Introduction
Trigeminal neuralgia (TGN) is a transient, recurrent
and intense pain in the area on the face where the trigeminal (TG)
nerve is distributed (1–3). Previous studies have demonstrated that
80–90% of primary TGN cases are induced by vascular compression of
the TG nerve at the root entry zone (REZ) (1,4).
Superior cerebellar artery (SCA) and the anterior inferior
cerebellar artery (AICA) are the predominant vessels responsible
for the compression, followed by venous vessels (5,6). Brain
arteriovenous malformations (bAVMs) often manifest as cerebral
hemorrhage, particularly in young patients; therefore, patients
with unruptured bAVMs have a 2–4% risk of rupture and bleeding each
year, and each bleeding event is associated with an ~18% risk of
mortality (7,8). In addition, bAVMs also manifest as
chronic headaches, epilepsy and neurological deficits (8–11).
However, there have been relatively few reports of cases of TGN
caused by bAVM (4,12). The present case report describes a
case of TGN caused by bAVMs in the cerebellopontine angle (CPA).
Facial pain was successfully relieved after the patient received
partial embolization treatment, which may be associated with the
blockade of the pulsatile compression of the feeding arteries, the
arterialized draining veins or the malformed niduses. Therefore,
TGN caused by bAVMs is a vascular nerve compression disease. In the
present case study and review, the underlying mechanism and
treatment strategy of TGN caused by bAVMs were discussed in the
context of present case, and a literature review was carried
out.
Case report
A 32-year-old male was admitted to the First
Hospital of Jilin University (Changchun, China) on December 6,
2014, suffering from recurrent pain in his right cheek for two
years, which had increased in severity over the previous 6 months.
The patient reported that the pain began three years previously as
a stabbing-like, intense pain that occurred suddenly on the right
lower lip and mandible without any prior signs; each episode of
pain lasted for 10–30 sec. The first period of pain lasted ~1 week
and was self-relieved without medication. The pain recurred
following a period of 6 months, and the duration of each episode of
pain extended to 2–3 min. The patient was administered 800 mg oral
carbamazepine (Novartis, Basel, Switzerland) daily, which was
effective at relieving the pain on the right side of the face of
the patient. In the 6 months prior to hospital admission, the
frequency of the pain in the right cheek of the patient increased.
The pain occurred >6 times every day and each episode lasted
10–30 min, and daily oral administration of 1,200 mg carbamazepine
was not effective at controlling the pain. Clinical examination
demonstrated that no significant trigger point could be palpated
and there were no abnormalities in corneal reflexes or facial
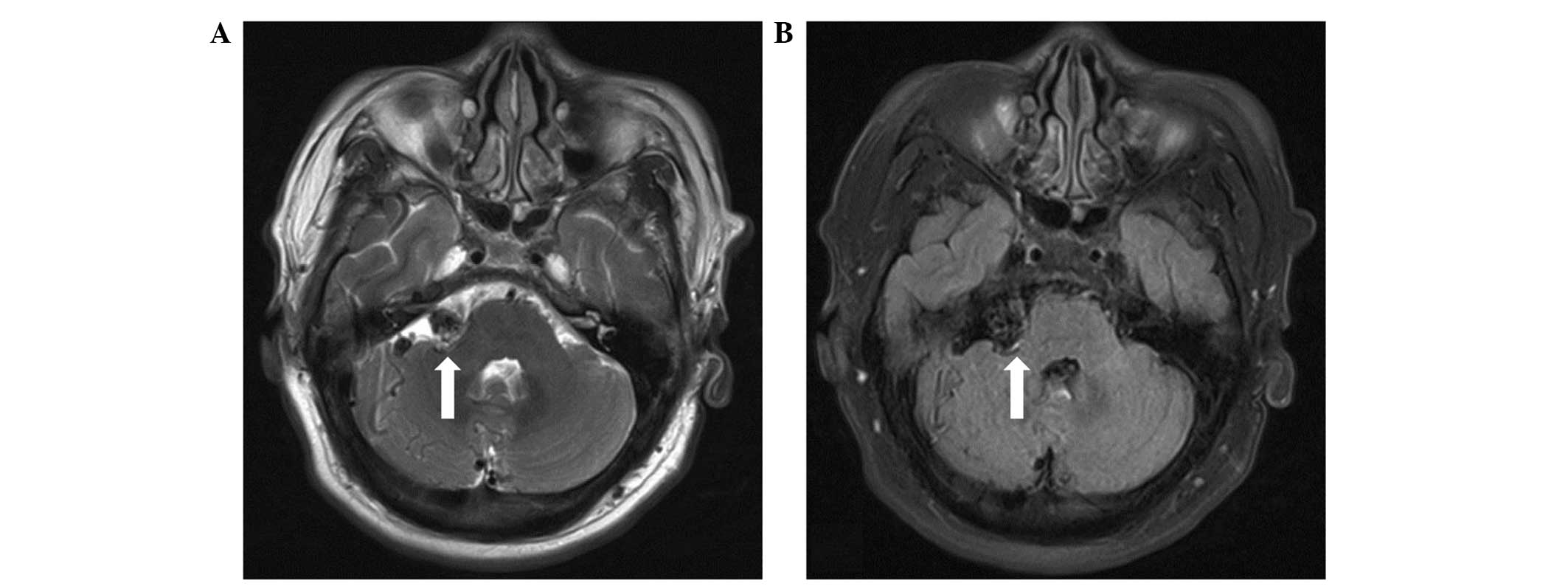
sensation. Subsequent magnetic resonance imaging (MRI) demonstrated
the presence of flow-void signals in the abnormal vessels in the
right CPA (Fig. 1). Digital
subtraction angiography (DSA) further confirmed the diagnosis of
bAVMs, and demonstrated that the malformed niduses were fed by the
right SCA and the AICA, and drained into the adjacent venous
sinuses on the same side (Fig. 2).
