Introduction
Diabetic nephropathy is a serious and progressive
complication of diabetes and occurs in 30–40% of diabetic patients
(1). Hyperglycemia is a crucial
factor for the induction of diabetic nephropathy. Although positive
effects on the development and progression of diabetic nephropathy
may be produced through strict control of blood glucose, blood
pressure and, in particular, blockade of the renin-angiotensin
system (2), these ainterventions are
not sufficient to significantly reduce the high incidence of
end-stage kidney damage caused by diabetes. Therefore, it is
important to develop novel therapies that allow for the prevention
and retardation of diabetic nephropathy.
The dried root (Radix) of Angelica sinensis
is commonly known as Danggui, and is a crude drug widely used in
traditional Chinese medicine for >2,000 years (3). Danggui is used to promote blood
circulation for the treatment of menstrual disorders, to modulate
the immune system, and the extracts of Radix A. sinensis
have been found to exhibit various levels of antioxidant capacity
(4). Radix Astragali, also
known as Huangqi, is the dried root of Astragalus
membranaceus, which belongs to the family of Fabaceae (5). Radix Astragali, which has also
been used for the treatment of diabetes for hundreds of years in
China, has been demonstrated to have an inhibitory effect on
metal-induced oxidative stress (6).
Danggui Buxue Tang (DBT) is a herbal decoction of Radix
Astragali and Radix A. sinensis (5:1) (7). DBT is speculated to possess a variety
of pharmacological actions, including regulation of immune
functions, stimulation of red blood cell production and enhancement
of cardiovascular function. Previous studies revealed that intake
of DBT ameliorated the symptoms of diabetes and decreased the
levels of blood glucose, plasma lipid, microalbuminuria, reduced
the body weight and improved renal function in streptozotocin
(STZ)-induced diabetic rats, indicating that DBT may be used as an
adjuvant therapy in the prevention of diabetic nephropathy
(8,9).
The glomerular tuft, a network of tangled
capillaries, is composed of three cell types: Endothelial cells at
the inside of the capillary, podocytes on the outside of the
capillary and glomerular mesangial cells (GMCs) supporting the
capillary loops (10). GMCs occupy a
central position in the renal glomerulus and are known to secrete
extracellular matrix (ECM) proteins, such as type IV collagen,
laminin and fibronectin (11). GMC
proliferation, hypertrophy and progressive accumulation of ECM
proteins lead to renal fibrosis, which is recognized to play a
major role in progressive renal failure in diabetic nephropathy
(12). Our previous study has
provided in vitro evidence that DBT effectively inhibits
high glucose-induced GMC proliferation and the expression of
laminin, type IV collagen and fibronectin in GMCs (13). Whether DBT displays a protective
effect on the ultrastructure of the renal glomerulus and
particularly GMCs requires further investigation.
Heparanase (HPA) is an endoglucuronidase which
functions at the cell-surface and within the ECM to degrade heparan
sulfate, the main polysaccharide of the glomerular basement
membrane (GBM) (14). Heparan
sulfate is believed to play a major role in the charge-selective
properties of the glomerular capillary wall, by binding to and
assembling structural basement membrane proteins, and thus
contributing to structural integrity and barrier function of the
basement membrane (15). A recent
study indicated that degradation and loss of heparan sulfate in the
GBM and overexpression of HPA were closely linked to the
development of diabetic nephropathy (16). However, whether DBT has beneficial
effects on reversing the increased level of HPA in diabetes
mellitus has not yet been elucidated.
Therefore, the aim of the present study was to
investigate the effects of DBT on high glucose-induced
ultrastructural alterations of the renal glomeruli in vivo
and the expression of HPA in the renal cortex in a STZ-induced
diabetic nephropathy rat model.
Materials and methods
Preparation of DBT
In preparation of DBT, exact quantities of Radix
Astragali and Radix A. sinensis were weighed at a
ratio of 5:1, and then mixed using a vortex. The herbs originated
from Gansu province (China) and were obtained from the Department
of Pharmacy of Zhongnan Hospital, Wuhan University (Wuhan, China).
As previously described (17), the
mixed herbs were extracted twice; initially boiled in water (1:6;
v/w) for 1 h, then the residue from first extraction was boiled in
water (1:8) for 1.5 h. Finally, the solutions were filtered using a
rotary evaporator (RE-52A; Shanghai Ya Rong Biochemical Instrument
Factory, Shanghai, China) and concentrated into doses of 0.5, 1.0
and 2.0 kg/l. The DBT decoction was stored at 4°C until required
for experimentation.
Animals
Male Sprague Dawley (SD) rats (n=60; age, 4–6 weeks;
weight, 170–200 g) were purchased from the Laboratory Animal Center
of Wuhan University (Certificate No. SCXK 2003-00004). The rats
were housed in a constant environment (temperature, 20–22°C;
humidity, 40–60%) under a 12-h light/dark cycle. This study was
approved by the Committee on the Use of Live Animal in Teaching and
Research of the Wuhan University, and all the animal experiments
were conducted in accordance with the stipulations of the Guide for
the Care and Use of Laboratory Animals (18).
Experimental protocols and
grouping
First, 52 SD rats were fed a high-fat and
high-glucose diet (Wuhan Biological Engineering Co., Ltd., Wuhan,
China) for two weeks, followed by fasting for 12 h (17). Then, the rats were administered a
single intravenous injection of STZ (30 mg/kg; Sigma-Aldrich, St.
