Introduction
In total, ~240 million individuals are chronically
infected with hepatitis B virus (HBV) worldwide (1). Interferon and nucleotide analogs (NAs)
are widely used to interrupt the progression of the disease and
prevent undesirable clinical outcomes (2). Of these two approaches, NAs are more
frequently selected due to their relatively rare side-effects and
for patients intolerant of immunomodulatory therapy. However, with
long-term NA monotherapy, the selective pressure imposed by the NAs
gradually favors an increase in viruses harboring resistant
variants to reverse transcriptase (RT), resulting in reduced
susceptibility and resistance to NAs (3,4).
Virological breakthrough (defined as a confirmed increase in the
HBV DNA level by >1 log10 copies/ml compared with the nadir HBV
DNA level during therapy), and biochemical breakthrough. may emerge
in clinical settings, followed by liver disease progression
(5–7).
Lamivudine (LAM)-resistant variants have posed a
major challenge in the management of patients with chronic
hepatitis B (CHB). Although LAM is not recommended as a first-line
therapy in current clinical practice guidelines due to the
relatively low genetic barrier to drug resistance, it was the first
NA to be developed and has been widely used as a first-line
monotherapy drug for 10 years (8,9).
Long-term LAM monotherapy frequently leads to drug resistance
characterized by increased viral replication in patients. The
incidence of LAM resistance is 14–32% after 1 year of treatment,
38% after 2 years and 53–76% after 3 years (10). The principal resistant variants
associated with LAM resistance are located in domain C of the YMDD
motif (11). These resistant
variants include rtM204V, rtM204I and the infrequently identified
rtM204S (11–13). The rise of LAM resistance has led to
a focus on rescue therapy, which involves switching therapies or
using LAM in combination with other NAs. When it initially became
available, adefovir (ADV) monotherapy or ADV-LAM combination
therapy was used as a rescue therapy following LAM treatment
failure for numerous patients with HBV infection, and these
strategies were included in the 2005 Asia-Pacific guidelines for
the management of patients with LAM-resistant variants (8,14).
Understanding the dynamics of resistant variants
under various antiviral pressures may contribute to improving
treatment strategy and preventing undesirable clinical outcomes.
The emergence of ADV-resistant variants in patients with LAM
resistance was more frequent in patients rescued with ADV
monotherapy than patients rescued with LAM-ADV combination therapy
(15–17). In rare cases in which LAM and
ADV-resistant variants developed during LAM-ADV sequential
monotherapy, ADV-entecavir (ETV) was considered as a promising
option following previous treatment failure with NAs, where more
potent drugs, such as tenofovir (TDF), have not been approved or
are not affordable by the majority of the population (18). Based on current knowledge of cross
resistance, NAs with an absence of cross-resistance are recommended
as rescue regimens in CHB patients with resistant variants
(9). However, to the best of our
knowledge, little is known regarding the dynamics of resistant
variants of LAM and ADV in patients who sequentially received
LAM-ADV monotherapy, and ADV-ETV combination therapy.
In this context, pyrosequencing was used in the
present study to investigate the dynamics of LAM-resistant variants
during antiviral therapy using ADV sequential monotherapy followed
by ADV-ETV combination therapy.
Materials and methods
Patients
A total of 55 patients with CHB were enrolled in the
present study between June 2007 and July 2008 at the Beijing Youan
Hospital, Capital Medical University (Beijing, China). The
inclusion criteria were as follows: i) HBsAg positive history for
≥6 months prior to treatment; ii) CHB male or female patients aged
≥16 years; iii) liver function with compensator phase (without
ascites, hepatic encephalopathy or upper gastrointestinal
bleeding); iv) not pregnant; and v) consent obtained prior to the
start of the study. The exclusion criteria were as follows: i)
Active liver disease or co-infection with another virus, including
hepatitis C, hepatitis D, human immunodeficiency viruses, or the
existence of autoimmune liver disease; ii) in addition to hepatitis
B, the patient had other major diseases of the organs, such as
severe heart disease or kidney disease; iii) renal dysfunction
(creatinine clearance <50 ml/min), since it would require that
the dosage of the antiviral drug be reduced; iv) poor compliance;
v) history of a malignancy, including hepatocellular carcinoma,
carcinoma in situ and atypical hyperplastic nodules, life
expectancy <1 year without liver transplant; vi) patients with
mental illness; vii the patients had received corticosteroids,
immunosuppressants or chemotherapeutic drugs ≤6 months prior to
enrollment; and viii) pregnant and breast-feeding women.
Of the 55 patients, only three with LAM-resistant
variants, including one female and two males, developed ADV
resistance and were selected for further analysis (Table I). These 3 patients had undergone
sequential monotherapy by daily oral administration of 10 mg ADV
(GlaxoSmithKline Co., Ltd., Tianjin, China). Following the
development of ADV resistance, 0.5 mg ETV (Sino-American Shanghai
Squibb Pharmaceutical Ltd., Shanghai, China) was orally
administered daily in combination with the ongoing ADV treatment as
a rescue therapy. CHB was diagnosed according to the guidelines of
the American Association for the Study of Liver Diseases (19) and histology was characterized
according to the Ishak scoring system (20). No patient was co-infected with the
hepatitis delta virus, hepatitis C virus, or.
 | Table I.Clinical features of chronic
hepatitis B patients with LAM-resistant variants treated with ADV
monotherapy followed by ADV-ETV combination therapy. |
Table I.
