Introduction
Angiotensin II type 1 receptor blockers (ARBs) are
widely and safely used as an alternative to angiotensin converting
enzyme inhibitors for the treatment of hypertension and
hypertension-related cardiovascular diseases without inducing cough
(1). In addition to their beneficial
effects against hypertension, ARBs have been found to exhibit
metabolic actions. In several clinical trials, the incidence of
new-onset type 2 diabetes was significantly lower in hypertensive
subjects treated with ARBs than in those treated with other
hypertensive therapies, which suggests potential antidiabetic
effects of angiotensin receptor blockade (2–4). In
addition, ARBs have been found to improve insulin sensitivity in a
3T3-L1 cell model of insulin resistance (5). The underlying mechanisms of the
insulin-sensitizing/antidiabetic effect of ARBs remain widely
unknown.
The peroxisome proliferator-activated receptor-γ
(PPARγ) is a clinically validated target for the treatment of
insulin resistance and functions as a transcription factor that
regulates the gene expression involved in carbohydrate and lipid
metabolism, thereby ameliorating type 2 diabetes (6). A study conducted by Benson et al
(7) demonstrated that telmisartan
exhibited selective PPARγ-modulating activity when tested at
concentrations typically achieved in plasma with conventional oral
dosing. The other clinically approved ARBs that were tested had
little or no effect on PPARγ (7,8),
although the oral administration of an extremely high dose of
irbesartan (50 mg/kg) was able to cause some activation of the
receptor (9).
Therefore, the present study was conducted to
investigate whether candesartan cilexetil exerts protective effects
on glucose and lipid metabolism and increases PPARγ levels in
adipose and liver tissues and thus improves insulin sensitivity in
a rat model of diet-induced obesity.
Materials and methods
Chemicals
Affinity-purified rabbit anti-PPARγ polyclonal
antibody was purchased from Abcam Inc. (Cambridge, MA, USA; cat.
no. ab19481). Human insulin (Novolin™ R) was from Novo Nordisk
(Copenhagen, Denmark). Candesartan cilexetil was provided by Takeda
Pharmaceutical Co., Ltd. (Osaka, Japan). Rabbit anti-β-actin
polyclonal antibody was from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA; cat. no. sc-1616). Peroxidase-conjugated
AffiniPure Goat Anti-Rabbit IgG (H+L) was obtained from Beijing
Zhongshan Golden Bridge Biotechnology Co., Ltd. (Beijing, China;
cat. no. ZB-2301).
Animals and treatment
Male Wistar rats were obtained at 6–7 weeks of age
from Vital River Laboratories Co., Ltd. (Beijing, China). The rats
were housed two per cage at 22±2°C and a relative humidity of
40–70% under a 12-h light/dark cycle with ad libitum access
to food and tap water until the start of experiments. Starting at
8–9 weeks of age, the animals were randomly distributed into three
groups: Normal chow group (NC group, n=15), high-fat diet group (HF
group, n=15) and high-fat diet with daily candesartan cilexetil
treatment group (HF + C group, n=15). The normal chow diet
consisted of (as a percentage of total kcal) 17% fat, 63%
carbohydrate and 10% protein, and a high-fat diet consisted of 27%
fat, 53% carbohydrate and 20% protein. Rats in both the HF group
and the HF + C group were initially fed a high-fat diet for 4
weeks, and then respectively received 8 mg/kg/day of either saline
(vehicle) or candesartan cilexetil by gavage for 28 consecutive
days, during which the high-fat diet was continued.
Blood was drawn from the retro-orbital venous
plexus, and blood glucose (BG) was immediately measured with a
Bayer Ascensia Breeze™ glucometer (Bayer HealthCare LLC, Mishawaka,
IN, USA), after which the blood was collected in a gel tube
containing a clotting accelerator and allowed to clot prior to
centrifugation for 10 min at 3,000 × g. Subsequently, serum was
collected and frozen at −80°C. Serum lipids and lipoproteins were
determined using Rat Triglyceride, Cholesterol, Low Density
Lipoprotein and High Density Lipoprotein kits (cat. nos. R6635,
R6955, R6953 and R6952, respectively; TSZ ELISA, Waltham, MA, USA),
according to the manufacturer's protocol. In addition, serum levels
of insulin (Insulin RIA kit; Linco Research, Inc., St. Charles, MO,
USA) and angiotensin II [ANG II enzyme-linked immunosorbent assay
(ELISA) kit; RapidBio, West Hills, CA, USA] were determined.
