physiopathological mechanism of DOX-induced cardiotoxicity is not
yet understood, cardiomyocyte apoptosis is acknowledged to play an
important role in DOX-associated cardiotoxicity (4,5).
MicroRNAs (miRs) are a class of endogenous
non-coding RNAs of ~22 nucleotides, which participate in the
regulation of protein-coding gene expression by binding to the
3′-untranslated regions of target mRNA at the post-transcriptional
level (6). It has been predicted
that there are >1,000 miRs in the human genome, and miRs are
estimated to regulate as many as 60% of all human mRNAs (7,8). It is
well recognized that miRs play important roles in a large variety
of physiological and pathological processes, such as embryonic
development, tumor formation and modulation of the inflammatory
response (9–11). Among the known miRs, miR-1 is a
muscle-enriched miR and highly expressed in the myocardium
(12). Therefore, aberrant
expression of miR-1 is closely associated with myocardial diseases.
It has been reported that miR-1 expression is markedly increased in
ischemia/reperfusion (IR) or hypoxia/reoxygenation-induced cardiac
myocyte apoptosis (13). In
addition, in miR-1 transgenic mice, cardiac myocyte apoptosis was
found to be exacerbated compared with that in wild-type mice when
subjected to myocardial IR injury; by contrast, knockdown of miR-1
using a locked nucleic acid-modified oligonucleotide against miR-1
significantly attenuated IR-induced cardiac myocyte apoptosis
(14). However, the role of miR-1 in
DOX-induced cardiomyocyte apoptosis is not understood.
Paeoniflorin (PEF), a monoterpene glucoside, is the
major active ingredient of Paeonia lactiflora Pall. (family
Paeoniaceae), which is a traditional Chinese herbal medicine that
has been used in China for >1,000 years. It has been reported
that PEF exerts multiple pharmacological activities, such as
inhibition of tumor invasion and metastasis (15), reduction of inflammatory factor
production (16), prevention of
insulin resistance (17) and
neuroprotective effects (18). Our
previous study demonstrated that PEF was able to inhibit
DOX-induced cardiomyocyte apoptosis by reducing the production of
reactive oxygen species (ROS) (19);
however, the downstream mechanism remains unclear.
In the present study, the role of miR-1 in
DOX-induced cell apoptosis was explored using a cultured H9c2 rat
cardiomyocyte cell line, and whether the anti-apoptotic effect of
PEF was related to a regulatory effect on miR-1 expression was
investigated.
Materials and methods
Materials
The H9c2 cell line was acquired from Academia Sinica
(Shanghai, China). PEF was obtained from Yangling Dongke Maidisen
Pharmaceutical Co., Ltd. (Xi'an, China). DOX was purchased from
Sigma-Aldrich (St. Louis, MO, USA). Dulbecco's modified Eagle's
medium (DMEM) and fetal bovine serum (FBS) were from Gibco (Thermo
Fisher Scientific, Inc., Waltham, MA, USA). mirVana miR isolation
kits and TaqMan™ miR Reverse Transcription kits were purchased from
Applied Biosystems (Thermo Fisher Scientific, Inc.). B-cell
lymphoma 2 (Bcl-2; ab59348) and β-actin (ab1801) antibodies were
from Abcam (Cambridge, UK). The following were purchased from
Beyotime Institute of Biotechnology (Jiangsu, China):
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
(MTT), Hoechst 33342 fluorescent dye and reactive oxygen species
(ROS) detection kit. The creatine kinase (CK) assay kit was
acquired from Nanjing Jiancheng Bioengineering Institute (Nanjing,
China).
Cell culture and treatment
H9c2 cells were cultured in DMEM containing 15%
(v/v) FBS. The medium was supplemented with 100 U/ml penicillin
(Guangzhou Baiyunshan Tianxin Pharmaceutical Co., Ltd., Guangdong,
China) and 100 µg/ml streptomycin (Harbin Pharmaceutical Group
Holding Co., Ltd., Heilongjiang, China). All cells were grown under
a humidified atmosphere of 5% CO2 at 37°C. The medium
was changed every 2–3 days. When cells reached 80% confluence, they
were made quiescent by serum starvation (0.5% FBS) for 24 h, and
then the cells were randomly allocated to 4 groups: i) The control
group (cultured in normal condition); ii) the DOX group (incubated
with 5 µmol/l DOX for 24 h); iii) the PEF + DOX group (cells were
treated with 100 µmol/l PEF for 2 h prior to exposure to 5 µmol/l
DOX for 24 h); and iv) the PEF group (incubated with 100 µmol/l PEF
for 26 h).
Evaluation of cell injury
Cell viability was assessed by MTT assay. Briefly,
following DOX treatment, the medium was aspirated, and the cells
were washed twice with PBS, then 0.5 mg/ml MTT solution was added
to each well and the plate was incubated for 4 h at 37°C in an
atmosphere of 5% CO2. After that, 100 µl
dimethysulfoxide (DMSO) was added to dissolve formazan. The
absorbance was read at 490 nm using a microplate reader (Molecular
Devices, Sunnyvale, CA, USA). Cell viability was expressed as a
percentage of the control. The activity of CK, an indicator of
cardiomyocyte injury, was detected using a commercially available
colorimetric assay kit according to the manufacturer's
protocol.