Clinical features of chronic
hepatitis B patients with LAM-resistant variants treated with ADV
monotherapy followed by ADV-ETV combination therapy.
| Characteristic | Patient 1 | Patient 2 | Patient 3 |
|---|
| Gender | Female | Male | Male |
| Age (years) | 53 | 24 | 45 |
| HBeAg | + | + | + |
| Duration of LAM
(months) | 21 | 24 | 23 |
| LAM-resistant
variants | M204I | M204I | L180M + M204V |
| HBV genotype | C | C | C |
| Viral load |
|
|
|
| (log10
copies/ml) | 6.88 | 6.60 | 8.70 |
| ALT
(U/l)a | 68.5 | 317.3 | 73.6 |
| AST
(U/l)a | 64.1 | 81.0 | 35.3 |
| Liver
histology (inflammation/fibrosis)b | 4/3 | 7/3 | 13/5 |
| During ADV
mono-therapy |
|
|
|
|
Duration of ADV(mo) | 19 | 19 | 30 |
|
ADV-resistant variants | A181V | A181T | N236T |
| During ADV-ETV
combination therapy |
|
|
|
| Viral
load at the start (log10copies/ml) | 4.04 | 6.21 | 6.01 |
|
Duration of ADV-ETV (mo) | 41 | 41 | 30 |
| Liver
histology at month 60 (inflammation/fibrosis)b | 2/2 | 3/2 | 7/4 |
The patients were followed-up from when they started
sequential monotherapy with ADV. The patients were consecutively
monitored every 3 months in the first year of therapy, and every 6
months thereafter throughout the course of treatment. During each
follow-up, serum specimens were collected for liver function tests,
viral marker tests, and HBV DNA quantification. Any remaining serum
samples were stored at −80°C for subsequent research. There were no
reported issues concerning medication non-compliance.
The present study was conducted in compliance with
the Declaration of Helsinki (21),
and was approved by the Medical Ethics Review Committee of the
Beijing Youan Hospital. All patients provided written-informed
consent authorizing access to their medical records and storage of
the remaining serum specimens for research use.
Measurement of liver function and HBV
DNA quantification
Alanine aminotransferase and aspartate
aminotransferase levels were measured using an Olympus Automatic
Biochemical Analyzer AU5400 (Olympus Corp., Tokyo, Japan) with a
cut-off value of 40 IU/l, according to the manufacturer's protocol.
The viral markers, including hepatitis B surface antigen (HBsAg),
anti-hepatitis B s antibody (HBsAb) hepatitis B e antigen (HBeAg),
and anti-hepatitis B e antibody (HBeAb), were determined using
commercial chemiluminescence immunoassay kits (cat. nos. S10980090,
S10980089, S10980088 and S10980087, respectively; Beijing Wantai
Biological Pharmacy Enterprise Co., Ltd., Beijing, China) on an
ARCHITECT i-20000SR automatic chemiluminescence immunoassay
analyzer (Abbott Laboratories, Chicago, IL, USA). Serum HBV DNA
levels were determined using a Cobas HBV Amplicor Monitor assay
(Roche Diagnostics, Pleasanton, CA, USA), with a lower limit of
detection of 2.46 log10 copies/ml (~50 IU/ml or 291
copies/ml), according to the manufacturer's protocol.
Detection of antiviral-resistant
mutations
The pyrosequencing assay was performed using the
PyroMark careHBV Drug Resistance Test kit (Qiagen China Co., Ltd.,
Shanghai, China) and a PyroMark Q24 MDx system (Qiagen GmbH,
Hilden, Germany), according to the manufacturer's protocols. HBV
DNA purification reagents (silica-gel membrane column and
extraction buffers), gene amplification primers, and sequencing
primers were included in the kit. The protocol was conducted as
previously described (22). A total
of 10 mutation sites (rtL169, rtV173, rtL180, rtA181, rtT184,
rtA194, rtS202, rtM204, rtN236 and rtM250) were analyzed on the
reverse transcription domain of HBV DNA polymerase that were
previously reported to be associated with HBV drug resistance
(23). In total. 30 samples obtained
from three patients were used for detection of these resistant
variants.
Results
Characteristics of patients with
LAM-resistant variants at the initiation of sequential monotherapy
with ADV
The three patients were 24–53 years-old. All
patients were treated with 100 mg LAM once daily for 21–24 months.