Although the patient only exhibited symptoms of TGN, the patient
was treated with interventional embolization, due to the risk that
the bAVMs may bleed in the future. Embolization was conducted under
general anesthesia with intravenous 0.05–0.1 mg/kg midazolam (Nhwa,
Jiangsu, China), 1.0–1.5 µg/kg remifentanil (Renfu, Yichang,
China), 0.2–0.3 mg/kg etomidate (Nhwa), 0.6 mg/kg rocuronium
(Organon, Brussels, Belgium), and was maintained with 0.6 mg/kg
propofol (Nhwa), 5–10 mg/(kg/h) and 0.1–0.2 µg/(kg/min)
remifentanil (Renfu, Yichang, China). Following arterial puncture
and sheath insertion in the femoral artery using the Seldinger
technique (13), a 6F-guiding
catheter (Cordis Co., Miami, FL, USA) was inserted into the left
vertebral artery. Under the guidance of a Mirage micro-wire (ev3,
Irvine, California, USA), a Marathon micro-catheter was inserted
into the bAVMs. The micro-catheter entered the bAVMs via the right
AICA and onyx-18 glue (ev3) was slowly injected. Onyx-18 glue
diffused well in the bAVMs and embolized the majority of the
malformations. Angiography was conducted immediately following
surgery, which demonstrated that the majority of the bAVMs had
disappeared and the draining veins were patent (Fig. 2). There were no complications
following the surgical procedure and the pain in the right cheek of
the patient was completely relieved one week following the surgical
procedure. The patient was satisfied with the outcome of the
embolization surgery. Post-surgical follow-ups were conducted for a
period of two years via telephone. The patient experienced no
further pain in his right cheek, and thus chose not to return to
the hospital for a follow-up reexamination. The present study was
approved by the Ethics Committee of the First Hospital of Jilin
University and patient informed consent was obtained prior to the
study.
Literature review
A total of 29 studies (4,12,14–40)
which reported TGN caused by bAVMs were retrieved from the PubMed
database, the majority of which were clinical reports that
described individual cases. We searched the PubMed database up to
2015 using the key words ‘AVM’, ‘bAVM’, ‘arteriovenous
malformation’, ‘cerebral arteriovenous malformation’, ‘brain
arteriovenous malformation’, ‘Cerebral vascular malformation’ in
combination with ‘trigeminal neuralgia’ and ‘TGN’. Abstracts were
reviewed to identify relevant literature, only the TGN caused by
bAVM related literatures were enrolled.
A total of 40 patients with TGN caused by bAVMs were
reported in these 29 studies (Table
I; 4,12,14-40). Excluding two patients for whom the information
was incomplete (27,33), there were 24 male and 14 female
patients (male:female ratio, ~1.71) aged between 23 and 69 years
with a mean age of 46.8±14.7 years. These patients presented with
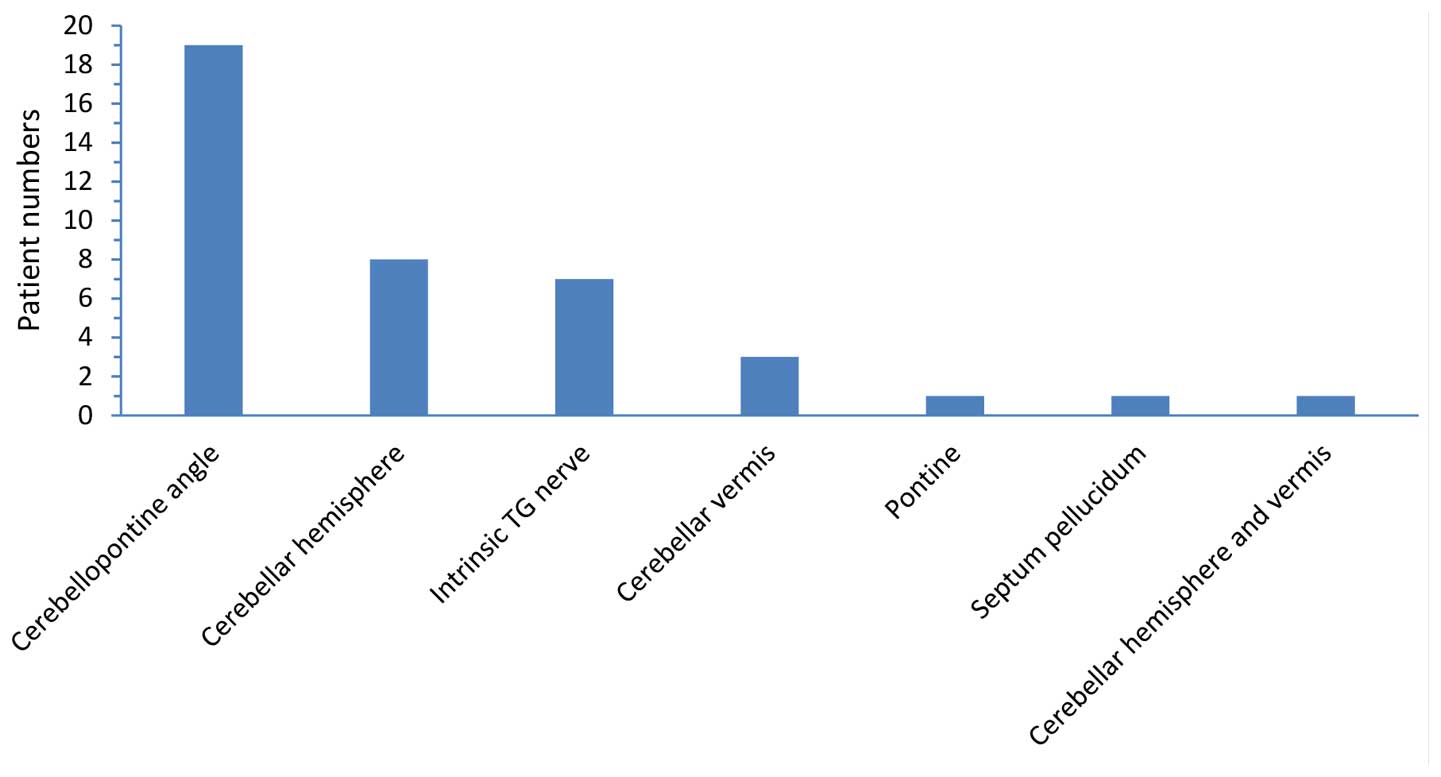
bAVMs located in the CPA (n=19)(12,16,17,20,23,27–33,36,38),
cerebellar hemisphere (n=8)(15,18,21,26,34,35,40), the
TG nerve (n=7)(22,25,39), the
cerebellar vermis (n=3)(4,14,24), the
pontine (n=1)(37), the septum
pellucidum (n=1)(19) and both the
cerebellar hemisphere and cerebellar vermis (n=1)(38) (Fig.