Louis, MO, USA) and continuously received the high-fat and
high-glucose diet. The rats were fasted for 12 h, and the diabetic
status was confirmed by monitoring blood glucose 72 h after the
injection using a Johnson blood glucose meter (Johnson &
Johnson Medical Equipment Co., Ltd., Shanghai, China). Rats with
fasting tail-blood glucose concentrations ≥7.0 mmol/l were
considered to have developed diabetes mellitus (19). The rats that were confirmed with
STZ-induced diabetes mellitus were randomly divided into five
groups, treated once per day with the follows: 2 ml normal saline
(Vehicle group); low-dose (0.5 kg/l) DBT (DBT-L group); medium-dose
(1.0 kg/l) DBT (DBT-M group); high-dose (2.0 kg/l) DBT (DBT-H
group); or 1.0 mg/ml gliclazide (Gliclazide group; Beijing Hailian
Pharmaceutical Co., Ltd., Beijing, China), respectively. Gliclazide
is an oral anti-diabetic drug and classified as a sulfonylurea, and
thus has been used as a positive control previously (20). It was expected that ~9 rats were to
be assigned into each group, based on our previous study (9). The dose selection was based on the
conversion to the human equivalent dose (21). In addition, the remaining 8 rats that
were fed with a normal diet and injected with normal saline instead
of STZ, thus serving as a normal control group (NC group).
Intragastric administration of normal saline, DBT or gliclazide was
performed on the designated groups of rats once daily for 6 weeks.
All rats were subsequently fasted overnight and sacrificed via an
overdose intravenously injected pentobarbital (100 mg/kg) after the
samples were collected. The kidneys were removed for the evaluation
of ultrastructural changes by electron microscopy and
immunohistochemistry for HPA expression.
Serum and urine studies
A 24-h urine collection was performed using
metabolic cages (Wuhan Biological Engineering Co., Ltd.). Fasting
blood glucose (FBG), blood urea nitrogen (BUN), serum creatinine
(Scr) and serum and urine β2-microglobulins (β2-MG) levels were
measured using an automatic biochemical analyzer (Hitachi 7020;
Hitachi, Ltd., Tokyo, Japan). Type IV collagen detection kits were
purchased from Wuhan Boster Biological Technology, Ltd. (Wuhan,
China).
Electron microscopy for ultrastructure
of the renal glomerular mesangium
For electron microscopy, the renal cortex was cut
into 5×5×5 mm pieces and fixed in 2.5% glutaraldehyde (Wuhan
Biological Engineering Co., Ltd.) for 2 h. After washing three
times in 0.1% phosphate-buffered saline (Wuhan Biological
Engineering Co., Ltd.), all kidney specimens were fixed in 1%
osmium tetroxide phosphate buffer solution (pH 7.2–7.4; Wuhan
Biological Engineering Co., Ltd.) for 1.5 h. Dehydration was
performed by sequential exposure to 50, 70 and 80% ethanol for 20
min each, and 100% ethanol twice for 15 min each. Sections were
then embedded in epoxy resin (Wuhan Biological Engineering Co.,
Ltd.) and cut into ultrathin sections, which were stained with
uranyl acetate and lead citrate (Wuhan Biological Engineering Co.,
Ltd.), and examined using an H-600 transmission electron microscope
(Hitachi, Ltd.).
Immunohistochemistry for HPA
expression
For immunohistochemistry for HPA expression, the
renal cortex was cut into a 0.2-cm block, which was fixed in 10%
formalin (Wuhan Biological Engineering Co., Ltd.) and embedded in
paraffin (Wuhan Biological Engineering Co., Ltd.). Then, the
embedded blocks were cut into 4-µm sections, which were dehydrated
in graded ethanol and incubated with a primary rabbit anti-rat HPA
monoclonal antibody (1:100; BA1630; Wuhan Boster Biological
Technology, Ltd.) at 4°C overnight. The sections were incubated
with biotinylated goat anti-rabbit IgG antibody as the secondary
antibody (1:100; BA1003; Wuhan Boster Biological Technology, Ltd.).
A slide known to be positive to HPA (Wuhan Biological Engineering
Co., Ltd.) was set as a positive control, and an irrelevant isotype
rabbit IG was used for a negative control. The peroxidase reaction
was visualized using 3,3′-diaminobenzidine tetrahydrochloride
(Wuhan Boster Biological Technology, Ltd.) as a peroxidase
substrate.
Images of immunostained sections were captured using
a Canon 1000D digital camera (Canon, Inc., Tokyo, Japan). The
positive immunostaining, which presented as brown or yellow
granules in the cytoplasm and/or cell membrane, was automatically
detected and measured for the staining intensity and area using a
computer equipped with HPIAS-1000 image analysis software (Wuhan
Qingping Image Technology, Co., Ltd., Wuhan, China). Five visual
fields were randomly selected and assessed for immunoreactive areas
at ×200 magnification. The ratio (%) of the HPA-positive glomerular
area to the total glomerular area assessed was calculated, and the
mean ratio of the five fields was defined to represent the level of
HPA expression.
Statistical analysis
All statistical analyses were performed using SPSS
software, version 13.0 (SPSS, Inc., Chicago, IL, USA). Quantitative
data are presented as the mean ± standard deviation. Student's
t-test and one-way analysis of variance methods were used for
analyzing the differences between two groups and among the six
groups, respectively. P<0.05 was considered to indicate a
statistically significant difference.