The emergence of LAM-resistant variants, such as rtM204 V/I and/or
rtL180 M, was observed in all patients. Their antiviral regimen was
switched to ADV monotherapy (10 mg/day) as a rescue therapy and no
patient required a dose reduction. The three patients developed ADV
resistance following LAM-ADV sequential monotherapy. The three
patients received a percutaneous liver biopsy at initiation of ADV
therapy and after 5 years therapy. Their clinical characteristics
at the initiation of ADV monotherapy are shown in Table I.
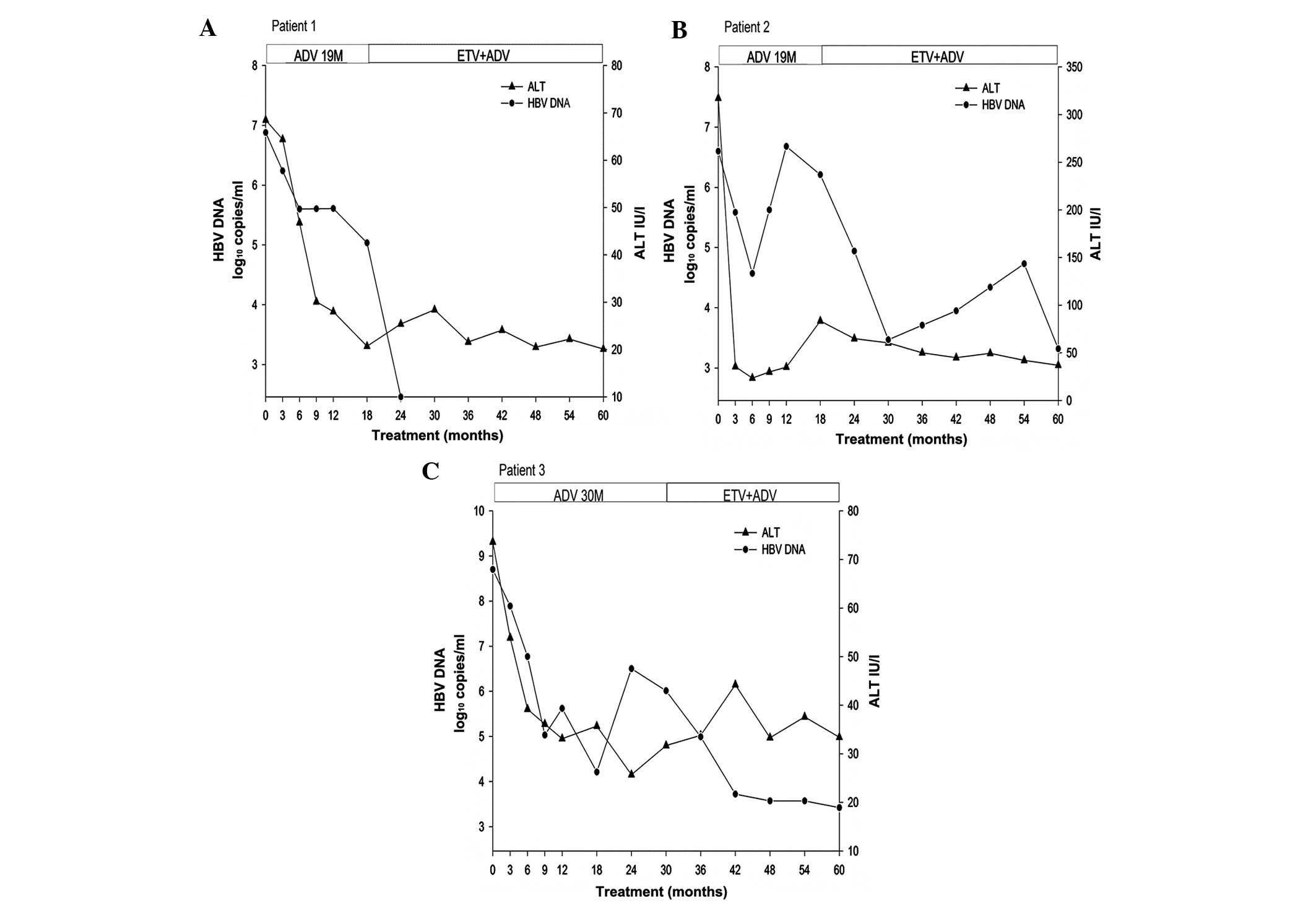
Clinical course of ADV monotherapy
followed by ADV-ETV combination therapy
The duration of ADV sequential monotherapy was 19–30
months. After a mean of 10 months (range 6–18 months) monotherapy
with ADV, viral load decreased to the lowest level during ADV
monotherapy, with an average decrease of 2.60 log10
copies/ml (range, 1.28–4.49 log10 copies/ml). Following
the passage to ADV-ETV combination therapy, the mean duration of
combination therapy was 37.33 months (range, 30–41 months). After a
mean duration of 25.33 months (range, 5–41 months) combination
therapy, viral load decreased to the lowest level during
combination therapy, with an average decrease of 3.0
log10 copies/ml (range, 2.59–3.28 log10
copies/ml). The clinical course of antiviral therapy in the three
patients is shown in Fig. 1.