3). Five of these patients had a confirmed history of repeated
hemorrhage (19,29,32,33,35). The
majority of these patients suffered from TGN on the same side as
the bAVM lesions; however, one patient suffered from TGN on the
contralateral side of the bAVMs lesions (40), and two patients suffered from
coexistent TGN and hemifacial spasm on the same side (14,16). In
terms of treatment, 2 patients received drug therapy (17) and 1 patient was without mention of
treatment (12). The other 37
patients received non-drug treatment (4,14–40), 11
patients received surgical resection alone (19,25,27–30,32), 2
patients received surgical resection following partial embolization
(23,26), 1 patient received surgical resection
following destructive neurosurgical manipulation of the TG
nerve(31), 3 patients received
surgical resection of bAVMs combined with microvascular
decompression (MVD; 14,15,18,20,24), 5 patients received an
interventional embolization treatment, 2 patients received
radiotherapy following embolization (4,21), 6
patients received MVD treatment alone (37–39), 2
patients received radiotherapy following MVD (36,40), 4
patients received destructive neurosurgical manipulation of the TG
nerve (16,17,33,38), and
1 patient received stereotactic radiotherapy (SRS; 22) (Fig. 4).
 | Table I.Literature review of patients with
trigeminal neuralgia caused by bAVMs. |
Table I.
Literature review of patients with
trigeminal neuralgia caused by bAVMs.
|
|
|
|
| History of drug
therapy | Non-drug
therapy |
|
|
|---|
|
|
|
|
|
|
|
|
|
|---|
| Author | Gender/age | bAVMs location | Compressing
vessels | Drugs | Therapeutic
effects | Treatment
methods | Complications | Therapeutic
effects | Notes | Refs. |
|---|
| Kikuchi et
al | Male/45 | CPA | Feeding
arteries | Not mentioned | Not mentioned | Resection of
bAVMs | Not mentioned | Immediate complete
relief | The patient had a
history of SAH 15 years prior | (29) |
| Figueiredo et
al | Male/18 | CPA | Not mentioned | Not mentioned | Poor | Resection of
bAVMs | Transient numbness
in V3 | Immediate complete
relief |
| (30) |
| Kawano et
al | Male/48 | CPA | Malformed niduses
and draining veins | Not mentioned | Pain aggravated
after 2 years | Glycerol rhizolysis
was performed on either side of the semilunar ganglion of the TG
nerve, and shown to be ineffective; subsequent resection of the
bAVM was performed | Not mentioned | Immediate complete
relief |
| (31,32) |
| Kawano et
al | Female/61 | CPA | Not mentioned | Not mentioned | Not mentioned | Resection of
bAVMs | Not mentioned | Not mentioned | The patient had a
history of hemorrhage in the left CPA 10 days prior to admission to
the hospital | (20) |
| Johnson and
Salmon | N/A | CPA | Not mentioned | Not mentioned | Not mentioned | Resection of the TG
nerve | Not mentioned | Pain relief | The patient had a
history of SAH and multiple events of transient loss of
consciousness | (33) |
| Niwa et
al | Female/44 | Cerebellar
hemisphere | Feeding arteries
and draining veins | Not mentioned | Not mentioned | Resection of bAVMs
and MVD | Not mentioned | Immediate complete
relief |
| (34) |
| Nishizawa et
al | Male/42 | Cerebellar
hemisphere | Feeding
arteries | Not mentioned | Not mentioned | TGN recurred 6
months after TG nerve was blocked. Resection of the bAVMs and MVD
was subsequently performed | Not mentioned | Immediate complete
relief | The patient had a
history of SAH 10 years prior | (35) |
| Edwards et
al | Male/38 | Intrinsic TG
nerve | Draining veins,
malformed niduses, and feeding arteries | Carbamazepine | Poor | Resection of the
bAVMs and MVD | Mild V1
hypesthesia | Immediate
relief |
| (25) |
|
| Female/55 | Intrinsic TG
nerve | Draining veins and
malformed niduses | Carbamazepine | Poor | Resection of
bAVMs | Mild V1–3
hypesthesia and corneal areflexia | Immediate
relief |
|
|
|
| Female/46 | Intrinsic TG
nerve | Draining veins and
malformed niduses | Carbamazepine | Poor | Resection of
bAVMs | Mild V1 & V2
hypesthesia and occasional mild dysesthesia | Immediate
relief |
|
|
|
| Female/35 | Intrinsic TG
nerve | Draining veins and
malformed niduses | Carbamazepine | Poor | Resection of
bAVMs | None | Immediate
relief | TGN recurred 4
years later; the be relieved by drugs |
|
|
| Female/36 | Intrinsic TG
nerve | Draining veins and
malformed niduses | Carbamazepine | Poor | Resection of
bAVMs | Small pontine
hematoma, transient ataxia, VII weakness, nausea, vomiting,
permanent V2 & V3 anesthesia, V1 hypesthesia, and corneal
areflexia | Immediate
relief |
| Mineura et
al | Male/21 | Cerebellar
hemisphere | Draining veins | Not mentioned | Not mentioned | Embolization and
resection of bAVMs | Mild cerebellar
ataxia | Complete
relief |
| (26) |
| Nomura et
al | N/A | CPA | Not mentioned | Not mentioned | Not mentioned | Resection of
bAVMs | Not mentioned | Complete
relief |
| (27) |
|
| Male/44 | CPA | Malformed
niduses | Not mentioned | Not mentioned | Resection of
bAVMs | Not mentioned | Complete
relief |
|
|
| Mendelowitsch et
al | Female/47 | CPA | Feeding
arteries | Not mentioned | Poor | Resection of
bAVMs | Pulmonary
embolism | Complete
relief |
| (28) |
| Anderson et
al | Female/39 | Intrinsic TG
nerve | Not mentioned | Not mentioned | Poor | SRS | Not mentioned | Complete
relief |
| (22) |
| Wanke et
al | Male/56 | CPA | Not mentioned | Carbamazepine | Poor | Embolization and
resection of bAVMs | Hydrocephalus and
SAH occurred after embolization. Surgical resection was
subsequently performed. Paresis of the III, VII and XII cranial
nerves occurred, and the trigeminal neural sensation was reduced.
