Introduction
B-type natriuretic peptide (BNP) is predominantly
secreted by ventricular cardiomyocytes in patients with chronic
cardiac disease (1). It serves a key
role in the regulation of circulation and the balance of water and
electrolytes (2), and it is closely
associated with the systolic and diastolic functions of myocardial
cells (3). At present, BNP is
considered to be an effective index of evaluation for myocardial
function (4). However, the levels of
serum N-terminal pro-BNP (NT-pro BNP) are closely associated with
BNP, and NT-pro BNP is stable enough to be detected using
laboratory tests (5).
Coronary heart disease (CAD) is one of the most
frequent causes of morbidity and mortality worldwide, and it is
well known that the predominant factor of CAD is atherosclerosis,
which is a chronic and progressive inflammatory disease (6). The pathogenesis of acute coronary
syndrome (ACS) includes the rupture or erosion of a vulnerable
plaque, which is characterized by infiltration of inflammatory
cells, a large necrotic core and a thin fibrous cap (7). It is believed that the earlier the
detection of UA, the better chances exist regarding treatment and
prognosis.
Numerous studies have demonstrated that the levels
of serum NT-pro BNP are associated with the severity and prognosis
of coronary atherosclerotic disease (8–11). At
present, computed tomography angiography (CTA) is the optimal
non-invasive method for assessing the burden and components of
coronary plaques (12). This
technique is highly consistent with optical coherence tomography
(OCT) and intravascular ultrasound (IVUS), which are widely
considered as the ‘gold standard’ methods for assessing plaque
characteristics (13). Currently,
coronary CTA (CCTA) is widely used to evaluate the risk and
stability of coronary atherosclerotic plaques in patients with
unstable angina (UA) (14–16).
Zhou et al (17) oserved that the concentration of BNP
was significantly higher in patients with carotid plaque compared
with those without carotid plaque, and the presence of the plaque
positively correlated with carotid intima-media thickness (17). Hong et al (18) demonstrated that patients with high
NT-pro-BNP expression levels had more vulnerable plaque components
(more non-calcified-containing lesions and a higher frequency of
thin-cap fibroatheroma, as assessed by virtual histology-IVUS). In
addition, Peng et al (19)
observed that patients with essential hypertension and moderate and
elevated BNP had significantly higher left ventricular mass index,
intima-media thickness, coronary calcification score (CCS) and
microalbuminuria levels compared with those with normal BNP
expression levels, as assessed by ultrasound and coronary CT
examinations (19). The above
studies were investigated the associations between carotid
atherosclerosis plaque burden, coronary artery plaque calcification
score and BNP/NT-pro BNP. Altintas et al (20) demonstrated that serum NT-pro BNP
expression level was associated with total plaque burden and
calcification volume in patients with stable chest pain (20). However, currently there are no
studies investigating the association between serum BNP/NT-pro BNP
expression level and characteristics of coronary artery plaque in
patients with ACS, in particular with regards to the burden and
composition of plaques, as assessed by CT.
The present study explores the association between
the levels of serum NT-pro BNP and the characteristics of
atherosclerotic plaques, as determined by CCTA.
Materials and methods
Subjects
A total of 202 patients (age range, 47–82 years;
mean age, 74.5 years; male to female ratio, 1.8:1) were recruited
for the present study between January 2013 and 2014 in the Chinese
General Hospital of the PLA (Beijing, China). Informed patient
consent was obtained prior to the collection of blood samples for
the detection of serum NT-pro BNP. The study was approved by the
Institutional Ethics Committee for human subjects, and was approved
by the ethics committee of the Chinese General Hospital of the PLA.
Written informed consent was obtained from the patients.
Inclusion criteria
Patients with stable angina pectoris (SAP) and UA,
diagnosed according to the AHA/ACC Guideline for the Management of
Patients with Non-ST-Elevation Acute Coronary Syndromes (21), were included in the study.
Exclusion criteria
Patients were excluded from the study if they
matched any of the following criteria: Acute myocardial infarction,
cardiomyopathy, valvular disease, cardiac dysfunction, coronary
revascularization (including percutaneous coronary intervention,
stenting and coronary artery bypass grafting), renal insufficiency,
iodine allergy, poor tolerance to CCTA examination, pregnancy and
minors.
Study design
All subjects underwent CCTA examination and blood
samples were taken for the detection of serum NT-pro BNP within
12–48 h following the onset of illness or admission to hospital.
Blood samples were drawn prior to the CTTA examination and stored
at −80°C until assays were performed. Arterial hypertension was
defined by the use of antihypertensive drugs prescribed by a
physician, or reported systolic blood pressures >140 mmHg.
Hyperlipidemia was defined by 200 mg/dl total cholesterol, or the
use of statins to treat hyperlipidemia at present or in the past.
Patients with UA (n=83) were analyzed as the experimental group,
and patients with SAP (n=62) and non-cardiac diseases (n=57) were
treated as control groups.
Scan protocol and image
reconstruction
Scans were performed according to SCCT guidelines
(22) for the performance of CCTA
with a multidetector computed tomography (CT) scanner (256 iCT;
Philips Healthcare, Andover, MA, USA). Helical scan data was
obtained using retrospective or prospective electrocardiographic
gating. Prior to performing the CCTA, 50–60 ml iodinated contrast
media (370 mg I/ml, Bayer AG, Leverkusen, Germany) was injected
followed by a 35–40 ml saline flush (4.5–5 ml/s). The threshold
tract trigger mode of the region of interest was adopted (23). Images were reconstructed at 40, 45
and 78% of the cardiac cycle, consistently and immediately
following the completion of a scan. The optimal phase
reconstruction was assessed by comparison with the phase that had
the least amount of coronary artery motion. The images were
evaluated using transaxial two-dimensional image stacks (raw data),
multiplanar reformations, maximum intensity projections, curved
multiplanar reformations and volume-rendering technique
reconstructions. If a coronary artery segment was uninterpretable
(characterized by artifacts, such as motion, beam hardening, metal
or calcium-related partial volume averaging, and adequacy of over
contrast concentration or opacification) (24), it was not included in the analysis.
IntelliSpace Portal 6 (Philips Healthcare, Andover, MA, USA) was
used to analyze the images.