During the 5 years of therapy, none of the patients
exhibited loss of HBeAg or HBsAg, or had seroconversion to HBeAb or
HBsAb. After 5 years of therapy, the inflammation and fibrosis
scores of the liver biopsies were of 4 on average (range, 2–6), and
showed a 1 point decrease according to the Ishak scoring
system.
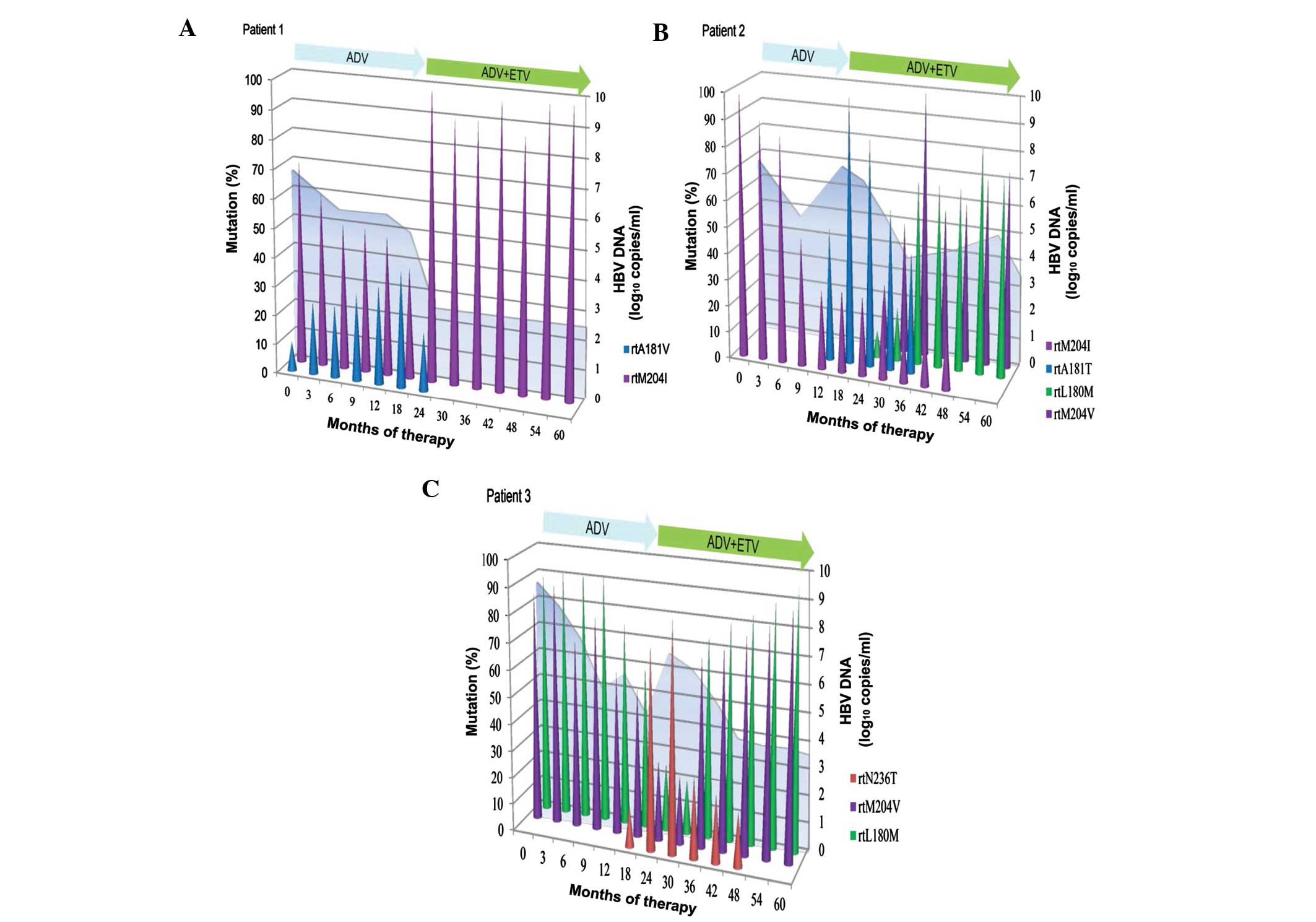
Dynamics of LAM-resistant variants
during ADV monotherapy followed by ADV-ETV combination therapy
The three patients had LAM-resistant variants
(rtM204I/V with or without rtL180 M) at the initiation of
sequential ADV monotherapy (Fig. 2).
During sequential monotherapy, the levels of LAM-resistant variants
(rtM204I/V with or without rtL180 M) gradually decreased as
treatment progressed, whereas ADV-resistant rtA181 V/T and rtN236T
gradually increased and became dominant in the viral populations
(Fig. 2). Following passage to an
ADV-ETV combination regimen, ADV-resistant rtA181 V/T and rtN236T
gradually decreased and became undetected in the viral population,
whereas LAM-resistant variant (rtM204I/V with or without rtL180 M)
was dominant in the viral population during combination therapy
(Fig. 2). ADV-resistant variants
rtA181 V/T and rtN236T went from being dominant at initiation of
ADV-ETV combination to undetectable, during an average duration of
combination therapy of 19.33 months (range, 11–24 months) (Fig. 2). After a mean of 37.33 months
(range, 30–41 months) combination treatment, LAM-resistant variants
(rtM204I/V with or without rtL180 M) remained dominant in the viral
population (Fig. 2).