Every function except the trigeminal neural sensation recovered
after 3 months | Complete relief
following resection |
| (23) |
| Athanasiou et
al | Male/56 | Cerebellar
vermis | Feeding
arteries | Carbamazepine | Little effect | Embolization | None | Complete
relief |
| (24) |
| García-Pastor et
al | Male/57 | CPA | Malformed
niduses | Not mentioned | Poor | The effect of
radiofrequency thermocoagulation of the TG nerve was poor;
micro-balloon compression of the TG nerve was subsequently
performed. | Not mentioned | Complete
relief |
| (38) |
|
| Male/68 | CPA | Malformed
niduses | Not mentioned | Poor | MVD | Not mentioned | Complete
relief |
|
|
|
| Male/40 | Cerebellar
hemisphere and vermis | Feeding
arteries | Not mentioned | Poor | MVD | Not mentioned | Complete
relief |
|
|
|
| Male/54 | CPA | Malformed
niduses | Not mentioned | Poor | MVD | Not mentioned | Complete
relief |
|
|
| Levitt et
al | Female/13 | Cerebellar
hemisphere | Not mentioned | Carbamazepine | Poor | Multiple
embolization treatments of bAVMs had no effect; the artery of the
foramen rotundum (the blood-supplying artery of the TG nerve V2
branch) was subsequently embolized, following which the pain was
relieved | A small area of
hypesthesia on the left cheek | Immediate relief
following embolization of the artery of the foramen rotundum |
| (18) |
| Talanov et
al | Male/16 | Septum
pellucidum | Draining veins | Not mentioned |
| Resection of
bAVMs | None | Not mentioned | Pain on the left
side of the TG nerve combined with hemorrhage | (19) |
| Simon et
al | Male/72 | CPA | Not mentioned | Carbamazepine | The pain increased
after 1 year | Embolization | None | Immediate complete
relief. The pain recurred 17 months later; but was relieved
following secondary embolization |
| (20) |
| Lesley | Male/55 | Cerebellar
hemisphere | Feeding arteries
and draining veins | No history of drug
therapy |
| Staged embolization
and SRS | None | Complete
relief |
| (21) |
| Ferroli et
al | Female/52 | Pontine | Draining veins | Carbamazepine | Poor | MVD | None | 50% free after 1
month |
| (37) |
|
| Male/38 | Cerebellar
hemisphere | Feeding arteries
and draining veins | Not mentioned | Poor | Radiofrequency
thermocoagulation of TG nerve was poor; MVD was subsequently
performed | Not mentioned | Complete
relief |
| Karibe et
al | Male/55 | Intrinsic TG
nerve | Feeding
arteries | Carbamazepine | Little effect | MVD | None complete
relief | Immediate and |
| (39) |
| Sato et
al | Female/49 | Cerebellar
hemisphere | Draining veins | Not mentioned | Not mentioned | MVD + SRS | None | Complete relief
after 1 year. The size of the bAVMs was reduced | The patient had
pain on the contralateral TG nerve | (40) |
| Mori et
al | Male/69 | Cerebellar
vermis | Feeding
arteries | Carbamazepine | The pain was
relieved for 5 years, then gradually reappeared | Embolization and
SRS | Temporary truncal
ataxia following embolization | Partial relief was
achieved following embolization. Complete pain relief was achieved
following subsequent radiotherapy |
| (4) |
| Yip et
al | Female/64 | CPA | Malformed
niduses | Carbamazepine | Poor | Not mentioned |
|
|
| (12) |
| Dou et
al | Female/24 | Cerebellar
vermis | Not mentioned | Carbamazepine | Poor | Embolization | None | HFS and TNG was
completely relieved | Combined with
HFS | (14) |
| Kono et
al | Male/53 | Cerebellar
hemisphere | Feeding
arteries | Anti-TN
medication | The pain increased
gradually | Embolization | None | The pain was
completely relieved after 1 month |
| (15) |
| Son et
al | Male/42 | CPA | Not mentioned | Non-specific | Poor | Radiofrequency
thermocoagulation of TG nerve and injection of carnitine | None | The pain was
relieved; carnitine was regularly injected to relieve facial spasm
(every 3–4 months) | Combined with
facial spasm | (16) |
| Machet et
al | Male/61 | CPA | Not mentioned | Oxcarbazepine | Follow-up was
conducted for 10 months; the pain was relieved | None | Not mentioned |
|
| (17) |
| Sumioka et
al | Male/66 | CPA | Feeding arteries
and malformed niduses | Not mentioned |
| MVD + SRS | Not mentioned | Immediate complete
relief. MRI showed that the bAVMs disappeared |
| (36) |
|
| Female/64 | CPA | Not mentioned | Carbamazepine | Follow-up was
conducted for 18 months; the pain was relieved | None | Not mentioned |
|
|
|
|
| Male/50 | CPA | Not mentioned | Carbamazepine | The pain was
partially relieved | Micro-balloon
compression of the TG nerve | Not mentioned | Pain partially
disappeared |
Discussion
TGN is one of the most common symptoms of
neurovascular compression, and is a demyelinating lesion of the
sensory fibers of the TG nerve caused by compression of the TG
nerve at REZ by the SCA and the AICA (1–4).