Image analysis and definition of
atherosclerotic plaque in the coronary artery
In each coronary artery segment, coronary
atherosclerosis was defined as the presence of tissue structures
>1 mm2 that existed within the coronary artery lumen
or adjacent to the coronary artery lumen that could be
discriminated from surrounding pericardial tissues, epicardial fat
or the vessel lumen itself. Coronary artery segments were
identified according to the 15-Segment American Heart Association
model (25). Calcification of
plaques was graded according to the following criteria:
Non-calcified plaque (<20% calcification; mean CT, ≤150 HU);
mixed plaque (20–80% calcification); and calcified plaque (>80%
calcification; mean CT, >130 HU).
Evaluation indicators
Images were analyzed for plaque and individual
aspects. Evaluation indicators for individual aspects included left
main-left anterior descending (LM-LAD) disease and obstructive
diseases (stenosis degree of the coronary artery of ≥50%), and the
number of segments of calcified plaque, non-calcified plaque and
mixed plaque. Coronary calcium scoring computer programs typically
identify pixels that exceed 130 HU as a level corresponding to
calcium on a non-contrast study Plaques were evaluated according to
the following criteria: CCS (Agaston score) (26); segment-involvement score (SIS; range
0–15); segment-stenosis score [SSS; range, 0–3; 0 = normal, 1 =
mild (<50%), 2 = moderate (≥50–70%), 3 = severe (≥70%);
obstructive disease = ≥2 points]; and the number of vessels (range,
0–4). Degrees of luminal stenosis were calculated on the basis of
the vessel diameter. SSS ranged between 0 and 45 points.
Blind method
Each image was analyzed by two radiologists with
experience of interpreting several thousands of CCTA scans. The
radiologists had no knowledge of the clinical diagnosis and
laboratory results of the patients.
Laboratory examination
Fasting blood samples (4.0 ml) were collected using
vacuum tubes and serum was separated 30 min following sample
extraction by centrifugation at 14,000 × g for 20 min at room
temperature. An automatic biochemical analyzer (7600DDP; Hitachi,
Ltd., Tokyo, Japan) was used to detect levels of serum NT-pro
BNP.
Statistical analysis
Patient demographic characteristics were presented
as the mean ± standard deviation or as medians (interquartile
ranges) for continuous variables, and as proportions (percentages)
for categorical variables. The levels of serum NT-pro BNP were
normally distributed with logarithmic transformation.
Non-parametric Mann-Whitney U or Kruskal-Wallis tests were used to
compare continuous variables, and the Pearson's χ2 test
was used to evaluate differences in frequencies. Correlations
between variables were analyzed using a linear and binary logistic
regression model. Receiver-operating characteristic (ROC) curve
analysis was used to evaluate the authenticity and reliability of
the study and estimate the optimal threshold (cut-off value
obtained from ROC). Statistical comparisons were performed using
SPSS version 19.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Baseline information and levels of
serum NT-pro BNP
There were no statistically significant differences
between patient parameters, including heart rate and blood
pressure, among the three groups (UA, SAP and non-cardiac disease
groups; Tables I and II). The analysis of serum NT-pro BNP
levels among the three groups is presented in Table III. Logarithmic values of NT-pro
BNP serum levels are as follows: Non-cardiac disease group
(1.57±0.57; n=57; 95% CI, 1.53–1.98); SAP group (1.93±0.50; n=62;
95% CI, 1.73–2.12); and UA group (2.16±0.53; n=83; 95% CI,
2.00–2.31). There were statistically significant differences in the
log(NT-pro BNP) values between the three groups (Table III).
 | Table I.Patient information. |
Table I.
Patient information.
| Parameter | NC | SAP | UA | F-value | P-value |
|---|
| Age |
56.8±11 |
60.4±9.6 |
62.7±11 | 2.724 | 0.070 |
| HR |
71.6±12 |
73.5±11 |
75.5±15 | 0.786 | 0.458 |
| SBP |
135±19 |
136±16 |
138±23 | 0.300 | 0.741 |
| DBP |
80.0±11 |
77.0±8.0 |
77.0±11 | 0.614 | 0.543 |
| BMI |
26.0±2.8 |
26.7±5.0 |
25.7±3.2 | 0.568 | 0.568 |
 | Table II.Patient information (n=202). |
Table II.
Patient information (n=202).
| Parameter | χ2 | P-value |
|---|
| Gender (male) | 4.626 | 0.099 |
| Hypertension | 1.001 | 0.606 |
| Diabetes
mellitus | 0.017 | 0.991 |
| Dyslipidemia | 0.588 | 0.745 |
| Family history | 0.115 | 0.944 |
| Smoking | 0.950 | 0.622 |
 | Table III.Levels of NT-pro BNP in the control
(non-cardiac disease), SAP and UA groups. |
Table III.
Levels of NT-pro BNP in the control
(non-cardiac disease), SAP and UA groups.
| Group | N | Log(NT-pro
BNP) | 95% CI | P-value |
|---|
| NC | 57 |
1.57±0.57 | 1.53–1.98 | 0.007a |
| SAP | 62 |
1.93±0.50b | 1.73–2.12 |
|
| UA | 83 |
2.16±0.53c,d | 2.00–2.31 |
|
Atherosclerotic plaque characteristics
assessed by CCTA
The data from the analysis of the atherosclerotic
plaque CCTA characteristics among the three groups is presented in
Table IV. There was no correlation
between the levels of serum NT-pro BNP and the characteristics of
atherosclerotic plaques, detected by CCTA, between the SAP and
control group.
 | Table IV.Univariant and multivariant analysis
of the association between serum NT-pro BNP levels and US. |
Table IV.
Univariant and multivariant analysis
of the association between serum NT-pro BNP levels and US.
| Parameter | NC | SAP | UA | P-value |
|---|
| Vessels |
1.26±1.46 |
2.26±1.13a |
2.13±1.27b,c | 0.008 |
| SIS |
1.96±2.67 |
2.89±2.01d |
3.56±3.22e,f | 0.019 |
| SSS |
3.04±0.99 |
3.78±3.87 |
5.33±5.72 | 0.066 |
| NCP |
1.33±2.62 |
1.72±1.34 |
2.53±2.34 | 0.458 |
| MP |
0.07±0.27 |
0.56±0.90g |
0.92±0.23h,i | 0.009 |
| CP |
0.89±1.69 |
1.15±1.46 |
2.33±3.97 | 0.108 |
| CCS |
27.8±12.6 |
148.3±102.4 |
287.5±259.2 | 0.101 |
Association between serum NT-pro BNP
expression levels and characteristics of plaques in patients with
UA
The correlation analysis between the levels of serum
NT-pro BNP and characteristics of atherosclerotic plaques detected
by CCTA in patients with UA is presented in Table V. In the UA group, log(NT-pro BNP)
had various degrees of linear correlation with the number of
vessels (r=0.462, P<0.001), SIS (r=0.475, P<0.001), SSS
(r=0.453, P<0.001), CCS (r=0.412, P<0.001), obstructive
disease (r=0.346, P<0.001) and the number of segments with
non-calcified plaque (r=0.235, P=0.017), mixed plaque (r=0.234,
P=0.017) and calcified plaque (r=0.431, P<0.001). However,
following the adjustment of various conventional risk factors,
log(NT-pro BNP) was not determined to be an independent risk factor
of UA (Table VI).
 | Table V.Correlation between N-terminal
pro-B-type natriuretic peptide levels and coronary computed
tomography angiography characteristics in patients with unstable
angina. |
Table V.