Insulin sensitivity index (ISI), a surrogate index of insulin
sensitivity, was calculated as follows: ISI = 1/(FPG × FINS), where
FPG is fasting plasma glucose (expressed in mmol/l) and FINS is
fasting insulin (expressed in mU/l) (10). All experimental procedures and
protocols conformed to guidelines outlined in the National
Institutes of Health Guide for the Care and Use of Laboratory
Animals (1996). The present study was approved by the Laboratory
Animal Welfare and Ethics Committee of Chinese PLA General Hospital
(Beijing, China).
Oral glucose tolerance tests
At the end of the 4-week treatment period, the rats
were fasted overnight prior to the oral glucose tolerance test
(OGTT). Animals underwent feeding with 2 g/kg body weight glucose
by gavage. Blood was drawn from a cut at the tip of the tail at 0,
30, 60 and 120 min after the glucose feeding, as previously
described (11). Plasma glucose
concentrations were determined. The area under the curve (AUC) for
glucose during the OGTT was calculated using the trapezoid method
(12).
Hyperinsulinemic-euglycemic clamp
analysis
At the end of the 4-week treatment period, conscious
rats received local anesthesia using 2% lidocaine hydrochloride
(Abbot Laboratories, North Chicago, IL, USA) on the tail root. The
tail artery and vein were catheterized for blood sampling and
infusion, respectively. Basic BG and basic insulin were measured.
Regular human insulin (Novolin™ R) was infused intravenously at a
rate of 0.25 U/kg·h. Hyperinsulinemic-euglycemic clamp analysis was
performed as described previously (13). Briefly, blood specimens (30 µl) were
obtained from the tail arterial catheter at 5-min intervals for
measuring plasma glucose levels by the glucose oxidase method using
the Bayer Ascensia Breeze™ glucometer. Based on these values, 10%
glucose solution was variably infused to maintain normal glucose
leves. A higher glucose infusion rate indicated a higher insulin
sensitivity of the peripheral tissue.
Western blot analysis
Rats were anesthetized following an overnight fast,
and within 10–15 min the abdominal cavity was opened. The perirenal
and epididymal adipose tissue, the liver and the heart were
removed, snap frozen in liquid nitrogen, weighed and stored at
−80°C until processed. Epididymal fat sample was subjected to
homogenization as previously described (14). Following centrifugation at 4°C for 20
min at 12,000 × g, the resultant supernatants were used for
immunoblotting. Total protein concentration was determined using
the bicinchoninic acid method (Pierce BCA Protein Assay kit; Thermo
Fisher Scientific, Inc., Waltham, MA, USA). Blots were incubated
overnight at 4°C with rabbit anti-PPARγ (1:400 dilution) and rabbit
anti-β-actin (1:200 dilution) polyclonal antibodies, followed by
incubation for 1 h at room temperature with peroxidase-conjugated
AffiniPure Goat Anti-Rabbit IgG (H+L) (1:5,000 dilution). The blots
were washed and then developed using an ECL Plus immunoblotting
detection kit (Applygen Technologies Inc., Beijing, China). The
protein bands were visualized by exposure of the membranes to Kodak
X-ray film (Kodak, Rochester, NY, USA). Band intensities were
scanned using a densitometer (Model GS-710; Bio-Rad Laboratories,
Inc., Hercules, CA, USA) and quantified by Quantity One software
(Bio-Rad Laboratories, Inc.).
Immunohistochemistry
Five-micrometer sections of 4%
paraformaldehyde-fixed paraffin-embedded liver tissues were
deparaffinized and hydrated. Endogenous peroxidase activity was
then blocked with 3% H2O2 in
phosphate-buffered saline for 10 min, and the tissues were
processed for heat-induced antigen retrieval in 0.01 mol/l citrate
buffer (pH 6.0) for 10 min. Primary rabbit-anti-PPARγ polyclonal
antibody was incubated at a dilution of 1/50–1/100 for 30 min at
room temperature before application to tissue sections. A two-step
immunohistological detection kit (cat. no. PV-6001; Beijing
Zhongshan Golden Bridge Biotechnology Co., Ltd.), including a goat
anti-rabbit IgG-HRP antibody, and a diaminobenzidine (DAB)
chromogen (DAB kit; Sigma-Aldrich, St. Louis, MO, USA), were used
to visualize specific binding. Five randomly selected high power
fields in the centrilobular or periportal areas were examined
randomly per section under a light microscope (Olympus CX31;
Olympus Corporation, Tokyo, Japan). The number of PPARγ positive
hepatocytes was counted in each section. The labeling indices,
expressed as the percentage of positive hepatocytes of the total
hepatocytes, were calculated.