Measurement of cardiomyocyte
apoptosis
Apoptotic cells were detected by Hoechst 33342
fluorescent dye using an inverted fluorescence microscope (Nikon
Eclipse TE-2000; Nikon Corporation, Tokyo, Japan). Briefly, after
DOX treatment, the medium was aspirated, and the cells were washed
twice with PBS. After that, cells were incubated with Hoechst 33342
(0.1 mg/ml) in the dark at 37°C for 20 min. Hoechst-stained nuclei
were detected by inverted fluorescence microscopy at an emission
wavelength of 521 nm. The data are expressed as a percentage of
apoptotic cells to total cells.
Determination of intracellular
ROS
The levels of intracellular ROS were measured using
2′,7′-dichlorofluorescein diacetate, a well characterized probe.
Briefly, following DOX treatment, the medium was aspirated and
cells were washed three times with serum-free DMEM. After that,
cells were incubated with 2′,7′-dichlorofluorescein diacetate at a
final concentration of 10 µM in the dark at 37°C for 20 min. The
fluorescence intensity was measured using a fluorospectrophotometer
(Shimadzu Corporation, Kyoto, Japan) at an excitation wavelength at
488 nm and an emission wavelength at 525 nm. The data are expressed
as a percentage of the control.
Quantitative polymerase chain reaction
(qPCR)
miR-1 expression
Following treatment, total RNA was isolated from
H9c2 cells using the mirVana miR isolation kit according to the
manufacturer's protocol. cDNA was synthesized from 1 µg total RNA
using the TaqMan™ miR reverse transcription kit (Promega
Corporation, Madison, WI, USA). cDNA was diluted 15-fold and then
used in PCR reactions. Reactions contained specific primers for
miR-1 or U6; the latter was used as an internal control. The primer
sequences were as follows: miR-1 forward,
5′-ACACTCCAGCTGGGGTGTGGAATGTA-3′ and reverse,
5′-TGGTGTCGTGGAGTCG-3; U6 forward,
5′-ACACTCCAGCTGGGGTGCTCGCTTCGGCAGCACA-3′ and reverse,
5′-AGGGTCCGAGGTATTC-3′. PCR was performed using TaqMan universal
PCR master mix (Applied Biosystems; Thermo Fisher Scientific, Inc.)
with the following conditions: 10 min at 95°C followed by 40 cycles
of 95°C for 15 sec and 60°C for 1 min. All amplification reaction
for each sample was carried out four times. The relative expression
values were normalized to the expression of U6 using the
2−ΔΔCq method (20).
Bcl-2 mRNA expression
Following treatment, total RNA was isolated using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and
quantified by measuring the optical density at 260 nm. 1 µg RNA
from each sample was reverse-transcribed to cDNA using an M-MLV
reverse transcriptase kit, and then cDNA was used for qPCR. Bcl-2
mRNA expression was quantitatively analyzed using an Applied
Biosystems 7300 Real-Time PCR system with a Power SYBR Green PCR
Master Mix kit (both Thermo Fisher Scientific, Inc.). PCR primers
were as follows: Bcl-2 primers: forward,
5′-CCGGGAGAACAGGGTATGATAA-3′ and reverse,
5′-CCCACTCGTAGCCCCTCTG-3′; glyceraldehyde 3-phosphate dehydrogenase
(GAPDH) primers: forward, 5′-TGGCCTCCAAGGAGTAAGAAAC-3′ and reverse,
5′-GGCCTCTCTCTTGCTCTCAGTATC-3′. The PCR amplification program
involved initial denaturation at 95°C for 10 min, followed by 40
cycles of denaturation at 95°C for 15 sec and annealing at 60°C for
60 sec. All amplification reactions for each sample were carried
out four times, and the relative expression values were normalized
to the expression value of GAPDH using the 2−ΔΔCq method
(20).
Western blot analysis
The protein expression of Bcl-2 was detected by
western blot analysis. Briefly, following the various treatments,
cells were lysed with ice-cold lysis buffer, and the protein
concentration was measured using a Bradford protein assay (Beyotime
Institute of Biotechnology, Inc.). Equal amounts of protein were
separated by 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis and transferred to a nitrocellulose membrane. After
transferring the protein samples, the membranes were blocked using
5% milk powder in Tris-buffered saline and Tween 20 at room
temperature for 1 h and then incubated with primary rabbit
polyclonal antibodies for Bcl-2 and β-actin (both 1:1,000 dilution)
overnight at 4°C, followed by incubation with the corresponding
goat anti-rabbit horseradish peroxidase-conjugated secondary
antibody (1:5,000 dilution; ab175773; Abcam) for 1 h at room
temperature. The protein bands were scanned and densitometrically
analyzed using an automatic image analysis system (Alpha Innotech
Corporation, San Loandra, CA, USA), and the results were normalized
to β-actin expression.
Statistical analysis
Data are presented as means ± standard error of the
mean. All values were analyzed using analysis of variance and the
Newman-Keuls Student's t-test. P<0.05 was considered to indicate
a statistically significant difference.