Correlation between N-terminal
pro-B-type natriuretic peptide levels and coronary computed
tomography angiography characteristics in patients with unstable
angina.
| Parameter | R-value | P-value |
|---|
| Vessel number | 0.462 | <0.001 |
| SIS | 0.475 | <0.001 |
| SSS | 0.453 | <0.001 |
| OD | 0.346 | <0.001 |
| NCP | 0.235 | 0.017 |
| MP | 0.234 | 0.017 |
| CP | 0.431 | <0.001 |
| CCS | 0.412 | 0.001 |
 | Table VI.Analysis of log(N-terminal pro-B-type
natriuretic peptide). |
Table VI.
Analysis of log(N-terminal pro-B-type
natriuretic peptide).
| Analysis | P-value | OR | 95% CI UL | 95% CI LL |
|---|
| Univariable | 0.008 | 2.892 | 1.326 | 6.309 |
| Multivariable | 0.053 | 2.723 | 0.985 | 7.526 |
Association between serum NT-pro BNP
expression levels and LM-LAD disease in patients with UA
There were statistically significant differences
between the prevalence of LM-LAD disease among the three groups.
LM-LAD was most common in patients with UA (Fig. 1; χ2=21.444, P<0.001)
and the levels of serum NT-pro BNP were significantly higher in
patients with UA and LM-LAD disease compared with UA patients
without LM-LAD disease (2.12±0.52 vs. 1.64±0.48; P<0.001;
Table VII).
 | Table VII.Levels of serum N-terminal pro-B-type
natriuretic peptide in LM-LAD disease in patients with unstable
angina. |
Table VII.
Levels of serum N-terminal pro-B-type
natriuretic peptide in LM-LAD disease in patients with unstable
angina.
| Group | Log(N-pro BNP)
(pg/ml) |
|---|
| With LM-LAD |
2.12±0.52a |
| Without LM-LAD |
1.64±0.48 |
Association between serum NT-pro BNP
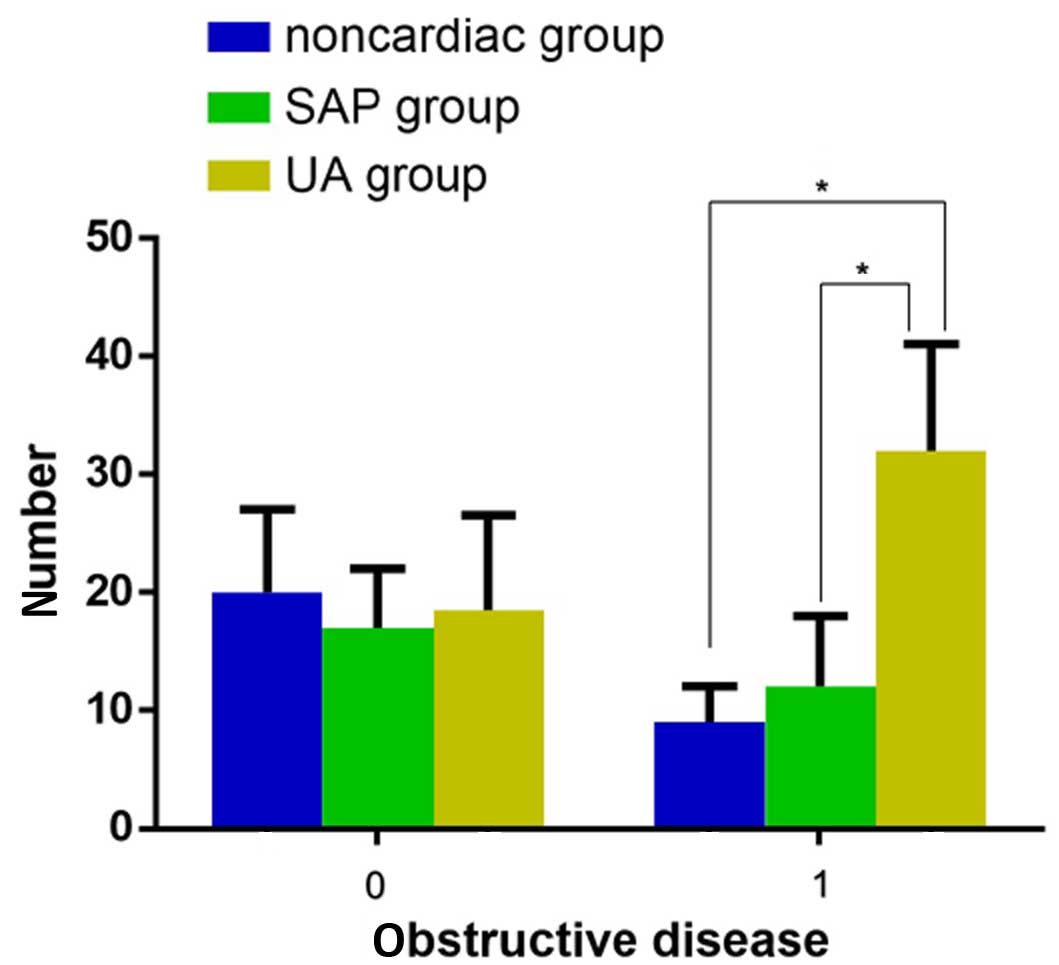
expression levels and obstructive disease in patients with UA
There were statistically significant differences
between the number of identified obstructive diseases among the
three groups, and patients in the UA group experienced the most
severe obstructive disease (Fig. 2;
χ2=10.113, P=0.006). In addition, in patients with UA
the levels of serum N-pro BNP in those with obstructive diseases
was significantly higher compared with those without obstructive
diseases (Table VIII; 1.78±0.49,
vs. 2.22±0.54; P<0.001); however, the levels of serum N-pro BNP
did not increase when the number of obstructive vessels increased
(P=0.400; Table IX).
 | Table VIII.Level of serum log(NT-pro BNP) in
patients with obstructive disease and unstable angina. |
Table VIII.