The dynamics of resistant variants in the three
patients in whom the pyrosequencing analyses were performed during
the sequential antiviral treatment are illustrated in Fig. 2. In Patient 1, in addition to
rtM204I, the serum sample taken at the initiation of sequential ADV
monotherapy harbored ADV-resistant rtA181 V. This resistant variant
existed as a minor subpopulation and in <10% of the viral
population. ETV was administered after 19 months of monotherapy,
and resistant rtA181 V gradually decreased and became undetectable
after 11 months of combination therapy. In Patient 2, resistant
rtA181T was rapidly selected and accompanied by virological
breakthrough following 12 months of ADV monotherapy. ADV-resistant
variant rtA181T then decreased and became undetected after 23
months of combination therapy. The dynamics of resistant variants
was characterized by successive waves, with the rtM204I variant
being initially dominant, then being replaced by the rtA181T
variant, and finally, by the rtL180 M and rtM204 V variants. The
biochemical breakthrough coincided with the shift from dominant
rtM204I to dominant rtA181T after 18 months. In Patient 3, the
viral load of the virus harboring rtN236T gradually increased for 6
months prior to virological breakthrough. The resistant variant
rtN236T became dominant and accompanied by virological breakthrough
after 24 months. ADV-resistant variant rtN236T decreased and became
undetected following 2 years of combination therapy. In contrast to
Patient 2, dominant resistant variants rtL180 M and rtM204 V were
replaced by dominant variant rtN236T following sequential ADV
monotherapy, and were then replaced by dominant variants rtL180 M
and rtM204 V during ADV-ETV combination therapy.
Discussion
Therapeutic regimens for the treatment of CHB have
evolved rapidly over the past few years (8,9).
Prolonged monotherapy with LAM is associated with the emergence of
LAM-resistant variants and the progression of liver disease
(9,24,25). ADV
sequential monotherapy as a rescue regimen was included in the 2005
Asia-Pacific consensus and guidelines for the management of
patients with LAM-resistant variants (8). Subsequent studies have suggested that
sequential NA monotherapy could promote the selection of
multidrug-resistant variants (17,26), but
little is known with regard to the dynamics of these resistant
variants during ADV-ETV combination therapy. In the present
longitudinal study, pyrosequencing was used to characterize the
dynamics of resistant variants in patients with LAM resistance
during ADV monotherapy followed by ADV-ETV combination therapy. The
results of the present study demonstrated that replication of
ADV-resistant variants, rtA181T/V and rtN236T, was inhibited by
combination therapy with ADV-ETV, whereas LAM-resistant rtL180 M
and rtM204I/V were persistent during 30–41 months of combination
therapy. Therefore, the results strongly suggest that LAM should be
carefully prescribed for NA-naïve patients, due to the intractable
issues following the emergence of resistant variants.
Treatment of CHB patients with resistant variants
remains a complex topic. In NAs-naïve patients, it has been
reported that the cumulative rate of ADV resistance was 0, 11 and
28% at 1, 3 and 5 years, respectively (17). In patients with LAM-resistant
variants, following sequential monotherapy with ADV, the cumulative
rate of ADV resistance was 18 and 25% after 1 and 2 years,
respectively (17,26). As for the outcome of the rescue
strategy, ADV-LAM combination therapy is more effective in reducing
viral load than switching to ADV monotherapy in patients with
LAM-resistant variants (27–31), However, previous longitudinal studies
(6,24,25,32)
demonstrated that the replication of LAM-resistant variants was not
fully inhibited by ADV monotherapy or LAM-ADV combination therapy.
These results may be attributed to an ADV-resistant variant, rtA181
V/T, which is responsible for cross-resistance to LAM and ADV
(11,32,33). A
previous study reported that LAM-resistant variants, rtM204I/V and
rtL180 M, were suppressed, but ADV-resistant variant rtA181 V/T
emerged after 13–19 months of LAM-ADV sequential monotherapy
(25). In the present study, the
data demonstrated that LAM-resistant variants rtL180 M and
rtM204I/M were suppressed, whereas ADV-resistant variant rtA181 V/T
or rtN236T was increased in patients with LAM resistance during
19–30 months of ADV sequential monotherapy. These results were
concordant with earlier findings from a previous study that
determined that LAM-resistant variants (rtM204I/V and rtL180 M)
decreased whereas ADV-resistant variants gradually increased during
ADV monotherapy (24). ADV
suppression of LAM-resistant variants, mainly due to rtL180 M and
rtM204I/M, does not significantly affect sensitivity to ADV.