Results
Effect of PEF on DOX-induced cell
injury
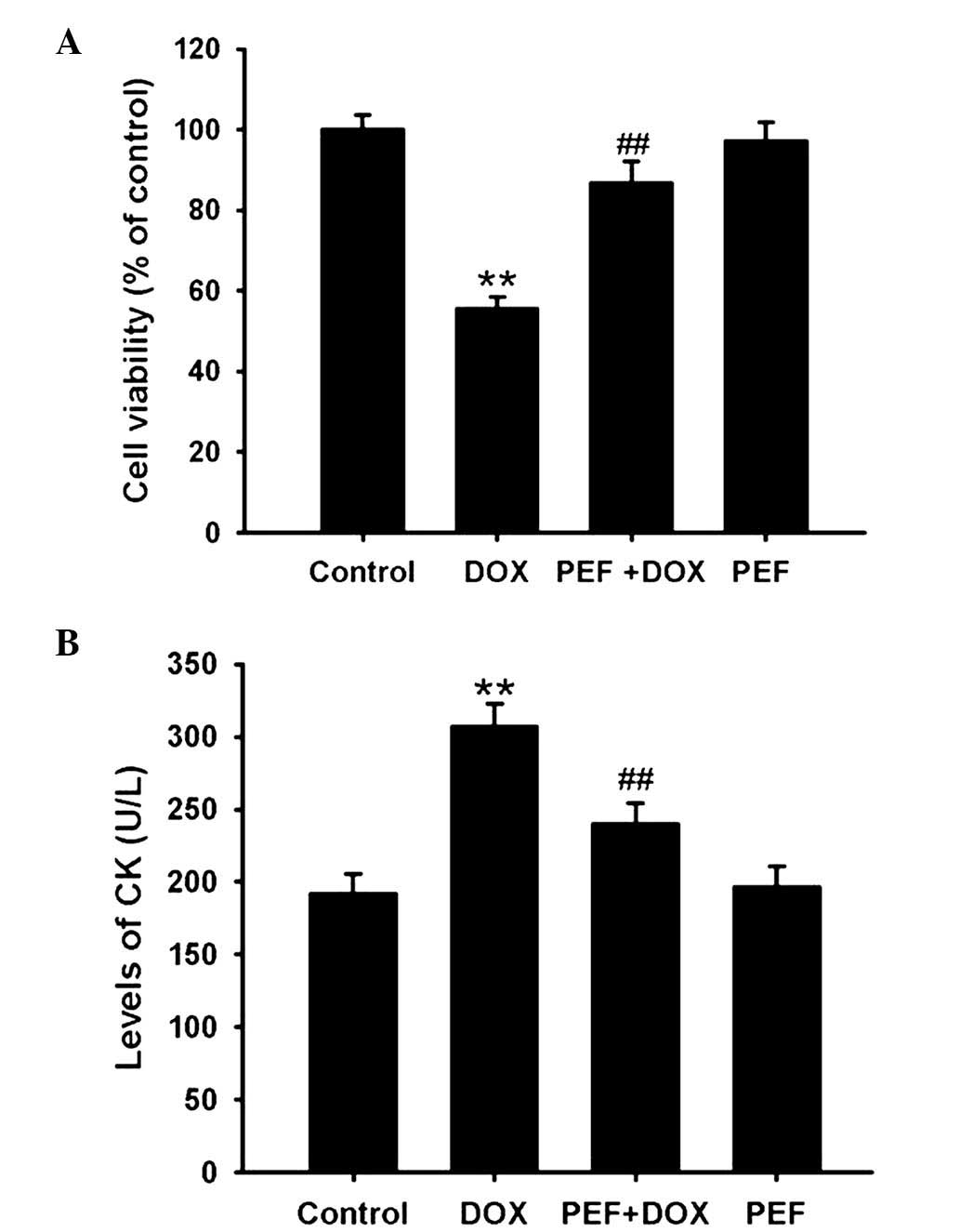
The MTT assay showed that cell viability was clearly
decreased following DOX treatment, and this reduction in viability
was significantly attenuated by pretreatment with PEF for 2 h
(P<0.01; Fig. 1A). In line with
the MTT assay results, the levels of CK were markedly increased
following incubation with DOX, and this effect of DOX was also
significantly inhibited by pretreatment with PEF (P<0.01;
Fig. 1B). However, PEF alone had no
effect on cell viability or the levels of CK.
Effect of PEF on DOX-induced
apoptosis
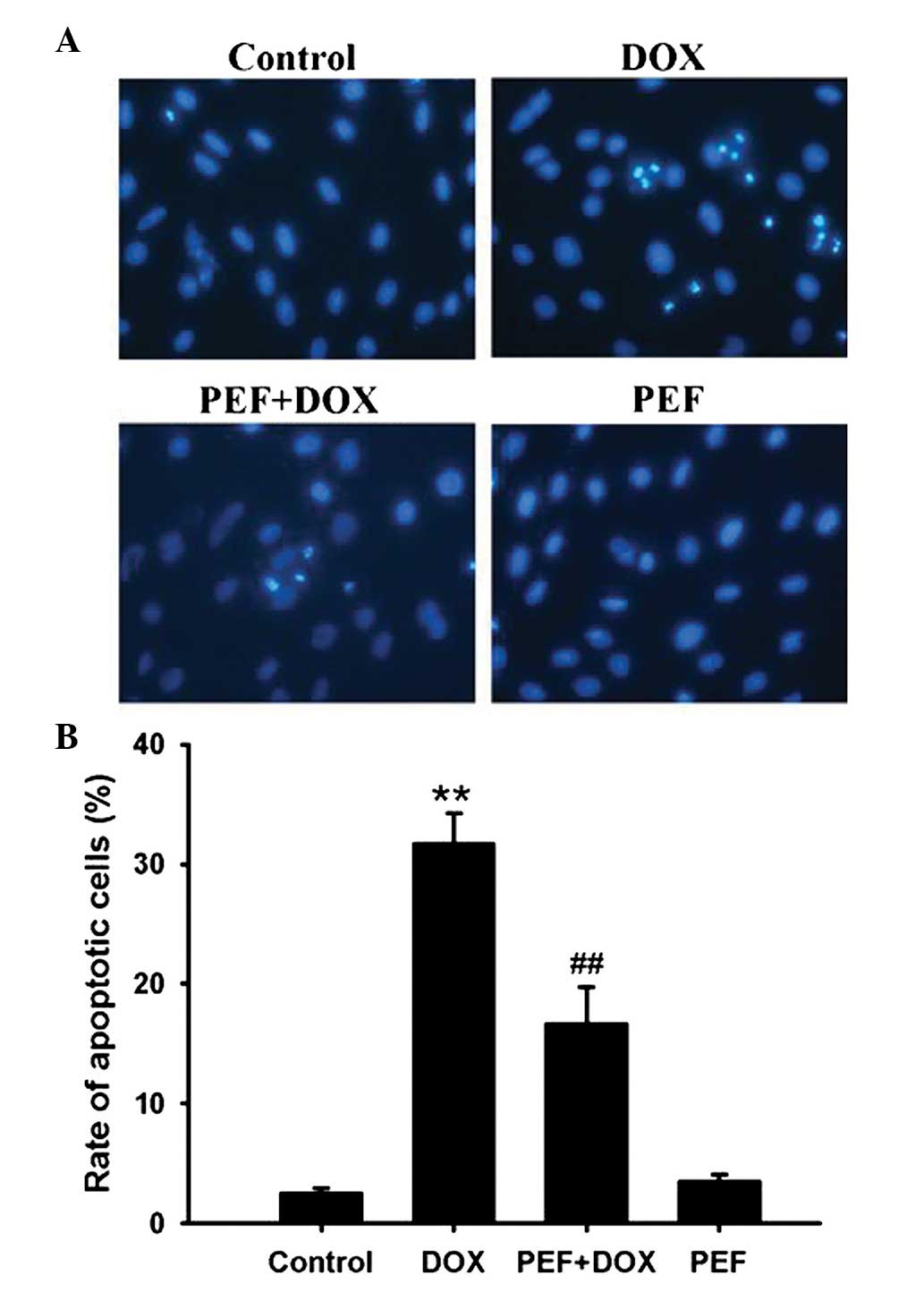