Level of serum log(NT-pro BNP) in
patients with obstructive disease and unstable angina.
| Disease status | Log(NT-pro
BNP) |
|---|
|
Non-obstructive |
1.78±0.49a |
| Obstructive |
2.22±0.54 |
 | Table IX.Level of serum NT-pro BNP (pg/ml) in
patients in the unstable angina group with OD. |
Table IX.
Level of serum NT-pro BNP (pg/ml) in
patients in the unstable angina group with OD.
| OD | N | Log(NT-pro
BNP) | 95% CI | P-value |
|---|
| Non-OD | 57 |
1.78±0.49 | 1.65–1.92 |
<0.001a |
| 1 OD | 102 |
2.09±0.51b | 1.89–2.38 |
|
| ≥2 OD | 43 |
2.12±0.56c,d | 2.07–2.53 |
|
ROC of serum NT-pro BNP expression
levels and indexes of CCTA plaque characteristics to identify
patients with UA
Using the SAP and non-cardiac disease groups as
control groups, ROC curves were plotted (Fig. 3). In patients with UA, the area under
the curve (AUC) was 0.656 (95% CI, 0.55–0.762; P=0.006) and the
optimal cut-off value was 1.74 (Fig.
3). The sensitivity, specificity, LR (likely ratio), Youden
index, OR (odds ratio), CI and Kappa values of NT-pro BNP in
patients with UA are presented in Table
X. In addition, the AUC of vessels, SIS, SSS and the number of
segments with calcified plaque, non-calcified plaque and mixed
plaque are presented in Table
XI.
 | Table X.N-pro BNP parameters of patients in
the unstable angina group. |
Table X.
N-pro BNP parameters of patients in
the unstable angina group.
| Parameter | NT-pro BNP |
|---|
| Sensitivity | 77.6% |
| Specificity | 51.9% |
| Likely positive
ratio | 1.61 |
| Likely negative
ration | 0.43 |
| Youden index | 0.295 |
| Odds ratio | 3.841 |
| 95% Confidence
interval | 2.077–7.102 |
 | Table XI.Receiver operating characteristic
analysis of vessels, SIS, SSS, and the number of segments with NCP,
MP and CP. |
Table XI.
Receiver operating characteristic
analysis of vessels, SIS, SSS, and the number of segments with NCP,
MP and CP.
| Parameter | AUC | P-value |
|---|
| Number of
vessels | 0.573 | 0.204 |
| SIS | 0.592 | 0.109 |
| SSS | 0.608 | 0.060 |
| NCP | 0.599 | 0.082 |
| MP | 0.591 | 0.112 |
| CP | 0.513 | 0.825 |
Discussion
In the present study, the results demonstrated that
the levels of serum NT-pro BNP have a positive correlation with
atherosclerotic plaque, and specifically calcified plaque. It has
been demonstrated that the pathogenesis of UA involves the rupture
or erosion of vulnerable plaques and secondary thrombosis (27), leading to an acute occlusion of
coronary arteries. Acute myocardial ischemia is categorized under
the Major Adverse Cardiovascular Events (MACE), which also includes
UA, acute myocardial infarction (AMI) and sudden cardiac death.
Numerous studies have demonstrated that there is cross-talk between
the endocrine and contractile functions of the heart, which acts as
a pump as well as a multifunctional and interactive organ (28–31).
Changes in ventricular wall tension, stimulation of
myocardial cells and myocardial ischemia can immediately induce the
secretion of BNP (32,33). When ventricular cardiomyocytes are
stimulated, pre-pro BNP is released and cleaved into active BNP and
inactive NT-pro BNP (34).
Currently, BNP and NT-pro BNP are primarily used for the screening
of heart disease, identifying the risk of congestive heart failure,
detecting dysfunction of the left ventricle, differentially
diagnosing dyspnea, and diagnosing acute and chronic heart failure
(8,9,11).
Research into acute coronary syndrome (ACS) and AMI
has demonstrated that levels of serum NT-pro BNP are closely
associated with the severity and prognosis of coronary artery
disease (35). Invasive examination,
coronary angiography, optical OCT and intravascular ultra-sound
IVUS are used in a large number of these studies (18,36–38).
However, in the present study it was observed that the majority of
patients with ACS preferred examination with noninvasive CCTA
rather than the invasive methods discussed above. Although there
are limitations to the detection of lipid necrotic cores and fiber
caps using CCTA, with the development of scanning and
post-processing techniques, CCTA may transform into a preferred
option compared with OCT and IVUS, which are currently considered
the ‘gold standard’ for detecting vulnerable plaques (13,15,39–40). In
addition, the rupture or erosion of vulnerable plaques may increase
the level of serum BNP and NT-pro BNP in a short period of time
(5,41,42).
Therefore, using CCTA in patients with UA to detect changes in
serum NT-pro BNP levels and the characteristics of atherosclerotic
plaques may have important clinical significance.
A previous study of IVUS demonstrated that the
levels of serum NT-pro BNP are significantly associated with
vulnerable plaques which have relatively large necrotic cores or
thin fibrous caps (43). In the
present study, high levels of serum NT-pro BNP were associated with
a high volume of calcified plaque. In particular, serum NT-pro BNP
levels were associated with the composition of atherosclerotic and
calcified plaque (correlation coefficient, 0.431), as compared with
non-calcified and mixed plaque that were not associated with serum
NT-pro BNP (OR=0.235 and 0.234 respectively). To the best of our
knowledge, these findings have not been previously reported.
The formation of atherosclerotic plaque is a complex
process comprised of three stages: The formation of lipid plaques,
fibrous plaques and complex lesions (44,45).
Plaques in various stages present different characteristics on a
CCTA, including low-density non calcified plaque, high-density non
calcified plaque, mixed plaque and calcified plaque (46). Considering the limitations of
detecting low-density and high-density non-calcified plaques using
CCTA (11), these two types of
plaque were considered to be non-calcified plaque in the present
study.