Data regarding the therapeutic regimen for patients
with LAM and ADV resistance remain limited (11,24,25,32,34). In
patients who have failed sequential LAM-ADV treatment, one recent
study reported that combination therapy with ETV-ADV was more
effective at reducing viral load compared with ADV-LAM combination
therapy and ETV monotherapy, but the difference was not significant
(35). Another recent study reported
that TDF-ETV is a potent therapeutic option for patients with LAM
and ADV resistance, and the cumulative probability of virological
suppression at 6 months was 75.0% in 28 patients (36). However, the efficacy and safety
profiles of these regimens were not well compared, therefore, to
date, the optimal treatment option for patients with LAM and ADV
resistance has not been identified. It has been reported (25) that LAM-ADV combination therapy may
not suppress the LAM and ADV-resistant variants that emerge during
LAM-ADV sequential monotherapy. In the present study, regardless of
the presence of LAM-resistant variants, it was observed that the
replication of ADV-resistant variants rtA181 V and/or rtN236T was
inhibited after 11–24 months of ADV-ETV combination therapy. A
resistant variant becoming undetectable is considered to be a good
response to rescue therapy (11,22,24,32,34,37).
However, previous studies have suggested that undetectable
resistant variants may be a transition phase in the selective
process of novel drug-resistant variants (34,38).
It should be noted that, even when the viral load
decreases following the addition of ETV to ongoing ADV therapy,
LAM-resistant variants rtL180 M and rtM204I/V persist after 30–41
months of ADV-ETV combination therapy. This result may be
predictable as rtM204 V results in partial cross resistance to LAM
and ETV (22,23,37).
However, it was demonstrated that LAM-resistant variants were
persistently dominant during ADV-ETV combination therapy in a
clinical study. Second, persistence of LAM-resistant variants
rtL180 M and rtM204 V increases the risk of ETV resistance, and
therefore a more potent antiviral regimen, such as TDF-ETV
combination therapy, may be considered in these rare cases.
Conversely, for patients with ETV resistance, physicians need to
inquire repeatedly about past medication history. Third,
histological benefits in the patients may be somewhat affected by
the emergence of drug-resistant variants during antiviral therapy.
In the present study, all patients exhibited an improvement in
inflammation scores and none of the patients showed progression of
fibrosis. The results suggest that ADV-ETV combination rescue
therapy may contribute to histological improvement. Whether
ADV-resistant variants re-emerge, or ETV-resistant variants
(rtT184, rtS202 or rtM250) are selected in these patients warrants
further investigation.
Although sensitive pyrosequencing was used in the
present study to detect an average of 10 time-point serum samples
for ≤5 years of treatment, the study presented certain limitations,
including the small number of patients and absence of treatment
failure during the short duration of combination therapy with
ADV-ETV. These limitations may make ADV-ETV combination an
inappropriate rescue therapy for patients with resistance to LAM
and ADV. To verify the efficacy and phenomenon of this salvage
regimen, further large cohort studies are needed.
In conclusion, following LAM-ADV sequential
monotherapy failure, ADV-ETV combination therapy partially
inhibited replication of HBV DNA in patients with LAM and ADV
resistance, as demonstrated by the decreased HBV DNA levels and
inhibition of ADV-resistant variants. However, LAM-resistant rtL180
M and rtM204I/V remained predominant during the 30–41 months of
ADV-ETV combination therapy. These results may be attributed to the
resistant variants that are responsible for some of the
cross-resistance to LAM and ETV. Therefore, careful monitoring and
more potent antiviral regimens should be considered in CHB patients
with multidrug resistance.
Acknowledgements
The present study was funded by grants from the
National Science and Technology Key Project (grant nos.
2012ZX10002004-006, 2012ZX10004904-003-001, 2013ZX10002002-006 and
2012ZX10002005); the Ministry of Science and Technology of China
(grant no. 2012ZX09301002-006); the High Technical Personnel
Training Item of the Beijing Health System (grant nos. 2011-3-083
and 2013-3-071); the Beijing Municipal Science & Technology
Commission (grant no. Z131107002213019); the Clinical Medicine
Development Special Fund, Beijing Municipal Administration of
Hospitals (grant no. XM201308); and the National Key Subject
Construction Project (grant no. WJWYA-2014-002).
Glossary
Abbreviations
Abbreviations:
|
ADV
|
adefovir
|
|
ALT
|
alanine aminotransferase
|
|
CHB
|
chronic hepatitis B
|
|
ETV
|
entecavir
|
|
HBV
|
hepatitis B virus
|
|
LAM
|
lamivudine
|
|
NA
|
nucleos(t)ide analog
|
|
RT
|
reverse transcriptase
|
References
|
1
|
Ott JJ, Stevens GA, Groeger J and Wiersma
ST: Global epidemiology of hepatitis B virus infection: New
estimates of age-specific HBsAg seroprevalence and endemicity.