Cardiomyocyte apoptosis was measured by Hoechst
33342 staining. The results showed that DOX treatment notably
increased the ratio of apoptotic cells compared with that in the
control group. However, pretreatment with PEF for 2 h significantly
inhibited DOX-induced cell apoptosis (P<0.01; Fig. 2). PEF alone had no effect on
cardiomyocyte apoptosis.
Effect of PEF on DOX-induced
intracellular ROS production
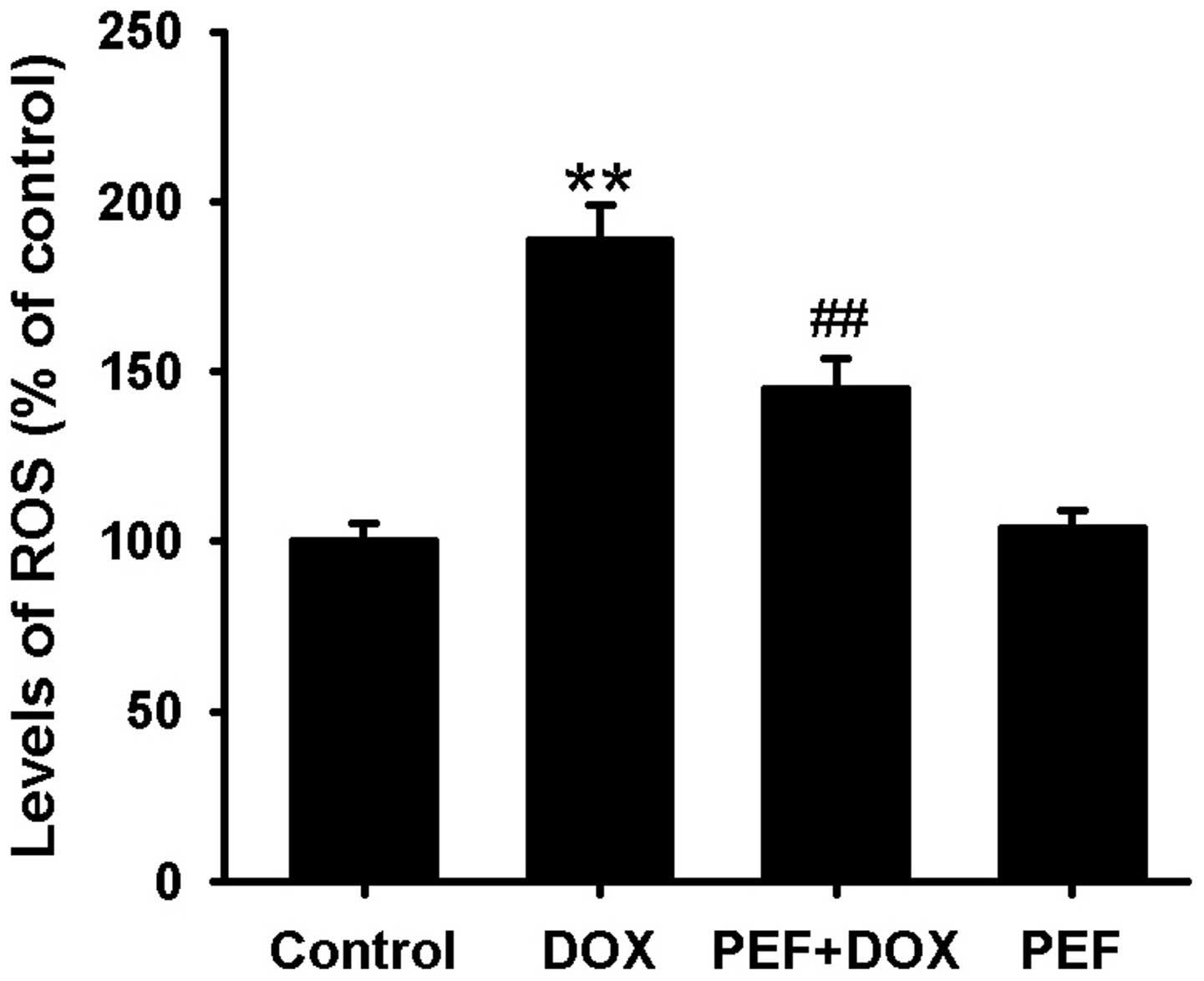
The levels of intracellular ROS, indicated by the
2′,7′-dichloroflurorescein fluorescence intensity, were notably
increased in DOX-treated cells compared with control cells.