Statistical analysis
Data are expressed as the mean ± standard deviation.
Comparisons between groups were performed by one-way analysis of
variance. P<0.05 was considered to indicate a statistically
significant difference.
Results
Characteristics of animals
studied
The initial body weights were similar in the groups
of 15 rats (data not shown). Although the rats in the HF group had
significantly higher body weights than those in the NC group from
the second week, body weights were lower in the HF + C group than
in the HF group from the third week of treatment until the end of
the experiment (Fig. 1). The
perirenal fat weight, epididymal fat weight and liver weight of the
HF + C group were significantly lower than those of the HF group,
but higher than those of the NC group (Table I). Although final heart weights and
kidney weights of rats in the HF group were slightly, but not
significantly, lower than those for rats in the NC group, heart
weights were significantly (P<0.05) higher than those for the HF
+ C group (Table I).
 | Table I.Effect of candesartan treatment on
final body weight, visceral organ weights and various biochemical
parameters in the three experimental groups. |
Table I.
Effect of candesartan treatment on
final body weight, visceral organ weights and various biochemical
parameters in the three experimental groups.
| Characteristic | NC group | HF group | HF + C group |
|---|
| Body weight, g |
461.33±36.48 |
518.40±28.30a |
478.33±34.79b |
| Perirenal fat
weight, g |
13.54±3.21 |
26.89±4.81a |
20.50±4.09a,c |
| Epididymal fat
weight, g |
7.64±1.72 |
11.50±1.95a |
9.61±1.83a,b |
| Heart weight,
g |
1.34±0.07 |
1.29±0.13 |
1.09±0.11a,c |
| Liver weight,
g |
13.04±1.63 |
17.86±1.76a |
15.72±1.70a,c |
| Kidney weight,
g |
2.46±0.24 |
2.28±0.17 |
2.25±0.18 |
| Triglyceride,
mmol/l |
1.82±0.67 |
1.27±0.24 |
1.30±0.37 |
| Total cholesterol,
mmol/l |
1.62±0.54 |
2.01±0.26d |
1.37±0.39b |
| Low-density
lipoprotein cholesterol, mmol/l |
0.07±0.03 |
0.72±0.15a |
0.62±0.23a |
| High-density
lipoprotein cholesterol, mmol/l |
0.75±0.13 |
0.6±0.06a |
0.68±0.11 |
| Serum angiotensin
II, pg/ml |
43.73±5.63 |
51.88±9.70d |
60.82±10.76c,d |
| Fasting blood
glucose, mmol/l |
6.18±0.73 |
6.38±0.66 |
5.76±0.92 |
| Fasting serum
insulin, mU/l |
1.57±0.98 |
4.76±2.75d |
3.03±1.37c,d |
| Insulin sensitivity
index, ×10−3 |
98.76±16.72 |
29.37±8.95d |
57.93±11.83c,d |
| Hyperinsulinemic
euglycemic clamp study, glucose infusion rate, mg/kg/min |
28.3±5.4 |
13.5±3.9a |
22.4±5.1b |
Serum lipid profile and angiotensin II
concentration
Although there was no statistically significant
difference in serum triglyceride levels among all three groups,
animals treated with 8 mg/kg candesartan cilexetil displayed
significant reductions in serum cholesterol (1.37±0.39 mmol/l in
the HF + C group vs. 2.01±0.26 mmol/l in the HF group; P<0.01)
(Table I). Serum angiotensin II
levels in the HF group were significantly higher than those in
controls (P<0.05); furthermore, the increase in angiotensin II
levels in the HF + C group was significantly more pronounced than
that in the HF group (P<0.05) due to angiotensin II type 1 (AT1)
receptor blockade (Table I).
Glucose and insulin
concentrations
Fasting blood glucose levels were comparable in the
NC and HF groups. Candesartan cilexetil showed a tendency to lower
the blood glucose levels in the high-fat-fed rats, but this effect
did not reach statistical significance (P>0.05; Table I). The fasting insulin levels in the
HF group were significantly higher compared with those in the NC
group (P<0.05; Table I). However,
animals receiving candesartan cilexetil displayed significant
reductions in insulin levels (36%; P<0.05) compared with those
in the HF group. As an indirect marker of peripheral insulin
action, the ISI was calculated for each animal. As shown in
Table I, the ISI of rats in the HF
group was substantially lower compared with that of rats in the NC
group, but the ISI of rats in the HF + C group was significantly
higher compared with that of the animals in the HF group
(P<0.05).