However, TGN caused by bAVMs remains rare, accounting for
0.22–1.78% of primary TGN cases (12,16,38). The
present case study described a case of TGN caused by bAVMs in a
middle-aged male patient suffering from recurrent pain in the right
cheek. MRI and DSA analyses demonstrated bAVMs in the CPA of the
posterior fossa on the affected side. As it remains difficult to
distinguishing the symptoms of TGN caused by bAVMs, particularly
TGN induced by insignificant bAVMs, which is usually negative in
angiography, the symptoms of primary TGN can be easily misdiagnosed
(12,25). However, the majority of patients can
be accurately diagnosed through MRI and cerebral angiography
(4,20,21,36).
Previous studies have demonstrated that combined use of
three-dimensional (3D) constructive interference in steady state
MRI and 3D time of flight angiography clearly demonstrate the
anatomic association between the TG nerve and the surrounding
vessels, as well as other neighboring structures (41,42). DSA
clearly shows the bAVMs, including the feeding arteries, niduses
and draining veins, and determines whether they are associated with
aneurysms, which is beneficial for the preoperative evaluation of
patients (17). Among the 40
previous case reports (4,12,14–40), 39
patients (97.5%) presented with bAVMs in the posterior fossa. bAVMs
located in the posterior fossa are more likely to result in TGN
(4,12,14–18,20–40).
García-Pastor (38) conducted a
study on 375 cases of bAVMs, which demonstrated that 1.3% of the
patients with bAVMs developed TGN, whereas 9.8% of the patients
with bAVMs in the posterior fossa developed TGN. The majority of
the patients suffered from TGN on the same side as the bAVMs, and
only one patient suffered from TGN on the contralateral side of the
lesions, which was predominantly caused by the compression of the
contralateral TG nerve REZ by the arterialized draining veins
(40). The aforementioned findings
also demonstrated that TGN caused by bAVMs is a symptom of
neurovascular compression, caused by the pulsatile compression of
vessels. Pulsatile compression of the vessels may be caused by the
feeding arteries of bAVMs, arterialized draining veins or
malformation niduses (38,40,43).
Among the 40 patients evaluated (4,12,14–40),
there were 27 patients (4,12,15,19,21,24–29,31,34–40) in
whom TGN was demonstrated to originate from vascular compression as
determined by angiography or during the surgical procedure. Of
these 27 patients, TGN was caused by compression by the feeding
arteries of bAVMs (n=8)(4,15,24,28,29,35,38,39), the
draining venous system (n=4)(19,26,37,40),
malformation niduses (n=5)(12,27,38),
simultaneous compression by the feeding arteries and the draining
venous system (n=3)(21,34,38),
simultaneous compression by the feeding arteries and the
malformation niduses (n=1)(36), the
simultaneous compression by the draining venous system and the
malformation niduses (n=5)(25,31) and
simultaneous compression by the feeding arteries, the draining
venous system and the malformation niduses (n=1) (25). Since the origin of vascular
compression could not be confirmed by image analysis in certain
patients, and these patients had also received drug therapy,
interventional embolization, and destructive neurosurgical
manipulation of the TG nerve, the incidence of TGN originating from
vascular compression may be even higher.
Currently, the majority of treatments for TGN caused
by bAVMs are determined on an individual basis, and there has been
no consistent conclusion as to the optimal treatment for TGN caused
by bAVMs (14–16). Drug therapy did not yield an ideal
therapeutic effect in the majority of cases, and the patients who
received drug therapy predominantly proceeded to seek non-drug
treatments (4,12,14–16,22,23,25,28,30–32).
Non-drug treatments include surgical resection of bAVMs, MVD, SRS,
and destructive neurosurgical manipulation of the TG nerve
(4,16,33,36,37).
Therefore, when selecting a treatment, it is necessary to
comprehensively consider whether the patient has a history of
hemorrhage, whether the bAVMs are associated with aneurysms and the
origin of the compression of the TG nerve.
Theoretically, complete surgical resection is the
ideal therapeutic option, as it eliminates the compression on the
TG nerve and avoids the potential risk of hemorrhage of bAVMs,
which is particularly important for patients with a history of
hemorrhage. In addition, the craniotomy for resection of bAVMs may
also confirm whether the feeding arteries and the draining veins
compress the TG nerve root; if necessary, MVD can be performed
simultaneously (44). Among the 40
patients evaluated (4,12,14–40),
42.5% of the patients received surgical resection (n=17), and
amongst these 17 patients (19,23,25–32,34,35), 11
patients received surgical resection alone (19,25,27–30,32), 3
patients received surgical resection combined with MVD (25,34,35), and
2 patients received surgical resection due to complications
following embolization (23,26), and 1 patient received surgical
resection due poor effect after destructive neurosurgical
manipulation of the TG nerve (31).
The pain caused by TGN was immediately relieved in all the patients
who received surgical resection. The longest duration of follow-up
visits in these previous studies was 109 months (25). According to the long-term follow-up
visits reported, relapsed pain was only reported in one patient at
a four-year post-surgical follow-up, and this pain was controlled
by 400 mg/day carbamazepine (25).
The majority of these lesions were located deep in the posterior
fossa adjacent to the brain stem and surrounding important nerves
(23,26,27).
Furthermore, there were numerous complications during surgical
resection, particularly for patients with bAVMs in the TG nerve
REZ. Edwards et al (25)
reported the treatment of five patients with bAVMs in the TG nerve
REZ through surgical resection, and following the surgical
procedure, four of these patients experienced varying levels of
dysesthesia of the TG nerve, and one patient also experienced
weakening of the facial neurological function, short-term ataxia
and cerebellar hematoma. Although the remission rate of TNG is very
high after surgical resection, there are also many complications.