However, pretreatment with PEF for 2 h significantly suppressed the
intracellular ROS production induced by DOX (P<0.01; Fig. 3). PEF alone had no such effect.
Effect of PEF on DOX-induced miR-1
expression
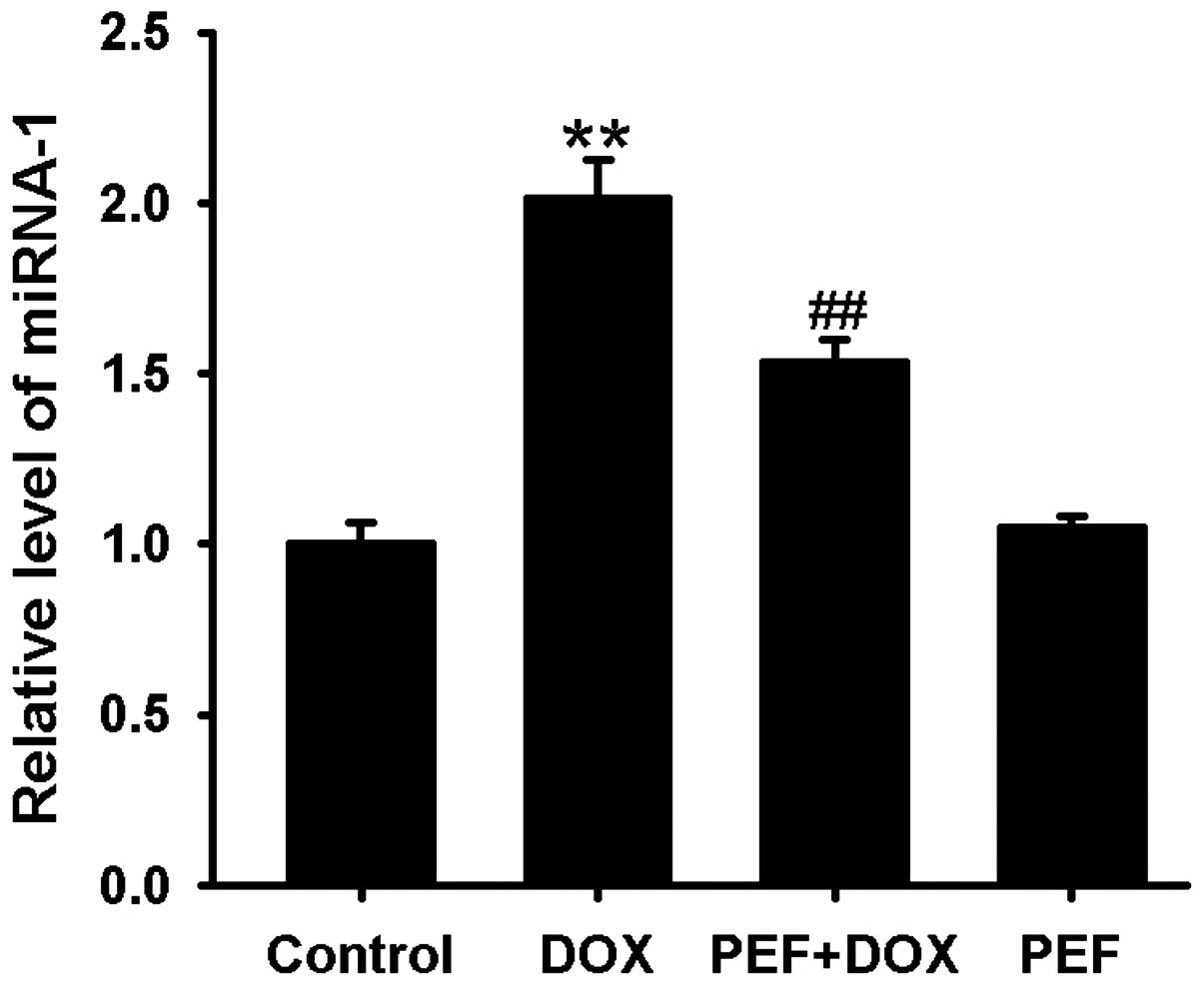
miR-1 expression was markedly upregulated after DOX
treatment compared with that in the control group. This effect of
DOX was significantly inhibited by pretreatment with PEF for 2 h
(P<0.01; Fig. 4). PEF alone had
no effect on miR-1 expression.