Glucose response during an OGTT in the candesartan
cilexetil treatment group is shown in Fig. 2A. Compared with the HF group,
treatment with 8 mg/kg candesartan cilexetil did not cause
significantly lower glucose values at 0 min, whereas treatment
resulted in lower glucose values (18, 7 and 13% lower,
respectively; all P<0.05) at the 30, 90 and 120 min time points.
The AUC values for glucose are presented in Fig. 2B; a reduction in this value reflects
an increase in insulin sensitivity (15). A significantly higher AUC for glucose
was calculated during the OGTT for the rats in the HF group
compared with the NC group (14.05±2.05 vs. 11.96±1.35 mmol.h/l;
P<0.05). Animals in the HF + C group had a lower glucose AUC
than those in the HF group (12.44±2.95 vs. 14.05±2.05 mmol·h/l;
P<0.05) during the OGTT.
Whole-body insulin sensitivity was evaluated using
the hyperinsulinemic-euglycemic clamp technique (Table I). During high-fat feeding in the HF
group, the glucose infusion rate (GIR) was 52.3% lower than that in
the NC group. However, the GIR was significantly enhanced following
candesartan cilexetil treatment (by 65.9%; P<0.01).
PPARγ expression in the epididymal
adipose tissue and liver tissue
To identify the potential activation of PPARγ
brought about by candesartan cilexetil, protein levels of PPARγ in
epididymal adipose tissue were assessed by western blotting.
High-fat feeding significantly reduced the amount of PPARγ in the
adipose tissue compared with that in rats of the NC group.
Candesartan cilexetil treatment together with a high-fat diet
caused a significant increase in PPARγ expression compared with
that in rats fed a high-fat diet and treated with saline (Figs. 3 and 4).
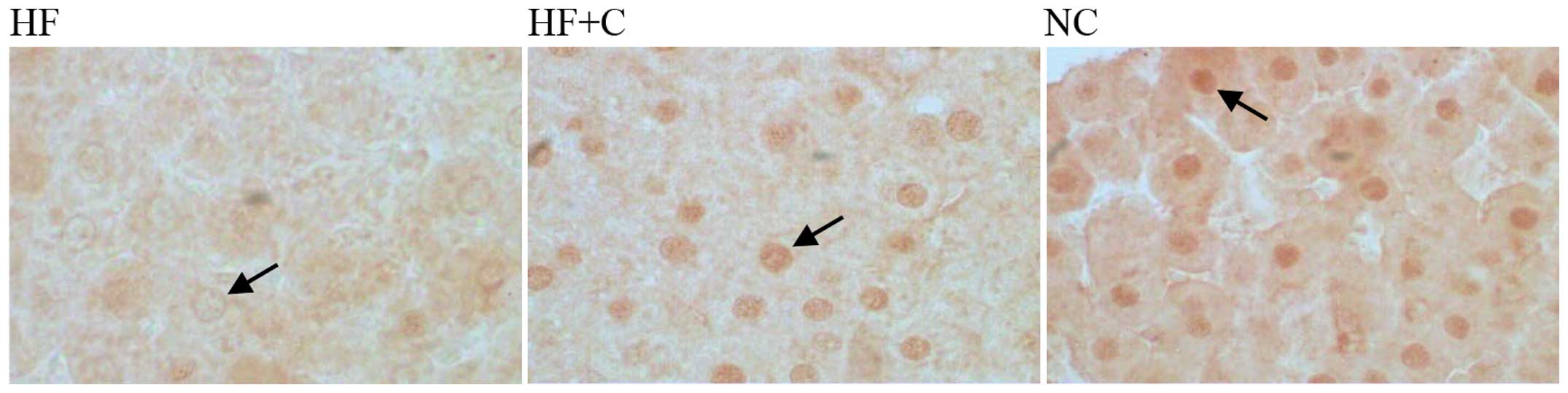
Subsequent immunohistochemical analysis of the liver
tissue is shown in Fig. 5.
Periportal and centrilobular hepatocytes exhibited low positivity
for PPARγ in the livers of rats in the HF group. However, levels of
PPARγ expression were increased significantly (P<0.05) in the
livers of rats treated with candesartan cilexetil (Figs. 5 and 6).
Discussion
In order to study the influence of the
renin-angiotensin system on adipose and liver tissue, diet-induced
obese rats were treated with long-term administration of
candesartan cilexetil. Absorbed candesartan cilexetil is completely
metabolized to candesartan. The absolute bioavailability is
relatively poor at 15% (candesartan cilexetil tablets) to 40%
(candesartan cilexetil solution) (16), and the dose administered (8
mg/kg/day) was chosen because it had been demonstrated to be
effective in a previous insulin-resistant obese rat model without
any overt signs of toxicity (17).