Results
General condition of the animals
Following the injection of STZ, six rats died or
failed to develop diabetes. Thus, the 46 rats that developed
STZ-induced diabetes mellitus were randomly distributed into the
Vehicle (n=10), DBT-H (n=9), DBT-M (n=9), DBT-L (n=9) and
Gliclazide (n=9) groups. The numbers of rats that died during the
next six weeks were three in Vehicle group and one in the DBT-L
group. After six weeks of high-glucose diet, the diabetic rats in
the Vehicle group showed typical diabetic symptoms, including
polydipsia, polyphagia, polyuria and body weight loss, with dull
fur and reduced activity.
Effects of DBT on FBG, BUN, Scr, serum
and urine β2-MG, and type IV collagen expression
All rats with STZ-induced diabetes mellitus had
elevated levels of FBG, BUN, Scr, serum and urine β2-MG, and type
IV collagen expression prior to treatment compared with rats in the
NC group (all P<0.05; Tables I
and II). Treatment with gliclazide
for six weeks significantly decreased the levels of FBG, BUN, Scr,
serum and urine β2-MG, and type IV collagen expression, as compared
with the vehicle group (P<0.05). Notably, DBT treatment at
different doses resulted in a similar reversal of these abnormal
parameters, although they were all above the normal levels.
 | Table I.Effects of DBT on fasting blood
glucose, serum creatinine and blood urea nitrogen in rats. |
Table I.
Effects of DBT on fasting blood
glucose, serum creatinine and blood urea nitrogen in rats.
|
|
| Fasting blood
glucose (mmol/l) | Serum creatinine
(µmol/l) | Blood urea nitrogen
(mmol/l) |
|---|
|
|
|
|
|
|
|---|
| Group | n | Before
treatment | 6 weeks after
treatment | Before
treatment | 6 weeks after
treatment | Before
treatment | 6 weeks after
treatment |
|---|
| Normal control | 8 | 5.3±1.12 | 5.2±1.53 | 19.85±3.21 | 18.11±3.22 | 7.1±1.21 | 6.9±1.42 |
| Vehicle | 7 |
16.8±1.21a | 17.3±1.12 |
61.21±4.12a | 75.91±5.12 |
10.3±2.11a | 13.23±2.34 |
| DBT-L | 8 |
17.3±0.98a |
12.2±1.31b,c |
62.31±3.52a |
52.11±2.34b,c |
11.8±2.13a |
9.21±1.02b,c |
| DBT-M | 9 |
17.1±1.11a |
13.1±1.51b,c |
63.11±2.32a |
49.13±1.28b,c |
12.4±2.29a |
9.11±1.13b,c |
| DBT-H | 9 |
16.9±1.02a |
13.4±1.22b,c |
64.10±3.13a |
46.32±4.19b,c |
13.9±2.01a |
8.32±0.12b,c |
| Gliclazide | 9 |
17.2±0.92a |
10.3±0.42b,c |
63.89±4.11a |
43.12±4.43b,c |
13.4±2.32a |
7.12±2.11b,c |
 | Table II.Effects of DBT on serum and urine
β2-microglobulin levels and type IV collagen expression in
rats. |