Thus it is a necessary to weigh the benefits and risks before the
operation.
It is sometimes difficult to perform complete
surgical resection of bAVMs, and this procedure may also be
unnecessary for some patients. Among the 40 patients (4,12,14–40)
with TGN caused by bAVMs, 55% of patients (n=22)(4,15,19,21,24–26,28,29,33–40)
presented with TGN which partially or completely originated from
the compression of feeding arteries or the draining veins, which
was confirmed by image analysis or during the surgical procedure.
Therefore, surgical resection of bAVMs was unnecessary in these
patients with TGN with no history of hemorrhage, and MVD surgery
would have been sufficient. MVD surgery of the TG nerve is >90%
effective and is capable of preserving neurological function
relatively well; thus, MVD surgery has become the first choice of
surgical treatment for primary TGN (45). Furthermore, bAVMs in the CPA, and
even malformed niduses in the TG nerve, may not necessarily result
in TGN (39,43,46).
Therefore, focusing on complete resection of bAVMs as the
predominant therapeutic option may cause further complications. The
mid-term results of a multicenter, randomized control study which
investigated the treatments of unruptured bAVMs demonstrated that
the effects of drug therapies are superior to those of invasive
therapies when treating unruptured bAVMs (47). Among the 40 patients evaluated in the
present review (4,12,14–40), 27%
of patients (n=11) received MVD surgery, with the longest duration
of follow-up being 18 months (36).
A 100% overall effective rate was achieved and no
surgery-associated complications occurred (34–40).
Interventional embolization can be used to reduce
the size of bAVMs by reducing blood supply to bAVMs (4,21,23,26).
This procedure is predominantly performed to surgically treat bAVMs
or as an adjuvant therapy prior to SRS (4,21,23,26).
However, when treating TGN caused by bAVMs, TGN may be effectively
relieved following partial embolization of bAVMs due to the change
in hemodynamics, which also demonstrates that TGN caused by bAVMs
is a symptom of neurovascular compression (20). Due to these characteristics,
interventional embolization was performed on the present patient
and TGN was completely relieved following successful embolization
of the majority of the bAVMs. Post-embolization follow-ups were
conducted for two years, and the therapeutic effect of this
treatment option remained satisfactory. Among the 40 patients
(4,12,14–40)
evaluated in the present review, 22.5% of patients (n=9)(4,14,15,18,20,21,23,24,26)
underwent interventional embolization and experienced significantly
relieved TGN following embolization. Among these 9 patients, 5
patients received an interventional embolization treatment alone
(14,15,18,20,24), 2
patients received subsequent SRS (4,21) and 2
patients received secondary surgical resection(23,26). The
longest duration of follow-up visits for patients who underwent
interventional embolization was six years (4). One patient reported relapsed pain 17
months post-embolization and the pain was subsequently relieved
following a secondary embolization (4). The pain relief may be associated with
the blocking of the pulsatile compression of the feeding arteries
of the bAVMs, the arterialized draining veins or the malformed
niduses that compressed the TG nerve following embolization, which
appears to have a similar mechanism to the MVD of the TG nerve.
However, caution should be taken with this therapeutic option as
interventional embolization and the change in hemodynamics
following embolization may induce ischemia, hemorrhage or
embolization of the venous system (4,23,26).
Among the aforementioned eight patients who received embolization,
one patient experienced transient ataxia (4), one patient received secondary surgical
resection of bAVMs due to subarachnoid hemorrhage and hydrocephalus
following the embolization (23),
and one patient suffered from severe paresis and sensory
disturbance due to the dilated draining veins following
embolization, which required surgical resection (26). Furthermore, since the rate of
complete embolization is restricted when treating bAVMs with
interventional therapy alone, and post-embolization recanalization
is relatively common, embolization alone may not result in a
long-term therapeutic effect. Simon et al (20) reported the case of a patient with TGN
caused by bAVMs whose pain was relieved following embolization;
however, the pain recurred 17 months later. It has also been
demonstrated that only short-term pain relief was achieved
following multiple embolization procedures (18), which may be associated with the
recanalization of bAVMs.
SRS is often performed following partial
embolization or combined with other treatment methods (4,21,36,40).
Among the 40 patients evaluated in the present review (4,12,14–40),
there was only one report (22) of
the achievement of an effective therapeutic effect of SRS alone. In
this case, the bAVMs were located inside the TG nerve and the size
of the malformation niduses was ~1.2×0.8×0.9 cm; the TGN was
completely relieved following 13 months of SRS (22). Notably, Levitt et al (18) treated a patient with TGN by
embolizing the supplying artery of the V2 branch of the TG nerve,
an artery of the foramen rotundum, which induced sustained relief
from TGN, but resulted in hypoesthesia of the cheeks on the same
side. Previous studies have also reported other palliative
destructive neurosurgical techniques of the TG nerve root (16,17,33,38),
including radiofrequency thermocoagulation, glycerol rhizolysis,
balloon compression and resection of the TG nerve; however, these
palliative therapies may result in complications, such as facial
numbness and weakened corneal reflexes (16,17,33,38).
Furthermore, the therapeutic effects of these palliative therapies
remain uncertain, and TGN has a relatively high recurrence rate
when treated with these therapeutic strategies (16,17,33,38).
In conclusion, surgical resection of the malformed
vessels is an ideal therapeutic option in theory, as this approach
relieves TGN and eliminates the risk of hemorrhage of bAVMs;
whereas MVD surgery is a recommended treatment for patients who
cannot receive surgical resection of bAVMs. Good therapeutic
outcomes have also been achieved with interventional embolization
in certain patients, including the patient described in the present
case study. Destructive neurosurgical manipulation of the TG nerve
may result in facial numbness and weakened corneal reflexes, and
has a high recurrence rate; therefore, this approach is only
recommended as an alternative for elderly surgery-intolerant
patients.