In the present study, it was demonstrated that high-fat feeding of
male Wistar rats caused insulin resistance, increased plasma total
cholesterol, low-density lipoprotein cholesterol, body weight and
adiposity. Treatment with candesartan cilexetil for 4 weeks
improved insulin sensitivity, and decreased plasma lipid levels and
adiposity. The induction of PPARγ activity demonstrates new
pleiotropic actions of candesartan cilexetil, providing a potential
mechanism for its insulin-sensitizing effects. Clinical observation
of a reduction in the development of diabetes mellitus with
candesartan cilexetil is supported by the Candesartan in
Heart-Failure Assessment of Reduction in Mortality and Morbidity
(CHARM) Preserved trial (18),
wherein the incidence of new-onset type 2 diabetes was
significantly lower in subjects given candesartan cilexetil than in
those given matching placebo, implicating an antidiabetic action of
candesartan cilexetil. However, notably, in other trials involving
candesartan cilexetil, the CHARM Alternative (19), the CHARM Added (20), and the Study on Cognition and
Prognosis in the Elderly (SCOPE) (21), there was no benefit of candesartan
cilexetil over placebo in the prevention of incident diabetes.
Candesartan cilexetil also failed to show any effect on glucose,
insulin or lipid levels in both the Candesartan Role in Obesity and
on Sympathetic System (CROSS) (22)
and Antihypertensive Treatment and Lipid Profile in a North Sweden
Efficacy Evaluation (ALPINE) (23).
As previously reported with AT1 receptor (AT1R)
blocker therapy (24), the data
obtained in the present study showed a marked postprandial 2 h
glucose level reduction and higher GIR in rats in the HF + C group
treated for 21 days with candesartan cilexetil. Fasting glucose
levels were similar among the three groups, whereas fasting insulin
levels in rats fed a high-fat diet were significantly higher than
those in the chow-fed controls. The observation of normoglycemia
with hyperinsulinemia indicates that the rats fed a high-fat diet
were insulin resistant. Increased glucose levels and AUC of glucose
were also observed following glucose loading in fat-fed rats.
Despite high insulin levels, the AUC of glucose in the HF group was
greater than that of HF + C group. However, the insulin levels of
rats treated with candesartan cilexetil were found to be
significantly lower compared with those of the HF control rats.
These results indicate that although candesartan cilexetil does not
affect insulin in normal conditions, under conditions of
hyperinsulinemia, it increases insulin sensitivity for effective
glucose disposal.
PPARs are ligand-activated transcription factors
that have a number of pleiotropic effects (25). The γ-subtype of the receptor (PPARγ)
plays an important role in carbohydrate and lipid metabolism and is
a therapeutic target in the treatment of insulin resistance,
diabetes and metabolic syndrome (26). PPARγ is mainly expressed in
adipocytes as well as hepatocytes and considered to be a key
regulator of adipocyte differentiation (27). Thiazolidinediones typically function
as full agonists of PPARγ, and improve insulin signaling and
insulin sensitivity (28). Some ARBs
such as telmisartan and irbesartan, are partial agonists of PPARγ
and are able to induce its activity and adipocyte differentiation
independent of their blocking properties. However, other ARBs have
failed to show any effects on PPARγ activity or adipogenesis
(29).
A major finding in the present study is that
candesartan cilexetil has PPARγ-activating properties. The 8
mg/kg/day dose of candesartan cilexetil in rats is approximately
twice the maximum recommended daily human dose of 32 mg on a
mg/m2 basis (this comparison assumes a human body weight
of 50 kg). That the dosage required in obese rats was much higher
than that recommended in humans may be due to inter-species
differences or to the fact that obese rats have not only glucose
and lipid metabolic disorders but also insulin resistance. The
mechanism by which candesartan cilexetil activates PPARγ remains to
be precisely defined. However, its relatively high volume of
distribution and lipophilicity, which have sufficiently high
penetration rates to gain access to the PPARγ-retinoid X receptor
complex within the cell nucleus, may be relevant. Once activated,
the complex influences the expression of the key target gene
glucose transporter-4 (GLUT-4) and increases glucose delivery to
skeletal muscle by improving insulin sensitivity (30). Consistent with the latter hypothesis,
we have observed that candesartan cilexetil shows other properties
of a PPARγ activator, which can induce small increases in GLUT-4
protein expression in the skeletal muscle of obesity-associated
rats (unpublished data). In addition, PPARγ-activating candesartan
cilexetil promotes adipocyte differentiation, resulting in the
redistribution of lipids from ectopic distribution to adipose
tissue and may increase adiponectin levels in humans (31), thereby contributing to the weight
loss and adiposity reduction of rats.