Vaccine. 30:2212–2219. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Peng CY, Chien RN and Liaw YF: Hepatitis B
virus-related decompensated liver cirrhosis: Benefits of antiviral
therapy. J Hepatol. 57:442–450. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Buti M: HBeAg-positive chronic hepatitis
B: Why do i treat my patients with Nucleos(t)ide analogs? Liver Int
34 Suppl. 1:108–111. 2014. View Article : Google Scholar
|
|
4
|
Viganò M, Mangia G and Lampertico P:
HBeAg-negative chronic hepatitis B: Why do I treat my patients with
nucleos(t)ide analogues? Liver Int 34 Suppl. 1:120–126. 2014.
View Article : Google Scholar
|
|
5
|
Yuen MF, Sablon E, Hui CK, Yuan HJ,
Decraemer H and Lai CL: Factors associated with hepatitis B virus
DNA breakthrough in patients receiving prolonged lamivudine
therapy. Hepatology. 34:785–791. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rodriguez C, Chevaliez S, Bensadoun P and
Pawlotsky JM: Characterization of the dynamics of hepatitis B virus
resistance to adefovir by ultra-deep pyrosequencing. Hepatology.
58:890–901. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zoulim F and Locarnini S: Hepatitis B
virus resistance to nucleos(t)ide analogues. Gastroenterology.
137:1593–1608, e1591–1592. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liaw YF, Leung N, Guan R, Lau GK, Merican
I, McCaughan G, Gane E, Kao JH and Omata M: Asian-Pacific consensus
update working party on chronic hepatitis B: Asian-Pacific
consensus statement on the management of chronic hepatitis B: A
2005 update. Liver Int. 25:472–489. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liaw YF, Kao JH, Piratvisuth T, Chan HL,
Chien RN, Liu CJ, Gane E, Locarnini S, Lim SG, Han KH, et al:
Asian-Pacific consensus statement on the management of chronic
hepatitis B: A 2012 update. Hepatol Int. 6:531–561. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lai CL, Dienstag J, Schiff E, Leung NW,
Atkins M, Hunt C, Brown N, Woessner M, Boehme R and Condreay L:
Prevalence and clinical correlates of YMDD variants during
lamivudine therapy for patients with chronic hepatitis B. Clin
Infect Dis. 36:687–696. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pallier C, Castéra L, Soulier A, Hézode C,
Nordmann P, Dhumeaux D and Pawlotsky JM: Dynamics of hepatitis B
virus resistance to lamivudine. J Virol. 80:643–653. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bozdayi AM, Uzunalimoğlu O, Türkyilmaz AR,
Aslan N, Sezgin O, Sahin T, Bozdayi G, Cinar K, Pai SB, Pai R, et
al: YSDD: A novel mutation in HBV DNA polymerase confers clinical
resistance to lamivudine. J Viral Hepat. 10:256–265. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Niesters HG, De Man RA, Pas SD, Fries E
and Osterhaus AD: Identification of a new variant in the YMDD motif
of the hepatitis B virus polymerase gene selected during lamivudine
therapy. J Med Microbiol. 51:695–699. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liaw YF, Lee CM, Chien RN and Yeh CT:
Switching to adefovir monotherapy after emergence of
lamivudine-resistant mutations in patients with liver cirrhosis. J
Viral Hepat. 13:250–255. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao P, Wang C, Huang L, Xu D and Li T:
Comparison of rescue strategies in lamivudine-resistant patients
with chronic hepatitis B. Antiviral Res. 96:100–104. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vassiliadis TG, Giouleme O, Koumerkeridis
G, Koumaras H, Tziomalos K, Patsiaoura K, Grammatikos N,
Mpoumponaris A, Gkisakis D, Theodoropoulos K, et al: Adefovir plus
lamivudine are more effective than adefovir alone in
lamivudine-resistant HBeAg- chronic hepatitis B patients: A 4-year
study. J Gastroenterol Hepatol. 25:54–60. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee YS, Suh DJ, Lim YS, Jung SW, Kim KM,
Lee HC, Chung YH, Lee YS, Yoo W and Kim SO: Increased risk of
adefovir resistance in patients with lamivudine-resistant chronic
hepatitis B after 48 weeks of adefovir dipivoxil monotherapy.
Hepatology. 43:1385–1391. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kang SH, Yim HJ, Kim HR, Kang K, Suh SJ,
Lee HJ, Yoon EL, Kim JH, Seo YS, Yeon JE and Byun KS: Comparison of
lamivudine plus adefovir therapy versus entecavir with or without
adefovir therapy for adefovir-resistant chronic hepatitis B. J Clin
Gastroenterol. 48:889–895. 2014.PubMed/NCBI
|
|
19
|
Lok AS and McMahon BJ: Chronic hepatitis
B. Hepatology. 45:507–539. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Goodman ZD: Grading and staging systems
for inflammation and fibrosis in chronic liver diseases. J Hepatol.