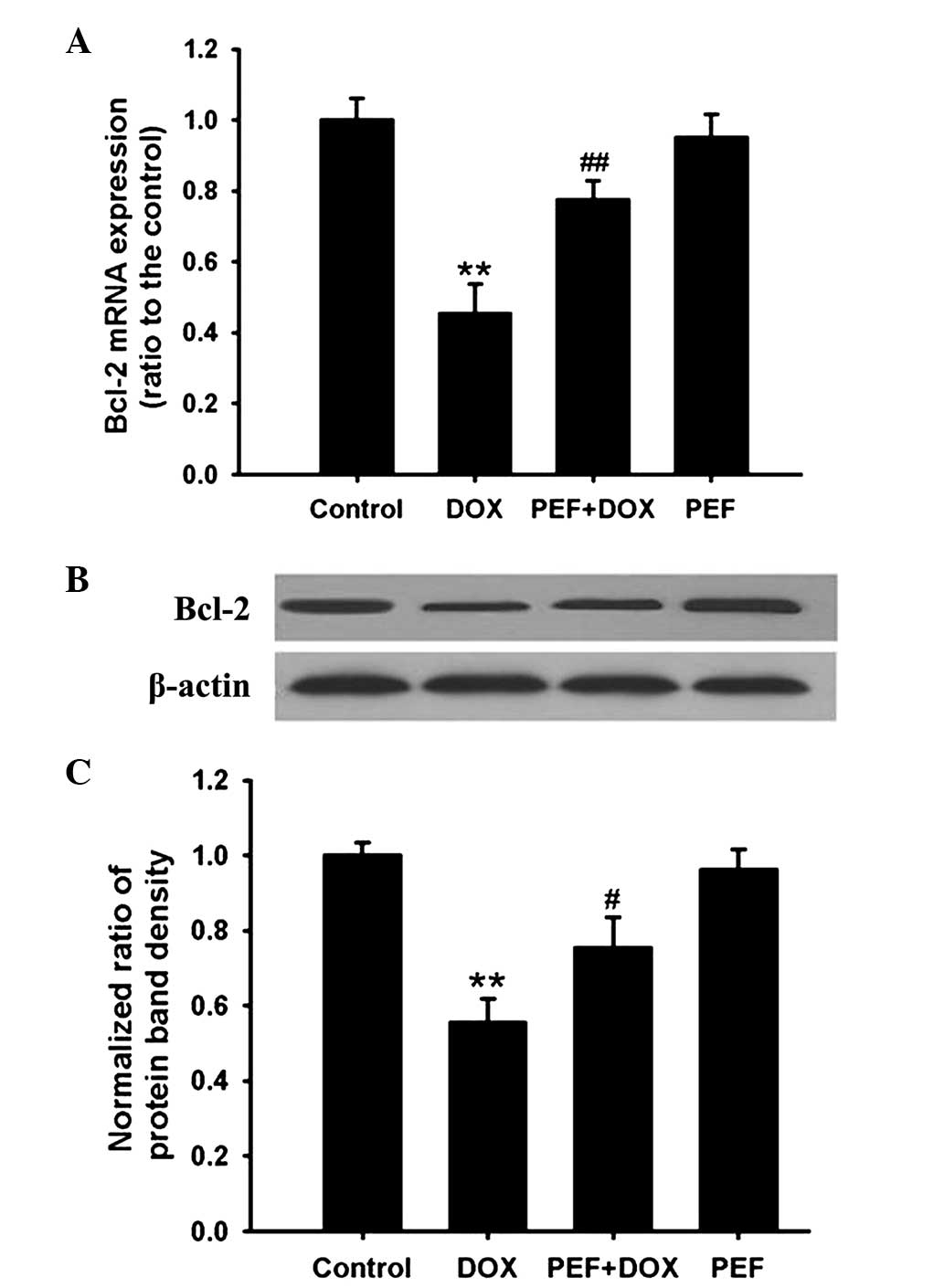
Effect of PEF on Bcl-2 expression
PCR analysis showed that the mRNA expression of
Bcl-2, an anti-apoptotic gene, was significantly downregulated in
DOX-treated cells (P<0.01). Consistent with these results, Bcl-2
protein expression was also markedly decreased after incubation
with DOX (P<0.01). However, these effects induced by DOX were
inhibited by pretreatment with PEF for 2 h (P<0.01 and
P<0.05, respectively; Fig. 5).
PEF alone had no effect on Bcl-2 expression.
Discussion
In the current study, it was found that DOX-induced
cardiomyocyte apoptosis was accompanied by increased intracellular
ROS production, upregulated miR-1 expression and downregulated
Bcl-2 mRNA and protein expression. These effects of DOX were
markedly inhibited by pretreatment with PEF. These results indicate
that the anti-apoptotic effect of PEF was associated with a
reduction in ROS production and downregulation of miR-1
expression.
It is acknowledged that cardiomyocyte apoptosis
plays an important role in DOX-induced cardiotoxicity (4,5).
However, the exact mechanism of DOX-induced cardiomyocyte apoptosis
is not fully understood. miR-1 is a muscle-specific miR and highly
expressed in the myocardium (12).
The abnormal expression of miR-1 is involved in a number of heart
diseases. It has been reported that changes in miR-1 expression are
associated with, for example, arrhythmia, myocardial infarction,
cardiac remodeling and heart failure (21). There is evidence that upregulated
miR-1 expression is closely associated with cardiomyocyte
apoptosis; in models of IR-, high glucose- or
H2O2-induced cardiomyocyte apoptosis, miR-1
expression has been shown to be significantly increased (13,22,23).
Further studies have found that the level of miR-1 is inversely
correlated with the expression of Bcl-2, which is an anti-apoptotic
protein that has been demonstrated to be a potential target of
miR-1 (13,23). Overexpression of miR-1 has been shown
to exacerbate cardiomyocyte apoptosis induced by IR in mouse
models, while knockdown of miR-1 expression significantly
attenuated cardiac injury (14). The
aforementioned studies suggest that upregulated expression of miR-1
plays a critical role in cardiomyocyte apoptosis via the
post-transcriptional repression of Bcl-2 expression, and reducing
miR-1 expression may be a promising strategy for the prevention of
cardiomyocyte apoptosis.