Obesity is one of the most common causes of insulin
resistance. Adipose tissue, particularly in the visceral
compartment, secretes various bioactive molecules that may directly
contribute to the development of obesity-related diseases. It is
acknowledged that adipose tissue is a complex and highly active
metabolic and endocrine organ (32),
producing proteins such as leptin, adiponectin and resistin
(33). Several peptides of the
renin-angiotensin system (34) are
also produced in adipose tissue. Angiotensin II is a main final
effector molecule of the renin-angiotensin system. The present
study confirms that serum angiotensin II levels are elevated in
diet-induced obese rats. This finding is consistent with earlier
observations that circulating levels of angiotensinogen, renin and
angiotensin II were increased in obese individuals (35). Systemic and local angiotensin II has
been shown to inhibit substrate delivery and cross-talk between
angiotensin and insulin receptor signaling pathways, which can be
restored by AT1R antagonism (36).
Beneficial effects of ARBs on impaired insulin signaling may
represent an additional molecular mechanism for insulin sensitizing
actions. Blockade of AT1 receptors leads to compensatory increases
in angiotensin II levels and the subsequent increased activation of
AT2 receptors (37). The AT2
receptor is now recognized as the counter-regulator of the AT1
receptor, exerting mostly beneficial actions (38). It has been reported that angiotensin
II stimulation can decrease the expression of PPARγ in cardiac
myofibroblasts (39), while PPARγ
causes a downregulation of AT1R gene expression via a
PPARγ-dependent mechanism in vascular smooth muscle cells (40). Therefore, PPARγ may also play a role
in the regulation of angiotensin II action. Candesartan cilexetil,
by virtue of its ability to block AT1Rs and to activate PPARγ, may
not only inhibit angiotensin II-mediated pathways of insulin
resistance, but also stimulate PPARγ pathways that help prevent
resistance.
There are several limitations of the present study.
First, the study did not demonstrate that candesartan cilexetil
stimulates PPARγ activation independent of AT1R blocking actions in
the absence of AT1R. Second, the effects of different ARBs with
PPARγ-activating properties were not compared. The superiority of
candesartan cilexetil for the enhancement of insulin sensitivity in
association with improvement of insulin resistance requires further
assessment.
In summary, the present study has demonstrated that
long-term administration of candesartan cilexetil, a specific ARB,
to insulin-resistant obese rats elicited a great improvement of
whole-body insulin sensitivity, at least in part because of PPARγ
activation. This angiotensin II receptor antagonism also resulted
in significant increases in PPARγ protein expression in adipose and
liver tissue. PPARγ activation by candesartan cilexetil may provide
novel therapeutic options in the treatment of patients with
metabolic syndrome.
Acknowledgements
This study has benefited from research funding from
National Natural Science Foundation of China (no. 30671095).
References
|
1.
|
Neutel JM: Choosing among
renin-angiotensin system blockers for the management of
hypertension: From pharmacology to clinical efficacy. Curr Med Res
Opin. 26:213–222. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Dahlöf B, Devereux RB, Kjeldsen SE, Julius
S, Beevers G, de Faire U, Fyhrquist F, Ibsen H, Kristiansson K,
Lederballe-Pedersen O, et al: LIFE Study Group: Cardiovascular
morbidity and mortality in the Losartan Intervention For Endpoint
reduction in hypertension study (LIFE): A randomised trial against
atenolol. Lancet. 359:995–1003. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Julius S, Kjeldsen SE, Weber M, Brunner
HR, Ekman S, Hansson L, Hua T, Laragh J, McInnes GT, Mitchell L, et
al: VALUE Study Group: Outcomes in hypertensive patients at high
cardiovascular risk treated with regimens based on valsartan or
amlodipine: The VALUE randomised trial. Lancet. 363:2022–2231.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Lindholm LH, Ibsen H, Borch-Johnsen K,
Olsen MH, Wachtell K, Dahlöf B, Devereux RB, Beevers G, de Faire U,
Fyhrquist F, et al: LIFE Study Group: Risk of new-onset diabetes in
the Losartan Intervention For Endpoint reduction in hypertension
study. J Hypertens. 20:1879–1886. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Clasen R, Schupp M, Foryst-Ludwig A,
Sprang C, Clemenz M, Krikov M, Thöne-Reineke C, Unger T and
Kintscher U: PPARgamma-activating angiotensin type-1 receptor
blockers induce adiponectin. Hypertension. 46:137–143. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Higgins LS and Depaoli AM: Selective
peroxisome proliferator-activated receptor gamma (PPARgamma)
modulation as a strategy for safer therapeutic PPARgamma
activation. Am J Clin Nutr. 91:267S–272S. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Benson SC, Pershadsingh HA, Ho CI,
Chittiboyina A, Desai P, Pravenec M, Qi N, Wang J, Avery MA and
Kurtz TW: Identification of telmisartan as a unique angiotensin II
receptor antagonist with selective PPARgamma-modulating activity.