47:598–607. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
World Medical Association: World Medical
Association Declaration of Helsinki: Ethical Principles for Medical
Research Involving Human Subjects. JAMA. 310:2191–2194. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee GH, Inoue M, Toh JK, Chong RH, Aung
MO, Koay ES and Lim SG: Two-step evolution of the hepatitis B
drug-resistant mutations in a patient who developed primary
entecavir resistance. Liver Int. 33:642–646. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lok AS, Zoulim F, Locarnini S,
Bartholomeusz A, Ghany MG, Pawlotsky JM, Liaw YF, Mizokami M and
Kuiken C: Hepatitis B Virus Drug Resistance Working Group:
Antiviral drug-resistant HBV: Standardization of nomenclature and
assays and recommendations for management. Hepatology. 46:254–265.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ijaz S, Arnold C, Dervisevic S, Mechurova
J, Tatman N, Tedder RS and Naoumov NV: Dynamics of
lamivudine-resistant hepatitis B virus during adefovir monotherapy
versus lamivudine plus adefovir combination therapy. J Med Virol.
80:1160–1170. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ko SY, Kim BK, Kwon SY, Kim KH, Kim JH,
Choe WH and Lee CH: Clonal evolution of hepatitis B virus
polymerase gene mutations during lamivudine-adefovir combination
treatment. World J Gastroenterol. 18:6437–6446; discussion p 6445.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yeon JE, Yoo W, Hong SP, Chang YJ, Yu SK,
Kim JH, Seo YS, Chung HJ, Moon MS, Kim SO, et al: Resistance to
adefovir dipivoxil in lamivudine resistant chronic hepatitis B
patients treated with adefovir dipivoxil. Gut. 55:1488–1495. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee SJ, Yim HJ, Hwang SG, Seo YS, Kim JH,
Yoon EL, Lee JM, Kim BH, Park SJ, Park YM, et al: Treatment of
lamivudine-resistant chronic hepatitis B infection: A multicenter
retrospective study. Scand J Gastroenterol. 48:196–204. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yim HJ, Seo YS, Yoon EL, Kim CW, Lee CD,
Park SH, Lee MS, Park CK, Chae HB, Kim MY, et al: Adding adefovir
vs. switching to entecavir for lamivudine-resistant chronic
hepatitis B (ACE study): A 2-year follow-up randomized controlled
trial. Liver Int. 33:244–254. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang M, Yuan L, Qiao B and Li Y: Two
rescue therapies in lamivudine-resistant patients with chronic
hepatitis B in the central China: Adefovir monotherapy and adefovir
plus lamivudine. Virus Genes. 48:32–37. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang ZB, Zhao SS, Huang Y, Dai XH, Zhou
RR, Yi PP, Chen RC, Li WT, Zhang BX, Li N and Fan XG: Comparison of
the efficacy of Lamivudine plus adefovir versus entecavir in the
treatment of Lamivudine-resistant chronic hepatitis B: A systematic
review and meta-analysis. Clin Ther. 35:1997–2006. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim HJ, Park JH, Park DI, Cho YK, Sohn CI,
Jeon WK and Kim BI: Rescue therapy for lamivudine-resistant chronic
hepatitis B: Comparison between entecavir 1.0 mg monotherapy,
adefovir monotherapy and adefovir add-on lamivudine combination
therapy. J Gastroenterol Hepatol. 25:1374–1380. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pallier C, Rodriguez C, Brillet R,
Nordmann P, Hézode C and Pawlotsky JM: Complex dynamics of
hepatitis B virus resistance to adefovir. Hepatology. 49:50–59.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Villet S, Pichoud C, Billioud G, Barraud
L, Durantel S, Trépo C and Zoulim F: Impact of hepatitis B virus
rtA181V/T mutants on hepatitis B treatment failure. J Hepatol.
48:747–755. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Deng XL, Li QL and Guo JJ: Dynamics of
lamivudine-resistant hepatitis B virus strains in patients with
entecavir rescue therapy. Virus Genes. 47:1–9. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Park MS, Kim BK, Kim KS, Kim JK, Kim SU,
Park JY, do Kim Y, Baartarkhuu O, Han KH, Chon CY and Ahn SH:
Antiviral efficacies of currently available rescue therapies for
multidrug-resistant chronic hepatitis B. Clin Mol Hepatol.
19:29–35. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lee YB, Lee JH, Lee DH, Cho H, Ahn H, Choi
WM, Cho YY, Lee M, Yoo JJ, Cho Y, et al: Efficacy of
entecavir-tenofovir combination therapy for chronic hepatitis B
patients with multidrug-resistant strains. Antimicrob Agents
Chemother. 58:6710–6716. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Villet S, Ollivet A, Pichoud C, Barraud L,
Villeneuve JP, Trépo C and Zoulim F: Stepwise process for the
development of entecavir resistance in a chronic hepatitis B virus
infected patient. J Hepatol. 46:531–538. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Villeneuve JP, Durantel D, Durantel S,
Westland C, Xiong S, Brosgart CL, Gibbs CS, Parvaz P, Werle B,
Trépo C and Zoulim F: Selection of a hepatitis B virus strain
resistant to adefovir in a liver transplantation patient. J
Hepatol. 39:1085–1089. 2003. View Article : Google Scholar : PubMed/NCBI
|