References
|
1
|
Love S and Coakham HB: Trigeminal
neuralgia: Pathology and pathogenesis. Brain. 124:2347–2360. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cruccu G, Gronseth G, Alksne J, Argoff C,
Brainin M, Burchiel K, Nurmikko T and Zakrzewska JM: American
Academy of Neurology Society; European Federation of Neurological
Society: AAN-EFNS guidelines on trigeminal neuralgia management.
Eur J Neurol. 15:1013–1028. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Headache Classification Committee of the
International Headache Society (IHS): The international
classification of headache disorders, 3rd edition (beta version).
Cephalalgia. 33:629–808. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mori Y, Kobayashi T, Miyachi S, Hashizume
C, Tsugawa T and Shibamoto Y: Trigeminal neuralgia caused by nerve
compression by dilated superior cerebellar artery associated with
cerebellar arteriovenous malformation: Case report. Neurol Med Chir
(Tokyo). 54:236–241. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ishikawa M, Nishi S, Aoki T, Takase T,
Wada E, Ohwaki H, Katsuki T and Fukuda H: Operative findings in
cases of trigeminal neuralgia without vascular compression:
Proposal of a different mechanism. J Clin Neurosci. 9:200–204.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
McLaughlin MR, Jannetta PJ, Clyde BL,
Subach BR, Comey CH and Resnick DK: Microvascular decompression of
cranial nerves: Lessons learned after 4400 operations. J Neurosurg.
90:1–8. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mast H, Young WL, Koennecke H-C, et al:
Risk of spontaneous haemorrhage after diagnosis of cerebral
arteriovenous malformation. The Lancet. 350:1065–1068. 1997.
View Article : Google Scholar
|
|
8
|
Nataraj A, Mohamed MB, Gholkar A, Vivar R,
Watkins L, Aspoas R, Gregson B, Mitchell P and Mendelow AD:
Multimodality treatment of cerebral arteriovenous malformations.
World Neurosurg. 82:149–159. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Whitehead KJ, Smith MC and Li DY:
Arteriovenous malformations and other vascular malformation
syndromes. Cold Spring Harb Perspect Med. 3:a0066352013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rodríguez-Hernández A, Kim H, Pourmohamad
T, Young WL and Lawton MT: University of California, San Francisco
Arteriovenous Malformation Study Project: Cerebellar arteriovenous
malformations: Anatomic subtypes, surgical results, and increased
predictive accuracy of the supplementary grading system.
Neurosurgery. 71:1111–1124. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mast H, Young WL, Koennecke HC, Sciacca
RR, Osipov A, Pile-Spellman J, Hacein-Bey L, Duong H, Stein BM and
Mohr JP: Risk of spontaneous haemorrhage after diagnosis of
cerebral arteriovenous malformation. Lancet. 350:1065–1068. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yip V, Michael BD, Nahser HC and Smith D:
Arteriovenous malformation: a rare cause of trigeminal neuralgia
identified by magnetic resonance imaging with constructive
interference in steady state sequences. QJM: monthly journal of the
Association of Physicians. 105:895–898. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Seldinger SI: Catheter replacement of the
needle in percutaneous arteriography: a new technique. Acta Radiol.
39:368–376. 1953. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dou NN, Hua XM, Zhong J and Li ST: A
successful treatment of coexistent hemifacial spasm and trigeminal
neuralgia caused by a huge cerebral arteriovenous malformation: A
case report. J Craniofac Surg. 25:907–910. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kono K, Matsuda Y and Terada T: Resolution
of trigeminal neuralgia following minimal coil embolization of a
primitive trigeminal artery associated with a cerebellar
arteriovenous malformation. Acta Neurochir (Wien). 155:1699–1701.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Son BC, Kim DR, Sung JH and Lee SW:
Painful tic convulsif caused by an arteriovenous malformation. Clin
Neuroradiol. 22:365–369. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Machet A, Aggour M, Estrade L, Chays A and
Pierot L: Trigeminal neuralgia related to arteriovenous
malformation of the posterior fossa: Three case reports and a
review of the literature. J Neuroradiol. 39:64–69. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Levitt MR, Ramanathan D, Vaidya SS, Hallam
DK and Ghodke BV: Endovascular palliation of AVM-associated
intractable trigeminal neuralgia via embolization of the artery of
the foramen rotundum. Pain Med. 12:1824–1830. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Talanov AB, Filatov Iu M, Eliava ShSh,
Novikov AE and Kulishova IaG: Arteriovenous malformation of septum
pellucidum in combination with persistent trigeminal neuralgia. Zh
Vopr Neirokhir Im N N Burdenko 50–53. discusion 53–54. 2009.(In
Russian).
|
|
20
|
Simon SD, Yao TL, Rosenbaum BP, Reig A and
Mericle RA: Resolution of trigeminal neuralgia after palliative
embolization of a cerebellopontine angle arteriovenous
malformation. Cent Eur Neurosurg. 70:161–163. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lesley WS: Resolution of trigeminal
neuralgia following cerebellar AVM embolization with Onyx.
Cephalalgia. 29:980–985. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Anderson WS, Wang PP and Rigamonti D: Case
of microarteriovenous malformation-induced trigeminal neuralgia
treated with radiosurgery. J Headache Pain. 7:217–221. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wanke I, Dietrich U, Oppel F and Puchner
MJ: Endovascular treatment of trigeminal neuralgia caused by
arteriovenous malformation: Is surgery really necessary? Zentralbl
Neurochir. 66:213–216. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Athanasiou TC, Nair S, Coakham HB and
Lewis TT: Arteriovenous malformation presenting with trigeminal
neuralgia and treated with endovascular coiling. Neurol India.