Hypertension. 43:993–1002. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Schupp M, Janke J, Clasen R, Unger T and
Kintscher U: Angiotensin type 1 receptor blockers induce peroxisome
proliferator-activated receptor-gamma activity. Circulation.
109:2054–2057. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Henriksen EJ, Jacob S, Kinnick TR, Teachey
MK and Krekler M: Selective angiotensin II receptor antagonism
reduces insulin resistance in obese Zucker rats. Hypertension.
38:884–890. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Li GW and Pan XR: A new
insulin-sensitivity index for the population-based study. Zhonghua
Nei Ke Za Zhi. 32:656–660. 1993.(In Chinese). PubMed/NCBI
|
|
11.
|
Sridhar MG, Vinayagamoorthi R, Arul
Suyambunathan V, Bobby Z and Selvaraj N: Bitter gourd (Momordica
charantia) improves insulin sensitivity by increasing skeletal
muscle insulin-stimulated IRS-1 tyrosine phosphorylation in
high-fat-fed rats. Br J Nutr. 99:806–812. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Allison DB, Paultre F, Maggio C, Mezzitis
N and Pi-Sunyer FX: The use of areas under curves in diabetes
research. Diabetes Care. 18:245–250. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Cheung A and Bryer-Ash M: Modified method
for the performance of glucose insulin clamp studies in conscious
rats. J Pharmacol Toxicol Methods. 31:215–220. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Kump DS and Booth FW: Sustained rise in
triacylglycerol synthesis and increased epididymal fat mass when
rats cease voluntary wheel running. J Physiol. 565:911–925. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Matsuda M and DeFronzo RA: Insulin
sensitivity indices obtained from oral glucose tolerance testing:
Comparison with the euglycemic insulin clamp. Diabetes Care.
22:1462–1470. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Burnier M and Brunner HR: Angiotensin II
receptor antagonists. Lancet. 355:637–645. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Tuncer I, Ozbek H, Ugras S and Bayram I:
Anti-fibrogenic effects of captopril and candesartan cilexetil on
the hepatic fibrosis development in rat. The effect of AT1-R
blocker on the hepatic fibrosis. Exp Toxicol Pathol. 55:159–166.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Yusuf S, Pfeffer MA, Swedberg K, Granger
CB, Held P, McMurray JJ, Michelson EL, Olofsson B and Ostergren J:
CHARM Investigators and Committees: Effects of candesartan in
patients with chronic heart failure and preserved left-ventricular
ejection fraction: The CHARM-Preserved trial. Lancet. 362:777–781.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Granger CB, McMurray JJ, Yusuf S, Held P,
Michelson EL, Olofsson B, Ostergren J, Pfeffer MA and Swedberg K:
CHARM Investigators and Committees: Effects of candesartan in
patients with chronic heart failure and reduced left-ventricular
systolic function intolerant to angiotensin-converting-enzyme
inhibitors: The CHARM-Alternative trial. Lancet. 362:772–776. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
McMurray JJ, Ostergren J, Swedberg K,
Granger CB, Held P, Michelson EL, Olofsson B, Yusuf S and Pfeffer
MA: CHARM Investigators and Committees: Effects of candesartan in
patients with chronic heart failure and reduced left-ventricular
systolic function taking angiotensin-converting-enzyme inhibitors:
The CHARM-Added trial. Lancet. 362:767–771. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Lithell H, Hansson L, Skoog I, Elmfeldt D,
Hofman A, Olofsson B, Trenkwalder P and Zanchetti A: SCOPE Study
Group: The Study on cognition and prognosis in the elderly (SCOPE):
Principal results of a randomized double-blind intervention trial.
J Hypertens. 21:875–886. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Grassi G, Seravalle G, Dell'Oro R, Trevano
FQ, Bombelli M, Scopelliti F, Facchini A and Mancia G: CROSS Study:
Comparative effects of candesartan and hydrochlorothiazide on blood
pressure, insulin sensitivity and sympathetic drive in obese
hypertensive individuals: Results of the CROSS study. J Hypertens.