Table II.
Effects of DBT on serum and urine
β2-microglobulin levels and type IV collagen expression in
rats.
|
|
| Serum
β2-microglobulin (mg/l) | Urine
β2-microglobulin (µg/l) | Type IV collagen
(ng/ml) |
|---|
|
|
|
|
|
|
|---|
| Group | n | Before
treatment | 6 weeks after
treatment | Before
treatment | 6 weeks after
treatment | Before
treatment | 6 weeks after
treatment |
|---|
| Normal control | 8 | 2.01±0.12 | 2.41±0.32 | 7.41±0.18 | 7.01±0.32 | 21.1±2.42 | 23.2±3.51 |
| Vehicle | 7 |
7.01±0.22a | 8.08±0.13 |
14.31±0.22a | 13.06±0.41 |
37.3±2.23a | 40.1±3.41 |
| DBT-L | 8 |
6.95±0.41a |
5.41±0.13b |
13.64±0.32a,c |
10.01±0.12b,c |
36.3±4.41a |
27.1±4.01b,c |
| DBT-M | 9 |
6.78±0.33a |
5.01±0.44b |
13.01±0.37a,c |
9.94±0.32b,c |
34.7±3.22a |
28.1±2.90b,c |
| DBT-H | 9 |
7.01±0.35a |
5.13±0.61b |
12.75±0.28a,c |
9.01±0.14b,c |
35.1±2.12a |
25.1±3.21b,c |
| Gliclazide | 9 |
7.03±0.22a |
6.78±0.12b |
13.01±0.42a,c |
10.01±0.32b,c |
33.9±3.41a |
27.1±2.42b,c |
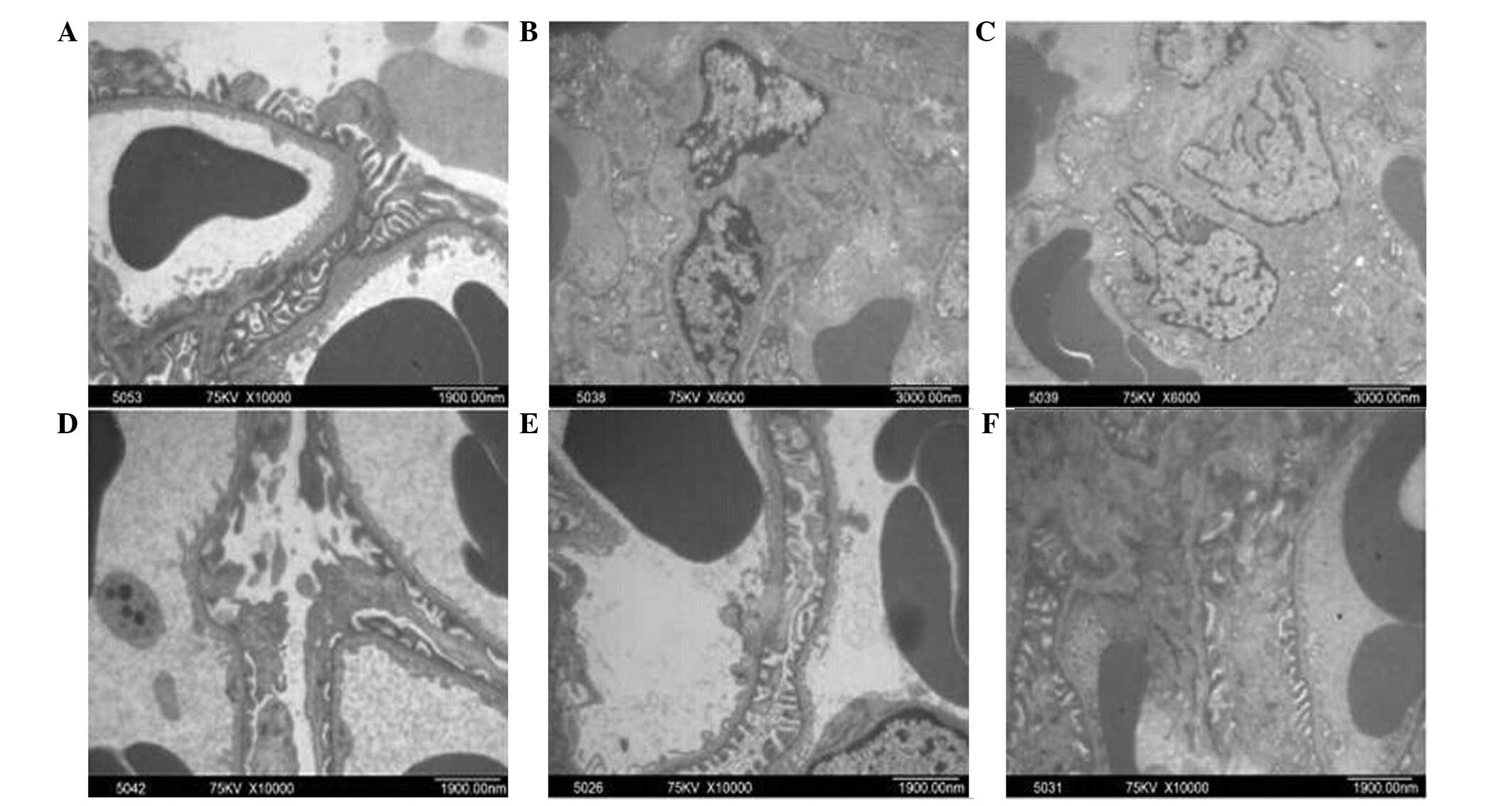
DBT treatment restores the changes in
the glomerular ultrastructure in diabetic rats
To assess the protective effects on the renal
glomerulus, ultrastructural analysis by electron microscopy was
performed in the renal cortex of diabetic rats after six weeks of
DBT treatment, with the untreated diabetic and normal rats as
controls. All rats in NC group showed normal glomerular
ultrastructure. Specifically, numerous thick primary processes
extended from the cell body of the podocytes. These then further
ramified into fine, finger-like secondary foot processes, which
interdigitated with foot processes from adjacent podocytes. The
foot processes formed a fence-like structure which was closely
attached to the GBM. Together with the GBM, the endothelium and
podocytes formed the filtration barrier, and the mesangium
consisting of GMCs and the matrix lied between the glomerular
capillaries (Fig. 1A). In the
Vehicle group, the secondary foot processes of podocytes became
irregular and displayed numerous areas of effacement and GBM
thickened with evident expansion of GMCs (Fig. 1B). However, in rats after six weeks
of DBT treatment, there was a notable improvement in the
ultrastructural abnormalities (Fig.
1C–E), particularly in those in the DBT-H group, where GBM
thickness, podocytes and GMCs were similar to those in the NC
group. Similar improvement in the ultrastructural abnormalities was
observed in rats in the Gliclazide group (Fig. 1F).