Salama et al (47) observed that calcified plaques were
commonly found in mid-to-late stage of atherosclerosis. In these
stages, the severity and extent of the disease is more serious than
in the early stages. In addition, the formation of atherosclerotic
plaque was a non-linear process, in which stable and unstable
plaques coexisted and calcified, and non-calcified and mixed
plaques could be observed in a patient at any time. Non-calcified
and mixed plaques can be frequently unstable and vulnerable to
rupture or erosion (48). However,
calcified plaques often coexist with more severe luminal stenosis
in mid- and late-stages of disease and are associated with
inflammatory processes (49,50). Once the rupture or erosion of
vulnerable plaque occurs, the degree of myocardial infarction can
be more serious than that of early stage disease in which only
non-calcified plaques exist. When plaques rupture or erode, the
level of serum NT-pro BNP increases (51–53).
The results in the present study suggested that the
increase of serum NT-pro BNP levels in patients with UA is a result
of the rupture or erosion of vulnerable plaques, and is relative to
the extent of lesion, degree of luminal stenosis and the number of
segments that are affected by calcified plaques. The present study
also demonstrated that the levels of serum NT-pro BNP in patients
with UA are significantly higher, as compared with those in control
groups, and that they are associated with the severity of the
lesion, the degree of luminal stenosis and LM-LAD disease, and the
number of segments affected by calcified plaques.
The results of the present study were concordant
with those of a previous study conducted by Palazzuoli et al
(22), who demonstrated that the
level of NT-pro BNP is positively correlated with SIS, SSS and the
number of involved vessels. Palazzuoli et al (54) observed that the level of NT-pro BNP
is higher in patients with >3 vessels implicated. Sadanandan
et al (55) observed that the
levels of serum NT-pro BNP increased in the coronary angiography
when the degree of luminal stenosis was >76% or LM-LAD was
present, and Radwan et al (41) reported similar findings when using
IVUS. It can therefore be speculated that high degree stenosis and
extensive lesions may weaken the compensatory abilities of the
myocardium, exacerbate myocardial ischemia and provoke the ongoing
release of NT-pro BNP and BNP. However, the same correlation in
patients with SAP was not observed in the current study.
Furthermore, the results from the present study were not concordant
with those reported by Weber et al (56), although that particular study did not
mention the incorporation of a time frame in which they extracted
blood samples, which is a critical stage in testing for serum
NT-pro BNP. However, an insufficient sample size in the present
study may have led to inaccurate results.
In the current study, the AUC of serum NT-pro BNP
levels in patients with UA was 0.656, which is marginally higher
than that of non-ST-elevated myocardial infarction, but lower than
that of ST-elevated myocardial infarction (47). The serum NT-pro BNM optimal cut-off
value was 55 pg/ml, sensitivity was 77.6% and specificity was
51.9%. There are large discrepancies between the results of the
present study and those reported by Liu et al (57), which may be due to the use of
different patient types; Liu et al (57) used patients with ACS. The levels of
serum NT-pro BNP were higher in patients with non-ST-elevated
myocardial infarction compared with patients with UA. In addition,
Liu et al (57) described
blood samples being drawn immediately following admission to
hospital, not at Tmax, which would lead to inaccurate
results; it is difficult to identify the Tmax in patients with
non-cardiac disease (58),
therefore, blood samples were taken 12–48 h following admission in
the present study. Furthermore, the use of different experimental
methods in the laboratory would also lead to inconsistent results
(8,59,60).
Univariant analysis in the present study concluded
that the OR of log(NT-pro BNP) was 3.841, but following
multivariant analysis, log(NT-pro BNP) was not categorized as an
independent risk factor of UA when atherosclerotic plaques were
detected by CCTA. In addition, the results of the present study
demonstrated that there were no statistically significant
differences between conventional risk factors of coronary heart
disease among the three experimental groups. Therefore, it can be
concluded that conventional risk factors have a limited value in
predicting MACE.
It has been demonstrated that the pathogenesis of UA
is not associated with the extent of lesions and the degree of
luminal stenosis, but rather with plaque stability (61,62). It
was observed that a large amount of non-calcified plaque was
unstable and vulnerable to rupture or erosion, and that the number
of segments with non-calcified plaque was higher in patients with
UA compared with the non-cardiac disease and SAP group. However, in
the present study there were no statistically significant
differences between the number of segments with non-calcified
plaque among the three patient groups. It can be speculated that
this was due to secondary thrombosis caused by plaque rupture or
erosion that may have activated fibrinolytic systems in the body.
Clots autolyzed subsequently. In coronary angiography, negative
results were often obtained; clot autolysis was further confirmed
by the HORIZONS-AMI Trial (63).
The limitations of the present study are as follows:
The study was a single center study, and further studies would be
required to acquire more accurate results; joint analysis with
regular blood biomarkers was not performed; and the prognostic
value of serum NT-pro BNP levels in patients with different
compositions of atherosclerotic plaques requires further
research.
In conclusion, the present study demonstrates that
the expression level of NT-pro BNP is associated with coronary
plaque, in particular calcified plaque, LM-LAD disease, and the
severity of vessel stenosis in patients with UA. It can be
suggested that NT-pro BNP is a marker of cardiac failure, and
indicates the severity of coronary artery atherosclerotic disease.
Although the expression level of NT-pro BNP was not an independent
risk factor of UA, it may be helpful in risk stratification in
patients with UA. Further research is required in order to explore
the value of NT-pro BNP and plaque detection by CCTA in the risk
stratification and prognosis of patients with UA.
Acknowledgements
The present study was supported by a grant from the
National Natural Science Foundation of China (no. 81371547).
References
|
1
|
Han ZJ, Wu XD, Cheng JJ, Zhao SD, Gao MZ,
Huang HY, Gu B, Ma P, Chen Y, Wang JH, et al: Diagnostic accuracy
of natriuretic peptides for heart failure in patients with pleural
effusion: A systematic review and updated meta-analysis. PLoS One.
10:e01343762015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Johnson KR and Olson KR: Responses of the
trout cardiac natriuretic peptide system to manipulation of salt
and water balance. Am J Physiol Regul Integr Comp Physiol.
296:R1170–R1179. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brutsaert DL: Cardiac dysfunction in heart
failure: The cardiologist's love affair with time. Prog Cardiovasc
Dis. 49:157–181. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stępień-Wałek AM and Wożakowska-Kapłon B:
The effect of left ventricle diastolic function on the secretion of
B-type natriuretic peptide at rest and directly after exercise test
in asymptomatic patients with diabetes or after myocardial
infarction with preserved left ventricular systolic function.