53:247–248. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Edwards RJ, Clarke Y, Renowden SA and
Coakham HB: Trigeminal neuralgia caused by microarteriovenous
malformations of the trigeminal nerve root entry zone: Symptomatic
relief following complete excision of the lesion with nerve root
preservation. J Neurosurg. 97:874–880. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mineura K, Sasajima H, Itoh Y, Kowada M,
Tomura N and Goto K: Development of a huge varix following
endovascular embolization for cerebellar arteriovenous
malformation. A case report. Acta Radiol. 39:189–192. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nomura T, Ikezaki K, Matsushima T and
Fukui M: Trigeminal neuralgia: Differentiation between intracranial
mass lesions and ordinary vascular compression as causative
lesions. Neurosurg Rev. 17:51–57. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mendelowitsch A, Radue EW and Gratzl O:
Aneurysm, arteriovenous malformation and arteriovenous fistula in
posterior fossa compression syndrome. Eur Neurol. 30:338–342. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kikuchi K, Kamisato N, Sasanuma J,
Watanabe K and Kowada M: Trigeminal neuralgia associated with
posterior fossa arteriovenous malformation and aneurysm fed by the
same artery. Case report. Neurol Med Chir (Tokyo). 30:918–921.
1990.(In Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Figueiredo PC, Brock M, De Oliveira AM
Júnior and Prill A: Arteriovenous malformation in the
cerebellopontine angle presenting as trigeminal neuralgia. Arq
Neuropsiquiatr. 47:61–71. 1989.PubMed/NCBI
|
|
31
|
Kawano H, Hayashi M, Kobayashi H, Tsuji T,
Kabuto M and Kubota T: Gas CT cisternography of trigeminal
neuralgia caused by AVM of the cerebellopontine angle. AJNR Am J
Neuroradiol. 8:161–162. 1987.PubMed/NCBI
|
|
32
|
Kawano H, Kobayashi H, Hayashi M, Tsuji T,
Kabuto M and Nozaki J: Trigeminal neuralgia caused by arteriovenous
malformation of the cerebellopontine angle-report of two cases. No
to Shinkei. 36:1175–1179. 1984.(In Japanese). PubMed/NCBI
|
|
33
|
Johnson MC and Salmon JH: Arteriovenous
malformation presenting as trigeminal neuralgia. Case report. J
Neurosurg. 29:287–289. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Niwa J, Yamamura A and Hashi K: Cerebellar
arteriovenous malformation presenting as trigeminal neuralgia. No
Shinkei Geka. 16:753–756. 1988.(In Japanese). PubMed/NCBI
|
|
35
|
Nishizawa Y, Tsuiki K, Miura K, Murakami
M, Kirikae M, Hakozaki S, Saiki I and Kanaya H: Multiple
arteriovenous malformations of left parietal lobe and left
cerebellar hemisphere with symptomatic trigeminal neuralgia: A case
report. No Shinkei Geka. 16(Suppl 5): S625–S630. 1988.(In
Japanese).
|
|
36
|
Sumioka S, Kondo A, Tanabe H and Yasuda S:
Intrinsic arteriovenous malformation embedded in the trigeminal
nerve of a patient with trigeminal neuralgia. Neurol Med Chir
(Tokyo). 51:639–641. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ferroli P, Acerbi F, Broggi M and Broggi
G: Arteriovenous micromalformation of the trigeminal root:
Intraoperative diagnosis with indocyanine green videoangiography:
Case report. Neurosurgery. 67(3 Suppl Operative): onsE309–onsE310;
discussion onsE310. 2010.PubMed/NCBI
|
|
38
|
García-Pastor C, López-González F,
Revuelta R and Nathal E: Trigeminal neuralgia secondary to
arteriovenous malformations of the posterior fossa. Surg Neurol.
66:207–211; discussion 211. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Karibe H, Shirane R, Jokura H and
Yoshimoto T: Intrinsic arteriovenous malformation of the trigeminal
nerve in a patient with trigeminal neuralgia: Case report.
Neurosurgery. 55:14332004.PubMed/NCBI
|
|
40
|
Sato K, Jokura H, Shirane R, Akabane T,
Karibe H and Yoshimoto T: Trigeminal neuralgia associated with
contralateral cerebellar arteriovenous malformation. Case
illustration. J Neurosurg. 98:13182003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Garcia M, Naraghi R, Zumbrunn T, Rösch J,
Hastreiter P and Dörfler A: High-resolution 3D-constructive
interference in steady-state MR imaging and 3D time-of-flight MR
angiography in neurovascular compression: A comparison between 3T
and 1.5T. AJNR Am J Neuroradiol. 33:1251–1256. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Linn J, Moriggl B, Schwarz F, Naidich TP,
Schmid UD, Wiesmann M, Bruckmann H and Yousry I: Cisternal segments
of the glossopharyngeal, vagus, and accessory nerves: Detailed
magnetic resonance imaging-demonstrated anatomy and neurovascular
relationships. J Neurosurg. 110:1026–1041. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Krischek B, Yamaguchi S, Sure U, Benes L,
Bien S and Bertalanffy H: Arteriovenous malformation surrounding
the trigeminal nerve-case report. Neurol Med Chir (Tokyo).
44:68–71. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Abla AA, Nelson J, Rutledge WC, Young WL,
Kim H and Lawton MT: The natural history of AVM hemorrhage in the
posterior fossa: Comparison of hematoma volumes and neurological
outcomes in patients with ruptured infra- and supratentorial AVMs.
Neurosurg Focus. 37:E62014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zakrzewska JM and Linskey ME: Trigeminal
neuralgia. Clin Evid (Online). 10:12072014.
|
|
46
|
Maher CO, Atkinson JL and Lane JI:
Arteriovenous malformation in the trigeminal nerve. Case report. J
Neurosurg. 98:908–912. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mohr JP, Parides MK, Stapf C, Moquete E,
Moy CS, Overbey JR, Al-Shahi Salman R, Vicaut E, Young WL, Houdart
E, et al: Medical management with or without interventional therapy
for unruptured brain arteriovenous malformations (ARUBA): A
multicentre, non-blinded, randomised trial. Lancet. 383:614–621.
2014. View Article : Google Scholar : PubMed/NCBI
|