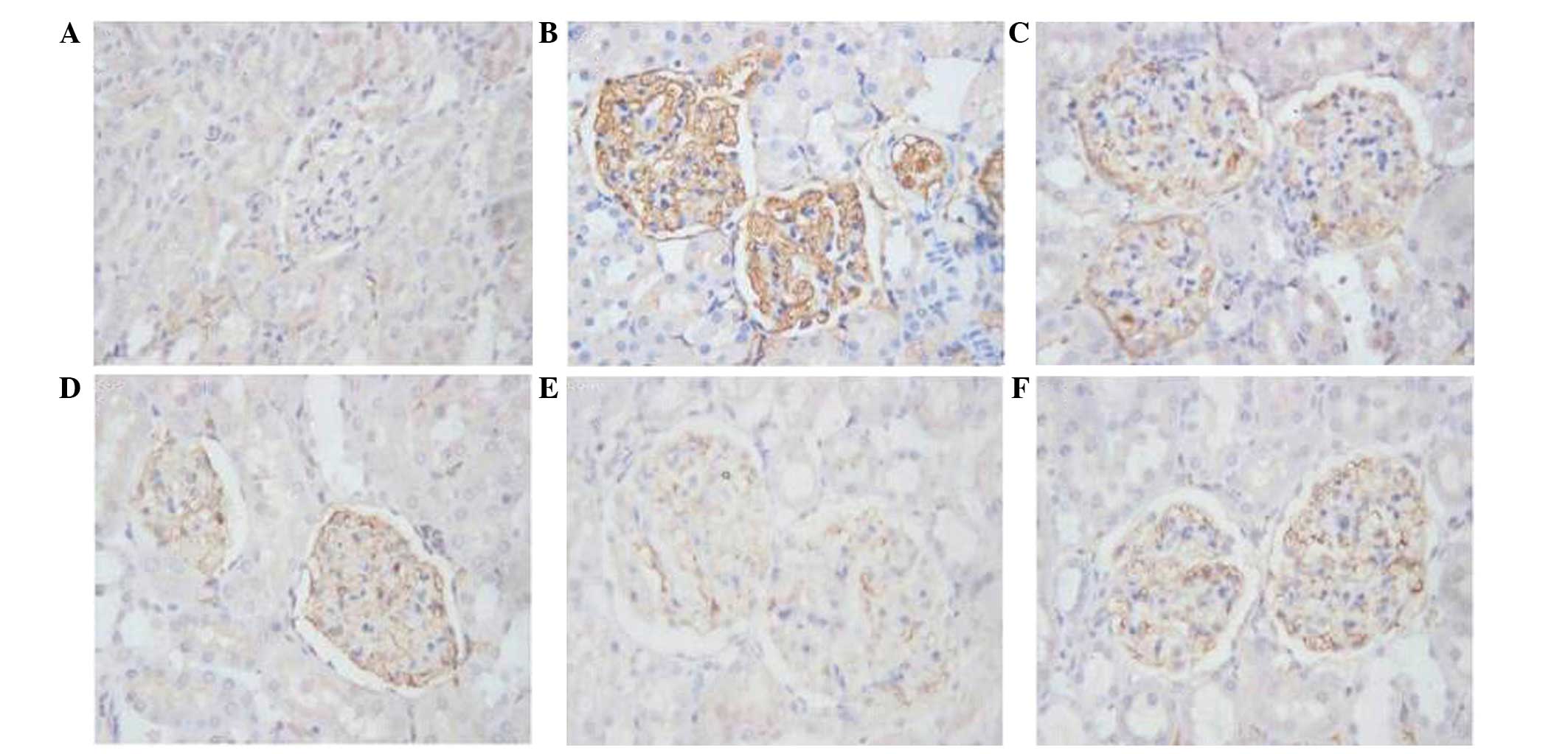
DBT treatment decreases HPA expression
in the renal cortex in diabetic rats
The positive immunohistochemical staining of HPA
protein was shown as brown signals in the cytoplasm and/or cell
membrane of podocytes, mesangial cells and tubular epithelial
cells. The positive signals were quantified using HPIAS-1000 image
analysis software, and the average values of HPA
immunohistochemical staining in the different groups are shown in
Table III. HPA expression was
negative or very low in glomeruli in the control group (Table III and Fig. 2A). The rats in the Vehicle group
exhibited a significantly higher HPA immunostaining in the
glomeruli (Table III and Fig. 2B). However, HPA protein expression
was significantly and dose-dependently decreased in the DBT groups,
as compared to the Vehicle group (Table III and Fig. 2C–E). HPA protein expression was also
significantly decreased in the Gliclazide group, as compared to the
Vehicle group (Table III and
Fig. 2F). Notably, the high-dose DBT
(2.0 kg/l) treatment showed an increased effect compared with
gliclazide with respect to decreasing HPA expression (36.25±3.86
vs. 52.00±2.71; P<0.05).
 | Table III.Expression of HPA in the kidney
tissues. |
Table III.
Expression of HPA in the kidney
tissues.
| Group | n | HPA expression
(%) |
|---|
| Normal control | 8 | 18.00±8.2 |
| Vehicle | 7 |
109.50±18.95a |
| DBT-L | 8 |
71.00±8.04a,b,c |
| DBT-M | 9 |
55.50±1.29a,b |
| DBT-H | 9 |
36.25±3.86a,b,c |
| Gliclazide | 9 |
52.00±2.71a,b |
Discussion
Development of diabetic nephropathy is characterized
by increased plasma levels of creatinine and BUN in STZ-induced
diabetic rats (22). STZ is a
powerful alkylating agent that has been widely accepted as an
inducer to produce diabetes mellitus in rodents (23). STZ interferes with numerous cellular
processes, such as glucose transport and glucokinase function, and
may induce DNA strand breakage (24). A single large dose of STZ (such as 30
mg/kg) may induce diabetes mellitus in rodents; however, this dose
has been associated with high mortality. In the present study, a
two-week high-glucose diet followed by a single injection of 30
mg/kg STZ induced diabetes mellitus in 88% (46/52) of the surviving
rats. Moreover, the subsequent six-week maintenance of the
high-glucose diet resulted in typical features of nephropathy in
the diabetic rats. Besides symptoms of hyperglycemia and obesity,
this model appeared to exhibit the most consistent and robust
increases in BUN and Scr; as such, it has been used as a useful
model of progressive diabetic renal disease.
The pathological characteristics of diabetic
nephropathy include the proliferation of GMCs, thickening of the
GBM, and accumulation of ECM proteins, followed by progressive
glomerulosclerosis and worsening of renal function (25–27). An
in vitro study has shown that GMCs are a major source of
free radicals following exposure to high glucose concentrations
(28). Hyperglycemia causes
oxidative stress and leads to an increase in a number of
complements, growth factors, and cytokines, such as transforming
growth factor (TGF)-β, and the excessive production and
accumulation of ECM proteins (29).