21:1761–1769. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Lindholm LH, Persson M, Alaupovic P,
Carlberg B, Svensson A and Samuelsson O: Metabolic outcome during 1
year in newly detected hypertensives: Results of the
Antihypertensive Treatment and Lipid Profile in a North of Sweden
Efficacy evaluation (ALPINE study). J Hypertens. 21:1563–1574.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Kinnick TR, Youngblood EB, O'Keefe MP,
Saengsirisuwan V, Teachey MK and Henriksen EJ: Modulation of
insulin resistance and hypertension by voluntary exercise training
in the TG(mREN2)27 rat. J Appl Physiol (1985). 93:805–812;
discussion 797. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Michalik L, Auwerx J, Berger JP,
Chatterjee VK, Glass CK, Gonzalez FJ, Grimaldi PA, Kadowaki T,
Lazar MA, O'Rahilly S, et al: International Union of Pharmacology.
LXI. Peroxisome proliferator-activated receptors. Pharmacol Rev.
58:726–741. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Balakumar P, Rose M and Singh M: PPAR
ligands: Are they potential agents for cardiovascular disorders?
Pharmacology. 80:1–10. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Kersten S, Desvergne B and Wahli W: Roles
of PPARs in health and disease. Nature. 405:421–424. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Spiegelman BM: PPAR-gamma: Adipogenic
regulator and thiazolidinedione receptor. Diabetes. 47:507–514.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Kurtz TW: Treating the metabolic syndrome:
Telmisartan as a peroxisome proliferator-activated receptor-gamma
activator. Acta Diabetol. 42(Suppl 1): S9–S16. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Morsing P, Adler G, Brandt-Eliasson U,
Karp L, Ohlson K, Renberg L, Sjöquist PO and Abrahamsson T:
Mechanistic differences of various AT1-receptor blockers in
isolated vessels of different origin. Hypertension. 33:1406–1413.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Koh KK, Quon MJ, Han SH, Chung WJ, Lee Y
and Shin EK: Anti-inflammatory and metabolic effects of candesartan
in hypertensive patients. Int J Cardiol. 108:96–100. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Frühbeck G, Gómez-Ambrosi J, Muruzábal FJ
and Burrell MA: The adipocyte: A model for integration of endocrine
and metabolic signaling in energy metabolism regulation. Am J
Physiol Endocrinol Metab. 280:E827–E847. 2001.PubMed/NCBI
|
|
33.
|
Banerjee RR and Lazar MA: Resistin:
Molecular history and prognosis. J Mol Med (Berl). 81:218–226.
2003.PubMed/NCBI
|
|
34.
|
Goossens GH, Blaak EE and van Baak MA:
Possible involvement of the adipose tissue renin-angiotensin system
in the pathophysiology of obesity and obesity-related disorders.
Obes Rev. 4:43–55. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Engeli S, Böhnke J, Gorzelniak K, Janke J,
Schling P, Bader M, Luft FC and Sharma AM: Weight loss and the
renin-angiotensin-aldosterone system. Hypertension. 45:356–362.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Motley ED, Eguchi K, Gardner C, Hicks AL,
Reynolds CM, Frank GD, Mifune M, Ohba M and Eguchi S:
Insulin-induced Akt activation is inhibited by angiotensin II in
the vasculature through protein kinase C-alpha. Hypertension.
41:775–780. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Furuhashi M, Ura N, Takizawa H, Yoshida D,
Moniwa N, Murakami H, Higashiura K and Shimamoto K: Blockade of the
renin-angiotensin system decreases adipocyte size with improvement
in insulin sensitivity. J Hypertens. 22:1977–1982. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
de Gasparo M, Catt KJ, Inagami T, Wright
JW and Unger T: International Union of Pharmacology. XXIII. The
angiotensin II receptors. Pharmacol Rev. 52:415–472.
2000.PubMed/NCBI
|
|
39.
|
Hao GH, Niu XL, Gao DF, Wei J and Wang NP:
Agonists at PPAR-gamma suppress angiotensin II-induced production
of plasminogen activator inhibitor-1 and extracellular matrix in
rat cardiac fibroblasts. Br J Pharmacol. 153:1409–1419. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Sugawara A, Takeuchi K, Uruno A, Ikeda Y,
Arima S, Kudo M, Sato K, Taniyama Y and Ito S: Transcriptional
suppression of type 1 angiotensin II receptor gene expression by
peroxisome proliferator-activated receptor-gamma in vascular smooth
muscle cells. Endocrinology. 142:3125–3134. 2001. View Article : Google Scholar : PubMed/NCBI
|