Kardiol Pol. Nov 12–2015.(Epub ahead of print). doi:
10.5603/KP.a2015.0216.
|
|
5
|
Morita E, Yasue H, Yoshimura M, Ogawa H,
Jougasaki M, Matsumura T, Mukoyama M and Nakao K: Increased plasma
levels of brain natriuretic peptide in patients with acute
myocardial infarction. Circulation. 88:82–91. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ellulu MS, Patimah I, Khaza'ai H, Rahmat
A, Abed Y and Ali F: Atherosclerotic cardiovascular disease: A
review of initiators and protective factors. Inflammopharmacology.
24:1–10. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lin E, Hashimoto B and Hwang W: Imaging of
subclinical atherosclerosis: questions and answers. Curr Probl
Diagn Radiol. 40:116–126. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Clerico A and Emdin M: Diagnostic accuracy
and prognostic relevance of the measurement of cardiac natriuretic
peptides: A review. Clin Chem. 50:33–50. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Doust JA, Glasziou PP, Pietrzak E and
Dobson AJ: A systematic review of the diagnostic accuracy of
natriuretic peptides for heart failure. Arch Intern Med.
164:1978–1984. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Roberts E, Ludman AJ, Dworzynski K,
Al-Mohammad A, Cowie MR, McMurray JJ and Mant J: NICE Guideline
Development Group for Acute Heart Failure: The diagnostic accuracy
of the natriuretic peptides in heart failure: Systematic review and
diagnostic meta-analysis in the acute care setting. BMJ.
350:h9102015. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Clerico A, Fontana M, Zyw L, Passino C and
Emdin M: Comparison of the diagnostic accuracy of brain natriuretic
peptide (BNP) and the N-terminal part of the propeptide of BNP
immunoassays in chronic and acute heart failure: A systematic
review. Clin Chem. 53:813–822. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Szilveszter B, Celeng C and
Maurovich-Horvat P: Plaque assessment by coronary CT. Int J
Cardiovasc Imaging. 32:161–172. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nakazato R, Otake H, Konishi A, Iwasaki M,
Koo BK, Fukuya H, Shinke T, Hirata K, Leipsic J, Berman DS and Min
JK: Atherosclerotic plaque characterization by CT angiography for
identification of high-risk coronary artery lesions: A comparison
to optical coherence tomography. Eur Heart J Cardiovasc Imaging.
16:373–379. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hoffmann U, Truong QA, Fleg JL, Goehler A,
Gazelle S, Wiviott S, Lee H, Udelson JE and Schoenfeld D: Design of
the rule out myocardial ischemia/infarction using computer assisted
tomography: A multicenter randomized comparative effectiveness
trial of cardiac computed tomography versus alternative triage
strategies in patients with acute chest pain in the emergency
department. Am Heart J. 163:330–338. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Puchner SB, Ferencik M, Maurovich-Horvat
P, Nakano M, Otsuka F, Kauczor HU, Virmani R, Hoffmann U and
Schlett CL: Iterative image reconstruction algorithms in coronary
CT angiography improve the detection of lipid-core plaque - a
comparison with histology. Eur Radiol. 25:15–23. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Puchner SB, Liu T, Mayrhofer T, Truong QA,
Lee H, Fleg JL, Nagurney JT, Udelson JE, Hoffmann U and Ferencik M:
High-risk plaque detected on coronary CT angiography predicts acute
coronary syndromes independent of significant stenosis in acute
chest pain: Results from the ROMICAT-II trial. J Am Coll Cardiol.
64:684–692. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou W, Ni Z, Yu Z, Shi B and Wang Q:
Brain natriuretic peptide is related to carotid plaques and
predicts atherosclerosis in pre-dialysis patients with chronic
kidney disease. Eur J Intern Med. 23:539–544. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hong YJ, Ahn Y, Sim DS, Yoon NS, Yoon HJ,
Kim KH, Park HW, Kim JH, Jeong MH, Cho JG, et al: Relation between
N-terminal pro-B-type natriuretic peptide and coronary plaque
components in patients with acute coronary syndrome: Virtual
histology-intravascular ultrasound analysis. Coron Artery Dis.
20:518–524. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Peng XL, Lin ZP, Zhang RK and Zhang ZW:
B-type natriuretic peptides and subclinical target organ damage in
essential hypertensive patients. Nan Fang Yi Ke Da Xue Xue Bao.
30:2347–2350. 2010.(In Chinese). PubMed/NCBI
|
|
20
|
Altintas S, Cardinaels EP, Versteylen MO,
Joosen IA, Seifert M, Wildberger JE, Crijns HJ, Nelemans PJ, Van
Dieijen-Visser MP, Mingels AM, et al: Unstable coronary plaque
characteristics are associated with high-sensitivity cardiac
troponin T and N-terminal pro-brain natriuretic peptide. J
Cardiovasc Comput Tomogr. 10:82–88. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Amsterdam EA, Wenger NK, Brindis RG, Casey
DE Jr, Ganiats TG, Holmes DR Jr, Jaffe AS, Jneid H, Kelly RF,
Kontos MC, et al: American College of Cardiology; American Heart
Association Task Force on Practice Guidelines; Society for
Cardiovascular Angiography and Interventions; Society of Thoracic
Surgeons; American Association for Clinical Chemistry: 2014 AHA/ACC
Guideline for the Management of Patients with Non-ST-Elevation
Acute Coronary Syndromes: A report of the American College of
Cardiology/American Heart Association Task Force on Practice
Guidelines. J Am Coll Cardiol. 64:e139–e228. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Taylor AJ, Cerqueira M, Hodgson JM, Mark
D, Min J, O'Gara P, Rubin GD, Kramer CM, Berman D, Brown A, et al:
American College of Cardiology Foundation Appropriate Use Criteria
Task Force; Society of Cardiovascular Computed Tomography; American
College of Radiology; American Heart Association; American Society
of Echocardiography; American Society of Nuclear Cardiology; North
American Society for Cardiovascular Imaging; Society for
Cardiovascular Angiography and Interventions; Society for
Cardiovascular Magnetic Resonance:
ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 appropriate use
criteria for cardiac computed tomography. A report of the American
College of Cardiology Foundation Appropriate Use Criteria Task
Force, the Society of Cardiovascular Computed Tomography, the
American College of Radiology, the American Heart Association, the
American Society of Echocardiography, the American Society of
Nuclear Cardiology, the North American Society for Cardiovascular
Imaging, the Society for Cardiovascular Angiography and
Interventions, and the Society for Cardiovascular Magnetic
Resonance. J Am Coll Cardiol. 56:1864–1894. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Abbara S, Arbab-Zadeh A, Callister TQ,
Desai MY, Mamuya W, Thomson L and Weigold WG: SCCT guidelines for
performance of coronary computed tomographic angiography: A report
of the Society of Cardiovascular Computed Tomography Guidelines
Committee. J Cardiovas Comput Tomogr. 3:190–204. 2009. View Article : Google Scholar
|
|
24
|
Raff GL, Abidov A, Achenbach S, Berman DS,
Boxt LM, Budoff MJ, Cheng V, DeFrance T, Hellinger JC and Karlsberg
RP: Society of Cardiovascular Computed Tomography: SCCT guidelines
for the interpretation and reporting of coronary computed
tomographic angiography. J Cardiovasc Comput Tomogr. 3:122–136.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Austen WG, Edwards JE, Frye RL, Gensini
GG, Gott VL, Griffith LS, McGoon DC, Murphy ML and Roe BB: A
reporting system on patients evaluated for coronary artery disease.