This increased production in GMCs has been implicated in the
development of diabetic nephropathy. Our previous in vitro
study showed that high glucose induced an increased synthesis of
various ECM proteins, including type IV collagen, laminin, and
fibronectin, in glomerular mesangial and epithelial cells (13). Furthermore, in the present study, the
high glucose-induced diabetic rats displayed abnormal thickening of
the GBM, loss of podocytes, effacement of foot processes and
expansion of mesangial cells. These findings clearly indicate that
the high glucose not only induces glomerular mesangial and
epithelial proliferation, but also causes ultrastructural changes
of the kidney.
Radix Astragali (Huangqi) is widely used in
China, and has been studied in animal models and patients with
various renal diseases for its effects on cytokines and reactive
oxygen species, ischemia/reperfusion injury and mechanisms of renal
fibrosis (30). A systematic review
revealed that Radix Astragali and its effective components
are may be able to FBG and albuminuria levels, reversing the
glomerular hyperfiltration and ameliorating the pathological
changes associated with early diabetic nephropathy in rat models
(31). DBT is traditionally used to
treat gynecological disorders and hypertension in China. DBT alone
appears to improve the renal functions and increase renal BMP-7
expression in STZ-induced diabetic rats (27). The present results showed that DBT
was effective at improving diabetic nephropathy. Similar to the
action of gliclazide, DBT treatment effectively attenuated the
increases in FBG, BUN, Scr, and serum and urine β2-MG in rats with
STZ-induced diabetes mellitus. The accumulation of ECM proteins,
including type IV collagen, plays an important role in the
pathogenesis of diabetic nephropathy (13). In the present study, DBT treatment
caused a reduction in type IV collagen expression compared with the
Vehicle group, suggesting that DBT may prevent kidney damage by
reducing the increase of ECM proteins.
DBT in combination with Radix Astragali
accelerates histological recovery of the kidney, and delays the
progression of renal fibrosis as much as the acetyl choline
inhibitor enalapril (32). This
appeared to occur via the suppression of TGF-β expression and
mitigation of the upregulated expression of type III and IV
collagens, fibronectin and laminin in a rat model of chronic
puromycin-induced nephrosis (33).
In the present study, DBT treatment substantially improved
diabetes-induced structural cellular alterations, such as the
proliferation of GMCs, loss of podocyte foot processes and abnormal
thickness of the GBM. In particular, the GBM thickness, numbers of
podocytes and GMCs were similar between the DBT-H and NC groups.
These results further support those obtained in our previous in
vitro study in which DBT had inhibitory effects on high
glucose-induced GMC proliferation and the expression of ECM
proteins (13).
Heparan sulfate proteoglycans (HSPG) are ubiquitous
macromolecules associated with cell surfaces and ECM, and represent
a major constituent of the GBM (34). HSPG in the kidneys are composed of an
agrin protein core that is covalently linked to the negatively
charged glycosaminoglycan heparan sulfate (35). HPA is an endoglycosidase which
cleaves heparan sulfate and hence participates in the degradation
and remodeling of the ECM. A previous study indicated that
increased glomerular expression of HPA was associated with overt
diabetic nephropathy (36). In
addition, the expression of HPA was upregulated in animal models of
proteinuric renal disease, including passive heymann nephritis,
puromycin nephrosis, anti-GBM nephritis and adriamycin nephropathy
(37). Furthermore, the
overexpression of HPA in transgenic mice led to proteinuria, while
treatment with a polyclonal anti-HPA antibody resulted in a
three-fold reduction of proteinuria in a model of anti-GBM disease
(37). In the present study, the
expression of HPA in the diabetic rat renal cortex was evaluated
using immunohistochemistry to investigate the effect of DBT
treatments on HPA expression. HPA expression was negative or low in
the glomeruli of the NC group. However, the rats in the Vehicle
group exhibited a significantly higher HPA immunostaining in the
glomeruli. These findings were consistent with our previous in
vitro study, which demonstrated that high glucose was able to
induce HPA expression in podocytes and a reduction of cell surface
heparan sulfate (38).
Overexpression of HPA may lead to an increased permeability of the
GBM to macromolecules, due to the degradation of glomerular heparan
sulfate and the activation of a variety of complements and
cytokines (39). To the best of our
knowledge, the present study is the first to demonstrate that DBT
treatment effectively inhibited the levels of HPA expression in
renal glomerular mesangium, indicating that DBT may preserve renal
functional and structural integrity. This may at least partially
occur via the inhibition of HPA expression, resulting in increased
heparin sulfate expression in the renal glomerular mesangium.
Notably, the high-dose DBT (2 kg/l) treatment was more effective
than gliclazide with respect to decreasing HPA expression. However,
heparan sulfate loss in proteinuric renal disease may be attributed
to a number of mechanisms other than HPA, including
depolymerization of heparin sulfate by increasing the formation of
free radicals, such as reactive oxygen species (ROS) and decreasing
antioxidant capacity (40).
Oxidative stress may facilitate the formation of advanced glycation
end products, which is a pathogenic factor in sustained
hyperglycemia-induced kidney injuries (41). DBT has been reported to be a native
free radical scavenger, and is able to decrease
hyperglycemia-induced ROS generation in the kidneys of diabetic
rats or in mesangial cells (28).
Therefore, anti-ROS treatments such as DBT may prevent
hyperglycemia-induced renal damage in diabetes mellitus.