The mechanism by which miR expression is regulated
is not fully clear. A number of factors, including ultraviolet
radiation and tertiary-butyl hydroperoxide can lead to changes in
the expression of multiple miRs, which is closely associated with
increased ROS production (24,25).
Schmelzer et al (26) have
demonstrated that bacterial lipopolysaccharide induces miR-146a
expression via a ROS-dependent pathway as this effect was inhibited
by pretreatment with the antioxidant ubiquinol-10. Furthermore, the
expression of multiple miRs, including miR-1, was found to change
after treatment with H2O2 (27,28).
These studies suggest that miR expression may be modulated by ROS.
A number of studies have demonstrated that DOX-induced
cardiomyocyte apoptosis is closely associated with excessive ROS
production (29,30). Therefore, we hypothesized that
DOX-induced cardiomyocyte apoptosis may be associated with
upregulation of miR-1 expression by an increase in ROS production.
In the present study, it was indeed found that DOX significantly
increased ROS generation concomitantly with upregulation of miR-1
expression. Thus, reduction of ROS production may be an effective
method for inhibiting DOX-induced cardiomyocyte apoptosis via the
downregulation of miR-1 expression.
PEF, a monoterpene glucoside extracted from the dry
root of Paeonia lactiflora, has been reported to exert
multiple pharmacological activities (15–18).
Studies have suggested that PEF has anti-oxidative activity
(19,31). Furthermore, our previous study
demonstrated that PEF was able to inhibit DOX-induced cardiomyocyte
apoptosis by reducing ROS production (19), but the downstream mechanism was not
clear. Since miR-1 expression is regulated by ROS, and PEF has an
anti-oxidative property, we speculated that the anti-apoptotic
effect of PEF may be associated with downregulation of miR-1
expression via inhibition of ROS production. In the present study,
it was confirmed that PEF markedly inhibited ROS generation, which
was accompanied by downregulation of miR-1 expression.
In summary, the results of the current study suggest
that the upregulation of miR-1 expression plays an important role
in DOX-induced cardiomyocyte apoptosis by the post-transcriptional
repression of Bcl-2 expression. Furthermore, the inhibitory effect
PEF against DOX-induced cardiomyocyte apoptosis may be associated
with the downregulation of miR-1 expression via a reduction in ROS
production.
Acknowledgements
This study was supported by the National Nature
Science Foundation of China (grant no. 81460613 to J.Z. Li,
81101476 to S.Y. Yu and 81260337 to L.J. Liu), the Guangxi Nature
Science Foundation of China (grant no. 2013GXNSFBA019126 to J.Z.
Li], the Bureau of Public Health of Guangxi Province (grant no.
Z2013206) and the Guangxi Administration of Traditional Chinese
Medicine (grant no. GZZC14-36).
References
|
1
|
Tacar O, Indumathy S, Tan ML,
Baindur-Hudson S, Friedhuber AM and Dass CR: Cardiomyocyte
apoptosis vs autophagy with prolonged doxorubicin treatment:
Comparison with osteosarcoma cells. J Pharm Pharmacol. 67:231–243.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rastegar H, Ashtiani Ahmadi H, Anjarani S,
Bokaee S, Khaki A and Javadi L: The role of milk thistle extract in
breast carcinoma cell line (MCF-7) apoptosis with doxorubicin. Acta
Med Iran. 51:591–598. 2013.PubMed/NCBI
|
|
3
|
Shi X, Li C, Gao S, Zhang L, Han H, Zhang
J, Shi W and Li Q: Combination of doxorubicin-based chemotherapy
and polyethylenimine/p53 gene therapy for the treatment of lung
cancer using porous PLGA microparticles. Colloids Surf B
Biointerfaces. 122:498–504. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gao S, Li H, Cai Y, Ye JT, Liu ZP, Lu J,
Huang XY, Feng XJ, Gao H, Chen SR, et al: Mitochondrial binding of
α-enolase stabilizes mitochondrial membrane: Its role in
doxorubicin-induced cardiomyocyte apoptosis. Arch Biochem Biophys.
542:46–55. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li D, Li J, An Y, Yang Y and Zhang SQ:
Doxorubicin-induced apoptosis in H9c2 cardiomyocytes by NF-κB
dependent PUMA upregulation. Eur Rev Med Pharmacol Sci.
17:2323–2329. 2013.PubMed/NCBI
|
|
6
|
Vimalraj S and Selvamurugan N: MicroRNAs
expression and their regulatory networks during mesenchymal stem
cells differentiation toward osteoblasts. Int J Biol Macromol.
66:194–202. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Palmero EI, de Campos SG, Campos M, de
Souza NC, Guerreiro ID, Carvalho AL and Marques MM: Mechanisms and
role of microRNA deregulation in cancer onset and progression.
Genet Mol Biol. 34:363–370. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Harries LW: MicroRNAs as Mediators of the
ageing process. Genes (Basel). 5:656–670. 2014.PubMed/NCBI
|
|
9
|
Eshel O, Shirak A, Dor L, Band M, Zak T,
Markovich-Gordon M, Chalifa-Caspi V, Feldmesser E, Weller JI,
Seroussi E, et al: Identification of male-specific amh duplication,
sexually differentially expressed genes and microRNAs at early
embryonic development of Nile tilapia (Oreochromis
niloticus). BMC Genomics. 15:7742014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Feng Y, Liu J, Kang Y, He Y, Liang B, Yang
P and Yu Z: MiR-19a acts as an oncogenic microRNA and is
up-regulated in bladder cancer. J Exp Clin Cancer Res. 33:672014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Roff AN, Craig TJ, August A, Stellato C
and Ishmael FT: MicroRNA-570-3p regulates HuR and cytokine
expression in airway epithelial cells. Am J Clin Exp Immunol.