Report of the Ad Hoc Committee for Grading of Coronary Artery
Disease, Council on Cardiovascular Surgery, American Heart
Association. Circulation. 51(Suppl): 5–40. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Agatston AS, Janowitz WR, Hildner FJ,
Zusmer NR, Viamonte M and Detrano R: Quantification of coronary
artery calcium using ultrafast computed tomography. J Am Coll
Cardiol. 15:827–832. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Luscher TF: Substrates of acute coronary
syndromes: new insights into plaque rupture and erosion. Eur Heart
J. 36:1347–1349. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Turer AT, Lewis GD, O'Sullivan JF,
Elmariah S, Mega JL, Addo TA, Sabatine MS, de Lemos JA and Gerszten
RE: Increases in myocardial workload induced by rapid atrial pacing
trigger alterations in global metabolism. PLoS One. 9:e990582014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sabatine MS, Liu E, Morrow DA, Heller E,
McCarroll R, Wiegand R, Berriz GF, Roth FP and Gerszten RE:
Metabolomic identification of novel biomarkers of myocardial
ischemia. Circulation. 112:3868–3875. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Turer AT, Stevens RD, Bain JR, Muehlbauer
MJ, van der Westhuizen J, Mathew JP, Schwinn DA, Glower DD, Newgard
CB and Podgoreanu MV: Metabolomic profiling reveals distinct
patterns of myocardial substrate use in humans with coronary artery
disease or left ventricular dysfunction during surgical
ischemia/reperfusion. Circulation. 119:1736–1746. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lewis GD, Wei R, Liu E, Yang E, Shi X,
Martinovic M, Farrell L, Asnani A, Cyrille M, Ramanathan A, et al:
Metabolite profiling of blood from individuals undergoing planned
myocardial infarction reveals early markers of myocardial injury. J
Clin Invest. 118:3503–3512. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Scardovi AB: Associazione Nazionale Medici
Cardiologi Ospedalieri; Società Italiana di Cardiologia Pediatrica;
Società Italiana di Nefrologia Pediatrica; Società Italiana
dell'Ipertensione Arteriosa; Società Italiana di Pediatria:
Clinical applications of brain natriuretic peptide testing. Ital
Heart J Suppl. 5:343–356. 2004.(In Italian). PubMed/NCBI
|
|
33
|
Meune C, Fulla Y, Martins E, Bergmann JF
and Devaux JY: B-type natriuretic peptide for the diagnostic and
prognostic assessment in cardiology. Its interest and perspectives
of application. Presse Med. 32:181–185. 2003.(In French).
PubMed/NCBI
|
|
34
|
Clerico A, Recchia FA, Passino C and Emdin
M: Cardiac endocrine function is an essential component of the
homeostatic regulation network: Physiological and clinical
implications. Am J Physiology Heart Circ Physiol. 290:H17–H29.
2006. View Article : Google Scholar
|
|
35
|
Mazzone M, Forte P, Portale G, Mancini F,
Ursella S, La Sala M, Testa A, Covino M, Pignataro G and Gentiloni
Silveri N: Brain natriuretic peptide and acute coronary syndrome.
Minerva Med. 96:11–18. 2005.PubMed/NCBI
|
|
36
|
McCullough PA, Peacock WF, O'Neil B, de
Lemos JA, Lepor NE and Berkowitz R: An evidence-based algorithm for
the use of B-type natriuretic testing in acute coronary syndromes.
Rev Cardiovasc Med 11 Suppl. 2:S51–S65. 2010.
|
|
37
|
Sun C, Zhi J, Bai X, Li X and Xia H:
Comparison of the efficacy of recombinant human brain natriuretic
peptide with saline hydration in preventing contrast-induced
nephropathy in patients undergoing coronary angiography with or
without concomitant percutaneous coronary intervention. Int J Clin
Exp Med. 8:14166–14172. 2015.PubMed/NCBI
|
|
38
|
Zürcher S, Honegger U, Wagener M, Lee G,
Stallone F, Marxer T, Puelacher C, Schumacher C, Sou SM, Twerenbold
R, et al: Delayed release of brain natriuretic peptide to identify
myocardial ischaemia. Eur J Clin Invest. 45:1175–1183. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sun Z and Xu L: Coronary CT angiography in
the quantitative assessment of coronary plaques. Biomed Res Int.
2014:3463802014.PubMed/NCBI
|
|
40
|
Obaid DR, Calvert PA, Gopalan D, Parker
RA, West NE, Goddard M, Rudd JH and Bennett MR: Dual-energy
computed tomography imaging to determine atherosclerotic plaque
composition: A prospective study with tissue validation. J
Cardiovasc Comput Tomogr. 8:230–237. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Radwan H, Selem A and Ghazal K: Value of
N-terminal pro brain natriuretic peptide in predicting prognosis
and severity of coronary artery disease in acute coronary syndrome.
J Saudi Heart Assoc. 26:192–198. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
James SK, Lindahl B, Siegbahn A,
Stridsberg M, Venge P, Armstrong P, Barnathan ES, Califf R, Topol
EJ, Simoons ML and Wallentin L: N-terminal pro-brain natriuretic
peptide and other risk markers for the separate prediction of
mortality and subsequent myocardial infarction in patients with
unstable coronary artery disease: A Global Utilization of
Strategies To Open occluded arteries (GUSTO)-IV substudy.