It should be noted that the present report is only a
preliminary observational study. Thus, further studies are required
to identify the specific active components present in DBT that are
responsible for the beneficial effects on the renal function, and
to elucidate the underlying mechanisms by which DBT improves and
prevents diabetes mellitus and diabetic nephropathy.
DBT appears to be a renal protective agent by
reversing renal ultrastructural changes in a
high-glucose/STZ-induced diabetic nephropathy rat model.
Furthermore, DBT exhibits beneficial effects by decreasing the
elevated HPA expression in the renal glomerular mesangium.
Therefore, DBT is potentially a novel therapeutic intervention for
the treatment of diabetic nephropathy.
Acknowledgements
This study was supported by funding from the
National Natural Science Foundation of China (grant no.
30973819).
References
|
1
|
Heerspink HJ and de Zeeuw D: The kidney in
type 2 diabetes therapy. Rev Diabet Stud. 8:392–402. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Abuissa H and O'Keefe J Jr: The role of
renin-angiotensin-aldosterone system-based therapy in diabetes
prevention and cardiovascular and renal protection. Diabetes Obes
Metab. 10:1157–1166. 2008.PubMed/NCBI
|
|
3
|
Zhang WL, Zheng KY, Zhu KY, Zhan JY, Bi
CW, Chen JP, Dong TT, Choi RC, Lau DT and Tsim KW: Chemical and
biological assessment of angelica roots from different cultivated
regions in a chinese herbal decoction danggui buxue tang. Evid
Based Complement Alternat Med. 2013:4832862013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu YC and Hsieh CL: Pharmacological
effects of Radix Angelica sinensis (Danggui) on cerebral
infarction. Chin Med. 6:322011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang Z, Wang J and Chan P: Treating type 2
diabetes mellitus with traditional chinese and Indian medicinal
herbs. Evid Based Complement Alternat Med.
2013:3435942013.PubMed/NCBI
|
|
6
|
Toda S, Yase Y and Shirataki Y: Inhibitory
effects of astragali radix, crude drug in Oriental medicines on
lipid peroxidation and protein oxidative modification of mouse
brain homogenate by copper. Phytother Res. 14:294–296. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gao QT, Choi RC, Cheung AW, Zhu JT, Li J,
Chu GK, Duan R, Cheung JK, Jiang ZY, Dong XB, et al: Danggui buxue
tang - a Chinese herbal decoction activates the phosphorylations of
extracellular signal-regulated kinase and estrogen receptor alpha
in cultured MCF-7 cells. FEBS Lett. 581:233–240. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang YW, Xie D, Xia B, Zhen RT, Liu IM
and Cheng JT: Suppression of transforming growth factor-beta1 gene
expression by Danggui buxue tang, a traditional Chinese herbal
preparation, in retarding the progress of renal damage in
streptozotocin-induced diabetic rats. Horm Metab Res. 38:82–88.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang YW, Xie D, Chen YX, Zhang HY and Xia
ZX: Protective effect of Gui Qi mixture on the progression of
diabetic nephropathy in rats. Exp Clin Endocrinol Diabetes.
114:563–568. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ha TS: Roles of adaptor proteins in
podocyte biology. World J Nephrol. 2:1–10. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chiang YY, Takebayashi S and Oberley TD:
In vitro analysis of extracellular matrix production by porcine
glomerular mesangial and vascular smooth muscle cells. Am J Pathol.
138:1349–1358. 1991.PubMed/NCBI
|
|
12
|
Lan T, Liu W, Xie X, Xu S, Huang K, Peng
J, Shen X, Liu P, Wang L, Xia P and Huang H: Sphingosine kinase-1
pathway mediates high glucose-induced fibronectin expression in
glomerular mesangial cells. Mol Endocrinol. 25:2094–2105. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ke HL, Zhang YW, Zhou BF and Zhen RT:
Effects of Danggui Buxue Tang, a traditional Chinese herbal
decoction, on high glucose-induced proliferation and expression of
extracellular matrix proteins in glomerular mesangial cells. Nat
Prod Res. 26:1022–1026. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bame KJ: Heparanases: endoglycosidases
that degrade heparan sulfate proteoglycans. Glycobiology.
11:91R–98R. 2011. View Article : Google Scholar
|
|
15
|
Jiang P, Kumar A, Parrillo JE, Dempsey LA,
Platt JL, Prinz RA and Xu X: Cloning and characterization of the
human heparanase-1 (HPR1) gene promoter: Role of GA-binding protein
and Sp1 in regulating HPR1 basal promoter activity. J Biol Chem.
277:8989–8998. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gil N, Goldberg R, Neuman T, Garsen M,
Zcharia E, Rubinstein AM, van Kuppevelt T, Meirovitz A, Pisano C,
Li JP, et al: Heparanase is essential for the development of
diabetic nephropathy in mice. Diabetes. 61:208–216. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang YW, Wu CY and Cheng JT: Merit of
Astragalus polysaccharide in the improvement of early diabetic
nephropathy with an effect on mRNA expressions of NF-kappaB and
IkappaB in renal cortex of streptozotoxin-induced diabetic rats. J
Ethnopharmacol. 114:387–392. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Clark JD, Gebhart GF, Gonder JC, Keeling
ME and Kohn DF: Special report: Guide for the care and use of
laboratory animals. ILAR J. 1997:41–48. 1997. View Article : Google Scholar
|
|
19
|
Nakamura T, Terajima T, Ogata T, Ueno K,
Hashimoto N, Ono K and Yano S: Establishment and pathophysiological
characterization of type 2 diabetic mouse model produced by
streptozotocin and nicotinamide. Biol Pharm Bull. 29:1167–1174.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schernthaner G, Grimaldi A, Di Mario U,
Drzewoski J, Kempler P, Kvapil M, Novials A, Rottiers R, Rutten GE
and Shaw KM: GUIDE study: Double-blind comparison of once-daily
gliclazide MR andglimepiride in type 2 diabetic patients. Eur J
Clin Invest. 34:535–542. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
U.S. Food and Drug Administration:
Guidance for Industry and Reviewers: Estimating the Safe Starting
Dose in Clinical Trials for Therapeutics in Adult Healthy
Volunteers. December;2002.