3:68–83. 2014.PubMed/NCBI
|
|
12
|
Malizia AP and Wang DZ: MicroRNAs in
cardiomyocyte development. Wiley Interdiscip Rev Syst Biol Med.
3:183–190. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kang B, Hong J, Xiao J, Zhu X, Ni X, Zhang
Y, He B and Wang Z: Involvement of miR-1 in the protective effect
of hydrogen sulfide against cardiomyocyte apoptosis induced by
ischemia/reperfusion. Mol Biol Rep. 41:6845–6853. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pan Z, Sun X, Ren J, Li X, Gao X, Lu C,
Zhang Y, Sun H, Wang Y, Wang H, et al: MiR-1 exacerbates cardiac
ischemia-reperfusion injury in mouse models. PLoS One.
7:e505152012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lu JT, He W, Song SS and Wei W:
Paeoniflorin inhibited the tumor invasion and metastasis in human
hepatocellular carcinoma cells. Bratisl Lek Listy. 115:427–433.
2014.PubMed/NCBI
|
|
16
|
Li JZ, Wu JH, Yu SY, Shao QR and Dong XM:
Inhibitory effects of paeoniflorin on
lysophosphatidylcholine-induced inflammatory factor production in
human umbilical vein endothelial cells. Int J Mol Med. 31:493–497.
2013.PubMed/NCBI
|
|
17
|
Kong P, Chi R, Zhang L, Wang N and Lu Y:
Effects of paeoniflorin on tumor necrosis factor-α-induced insulin
resistance and changes of adipokines in 3T3-L1 adipocytes.
Fitoterapia. 91:44–50. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang K, Zhu L, Zhu X, Zhang K, Huang B,
Zhang J, Zhang Y, Zhu L, Zhou B and Zhou F: Protective effect of
paeoniflorin on Aβ25-35-induced SH-SY5Y cell injury by preventing
mitochondrial dysfunction. Cell Mol Neurobiol. 34:227–234. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li JZ, Yu SY, Wu JH, Shao QR and Dong XM:
Paeoniflorin protects myocardial cell from doxorubicin-induced
apoptosis through inhibition of NADPH oxidase. Can J Physiol
Pharmacol. 90:1569–1575. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCt method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li J, Dong X, Wang Z and Wu J: MicroRNA-1
in cardiac diseases and cancers. Korean J Physiol Pharmacol.
18:359–363. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu XY, Song YH, Geng YJ, Lin QX, Shan ZX,
Lin SG and Li Y: Glucose induces apoptosis of cardiomyocytes via
microRNA-1 and IGF-1. Biochem Biophys Res Commun. 376:548–552.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tang Y, Zheng J, Sun Y, Wu Z, Liu Z and
Huang G: MicroRNA-1 regulates cardiomyocyte apoptosis by targeting
Bcl-2. Int Heart J. 50:377–387. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cha HJ, Kim OY, Lee GT, Lee KS, Lee JH,
Park IC, Lee SJ, Kim YR, Ahn KJ, An IS, et al: Identification of
ultraviolet B radiation-induced microRNAs in normal human dermal
papilla cells. Mol Med Rep. 10:1663–1670. 2014.PubMed/NCBI
|
|
25
|
Fatemi N, Sanati MH, Shamsara M, Moayer F,
Zavarehei MJ, Pouya A, Sayyahpour F, Ayat H and Gourabi H:
TBHP-induced oxidative stress alters microRNAs expression in mouse
testis. J Assist Reprod Genet. 31:1287–1293. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schmelzer C, Kitano M, Rimbach G,
Niklowitz P, Menke T, Hosoe K and Döring F: Effects of ubiquinol-10
on microRNA-146a expression in vitro and in vivo. Mediators
Inflamm. 2009:4154372009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Simone NL, Soule BP, Ly D, Saleh AD,
Savage JE, Degraff W, Cook J, Harris CC, Gius D and Mitchell JB:
Ionizing radiation-induced oxidative stress alters miRNA
expression. PLoS One. 4:e63772009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen T, Ding G, Jin Z, Wagner MB and Yuan
Z: Insulin ameliorates miR-1-induced injury in H9c2 cells under
oxidative stress via Akt activation. Mol Cell Biochem. 369:167–174.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang S, Liu X, Bawa-Khalfe T, Lu LS, Lyu
YL, Liu LF and Yeh ET: Identification of the molecular basis of
doxorubicin-induced cardiotoxicity. Nat Med. 18:1639–1642. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ma J, Wang Y, Zheng D, Wei M, Xu H and
Peng T: Rac1 signalling mediates doxorubicin-induced cardiotoxicity
through both reactive oxygen species-dependent and -independent
pathways. Cardiovasc Res. 97:77–87. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao Y, Zhou G, Wang J, Jia L, Zhang P, Li
R, Shan L, Liu B, Song X, Liu S and Xiao X: Paeoniflorin protects
against ANIT-induced cholestasis by ameliorating oxidative stress
in rats. Food Chem Toxicol. 58:242–248. 2013. View Article : Google Scholar : PubMed/NCBI
|