Circulation. 108:275–281. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hong YJ, Ahn Y, Sim DS, Yoon NS, Yoon HJ,
Kim KH, Park HW, Kim JH, Jeong MH, Cho JG, et al: Relation between
N-terminal pro-B-type natriuretic peptide and coronary plaque
components in patients with acute coronary syndrome: virtual
histology-intravascular ultrasound analysis. Coron Artery Dis.
20:518–524. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Beaufrere H, Nevarez JG, Holder K, Pariaut
R, Tully TN and Wakamatsu N: Characterization and classification of
psittacine atherosclerotic lesions by histopathology, digital image
analysis, transmission and scanning electron microscopy. Avian
Pathol. 40:531–544. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Stary HC, Chandler AB, Dinsmore RE, Fuster
V, Glagov S, Insull W Jr, Rosenfeld ME, Schwartz CJ, Wagner WD and
Wissler RW: A definition of advanced types of atherosclerotic
lesions and a histological classification of atherosclerosis. A
report from the Committee on Vascular Lesions of the Council on
Arteriosclerosis, American Heart Association. Circulation.
92:1355–1374. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Saremi F and Achenbach S: Coronary plaque
characterization using CT. AJR Am J Roentgenol. 204:W249–W260.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Salama RH, El-Moniem AE, El-Hefney N and
Samor T: N-Terminal PRO-BNP in acute coronary syndrome patients
with ST elevation versus non ST elevation in Qassim region of Saudi
Arabia. Int J Health Sci (Qassim). 5:136–145. 2011.PubMed/NCBI
|
|
48
|
Maurovich-Horvat P, Ferencik M, Voros S,
Merkely B and Hoffmann U: Comprehensive plaque assessment by
coronary CT angiography. Nat Rev Cardiol. 11:390–402. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Min JK, Lin FY, Dunning AM, Delago A, Egan
J, Shaw LJ, Berman DS and Callister TQ: Incremental prognostic
significance of left ventricular dysfunction to coronary artery
disease detection by 64-detector row coronary computed tomographic
angiography for the prediction of all-cause mortality: results from
a two-centre study of 5330 patients. Eur Heart J. 31:1212–1219.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Sozzi FB, Civaia F, Rossi P, Robillon JF,
Rusek S, Berthier F, Bourlon F, Iacuzio L, Dreyfus G and Dor V:
Long-term follow-up of patients with first-time chest pain having
64-slice computed tomography. Am J Cardiol. 107:516–521. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Shmilovich H, Cheng VY, Tamarappoo BK, Dey
D, Nakazato R, Gransar H, Thomson LE, Hayes SW, Friedman JD,
Germano G, et al: Vulnerable plaque features on coronary CT
angiography as markers of inducible regional myocardial
hypoperfusion from severe coronary artery stenoses.
Atherosclerosis. 219:588–595. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Schlett CL, Nance JW Jr, Schoepf UJ,
O'Brien TX, Ebersberger U, Headden GF, Hoffman U and Bamberg F:
Differences in coronary artery disease by CT angiography between
patients developing unstable angina pectoris vs. major adverse
cardiac events. Eur J Radiol. 83:1113–1119. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chow BJ, Wells GA, Chen L, Yam Y,
Galiwango P, Abraham A, Sheth T, Dennie C, Beanlands RS and Ruddy
TD: Prognostic value of 64-slice cardiac computed tomography
severity of coronary artery disease, coronary atherosclerosis, and
left ventricular ejection fraction. J Am Coll Cardiol.
55:1017–1028. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Palazzuoli A, Gennari L, Calabria P,
Quatrini I, Vecchiato L, De Paola V, Campagna MS, Palazzuoli V and
Nuti R: Relation of plasma brain natriuretic peptide levels in
non-ST-elevation coronary disease and preserved systolic function
to number of narrowed coronary arteries. Am J Cardiol.
96:1705–1710. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Sadanandan S, Cannon CP, Chekuri K, Murphy
SA, Dibattiste PM, Morrow DA, de Lemos JA, Braunwald E and Gibson
CM: Association of elevated B-type natriuretic peptide levels with
angiographic findings among patients with unstable angina and
non-ST-segment elevation myocardial infarction. J Am Coll Cardiol.
44:564–568. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Weber M, Dill T, Arnold R, Rau M, Ekinci
O, Müller KD, Berkovitsch A, Mitrovic V and Hamm C: N-terminal
B-type natriuretic peptide predicts extent of coronary artery
disease and ischemia in patients with stable angina pectoris. Am
Heart J. 148:612–620. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Liu B, Fu Q, Yan QN, Jin W, Tao DP, Hua JH
and Li ZL: Value of biochemical marker detection in risk
stratification in patients with acute coronary syndrome. Nan Fang
Yi Ke Da Xue Xue Bao. 30:1015–1019. 2010.PubMed/NCBI
|
|
58
|
Lewandrowski K, Chen A and Januzzi J:
Cardiac markers for myocardial infarction. A brief review. Am J
Clin Pathology. 118(Suppl): S93–S99. 2002.
|
|
59
|
Clerico A, Prontera C, Emdin M, Passino C,
Storti S, Poletti R, Zyw L and Zucchelli GC: Analytical performance
and diagnostic accuracy of immunometric assays for the measurement
of plasma B-type natriuretic peptide (BNP) and N-terminal proBNP.
Clin Chem. 51:445–447. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Apple FS, Panteghini M, Ravkilde J, Mair
J, Wu AH, Tate J, Pagani F, Christenson RH and Jaffe AS: Committee
on Standardization of Markers of Cardiac Damage of the IFCC:
Quality specifications for B-type natriuretic peptide assays. Clin
Chem. 51:486–493. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Dey D, Achenbach S, Schuhbaeck A,
Pflederer T, Nakazato R, Slomka PJ, Berman DS and Marwan M:
Comparison of quantitative atherosclerotic plaque burden from
coronary CT angiography in patients with first acute coronary
syndrome and stable coronary artery disease. J Cardiovasc Comput
Tomogr. 8:368–374. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Toutouzas K, Stathogiannis K, Synetos A,
Karanasos A and Stefanadis C: Vulnerable atherosclerotic plaque:
From the basic research laboratory to the clinic. Cardiology.
123:248–253. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Larsen AI, Nilsen DW, Yu J, Mehran R,
Nikolsky E, Lansky AJ, Caixeta A, Parise H, Fahy M, Cristea E, et
al: Long-term prognosis of patients presenting with ST-segment
elevation myocardial infarction with no significant coronary artery
disease (from the HORIZONS-AMI trial). Am J Cardiol. 111:643–648.
2013. View Article : Google Scholar : PubMed/NCBI
|