|
|
22
|
Wang GG, Lu XH, Li W, Zhao X and Zhang C:
Protective effects of luteolin on diabetic nephropathy in
STZ-induced diabetic rats. Evid Based Complement Alternat Med.
2011:3231712011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
King AJ: The use of animal models in
diabetes research. Br J Pharmacol. 166:877–894. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Like AA and Rossini AA:
Streptozotocin-induced pancreatic insulitis: New model of diabetes
mellitus. Science. 193:415–417. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kanwar YS, Wada J, Sun L, Xie P, Wallner
EI, Chen S, Chugh S and Danesh FR: Diabetic nephropathy: Mechanisms
of renal disease progression. Exp Biol Med (Maywood). 233:4–11.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wolf G: New insights into the
pathophysiology of diabetic nephropathy: From haemodynamics to
molecular pathology. Eur J Clin Invest. 34:785–796. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dronavalli S, Duka I and Bakris GL: The
pathogenesis of diabetic nephropathy. Nat Clin Pract Endocrinol
Metab. 4:444–452. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yeh CH, Chang CK, Cheng KC, Li YX, Zhang
YW and Cheng JT: Role of bone morphogenetic proteins-7 (BMP-7) in
the renal improvement effect of DangGui (Angelica sinensis)
in type-1 diabetic rats. Evid Based Complement Alternat Med.
2011:7967232011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Giacco F and Brownlee M: Oxidative stress
and diabetic complications. Circ Res. 107:1058–1070. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhong Y, Deng Y, Chen Y, Chuang PY and
Cijiang He J: Therapeutic use of traditional Chinese herbal
medications for chronic kidney diseases. Kidney Int. 84:1108–1118.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang J, Xie X, Li C and Fu P: Systematic
review of the renal protective effect of Astragalus
membranaceus (root) on diabetic nephropathy in animal models. J
Ethnopharmacol. 126:189–196. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen X, Cao A, Wang L, Yin P, Zhang X and
Peng W: Prevention and treatment of diabetic nephropathy using
traditional Chinese medicine. J Integr Nephrol Androl. 1:53–57.
2014. View Article : Google Scholar
|
|
33
|
Wang H, Li J, Yu L, Zhao Y and Ding W:
Antifibrotic effect of the Chinese herbs, Astragalus
mongholicus and Angelica sinensis, in a rat model of
chronic puromycin aminonucleoside nephrosis. Life Sci.
74:1645–1658. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shimomura H and Spiro RG: Studies on
macromolecular components of human glomerular basement membrane and
alterations in diabetes. Decreased levels of heparan sulfate
proteoglycan and laminin. Diabetes. 36:374–381. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rops AL, van der Vlag J, Lensen JF,
Wijnhoven TJ, van den Heuvel LP, van Kuppevelt TH and Berden JH:
Heparan sulfate proteoglycans in glomerular inflammation. Kidney
Int. 65:768–785. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
van den Hoven MJ, Rops AL, Bakker MA, Aten
J, Rutjes N, Roestenberg P, Goldschmeding R, Zcharia E, Vlodavsky
I, van der Vlag J and Berden JH: Increased expression of heparanase
in overt diabetic nephropathy. Kidney Int. 70:2100–2108. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shafat I, Agbaria A, Boaz M, Schwartz D,
Baruch R, Nakash R, Ilan N, Vlodavsky I and Weinstein T: Elevated
urine heparanase levels are associated with proteinuria and
decreased renal allograft function. PLoS One. 7:e440762012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Maxhimer JB, Somenek M, Rao G, Pesce CE,
Baldwin D Jr, Gattuso P, Schwartz MM, Lewis EJ, Prinz RA and Xu X:
Heparanase-1 gene expression and regulation by high glucose in
renal epithelial cells: A potential role in the pathogenesis of
proteinuria in diabetic patients. Diabetes. 54:2172–2178. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
van den Hoven MJ, Rops AL, Bakker MA, Aten
J, Rutjes N, Roestenberg P, Goldschmeding R, Zcharia E, Vlodavsky
I, van der Vlag J and Berden JH: Increased expression of heparanase
in overt diabetic nephropathy. Kidney Int. 70:2100–2108. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kramer A, van den Hoven M, Rops A,
Wijnhoven T, van den Heuvel L, Lensen J, van Kuppevelt T, van Goor
H, van der Vlag J, Navis G and Berden JH: Induction of glomerular
heparanase expression in rats with adriamycin nephropathy is
regulated by reactive oxygen species and the renin-angiotensin
system. J Am Soc Nephrol. 17:2513–2520. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tan AL, Forbes JM and Cooper ME: AGE, RAGE
and ROS in diabetic nephropathy. Semin Nephrol. 27:130–143. 2007.
View Article : Google Scholar : PubMed/NCBI
|